Abstract
Delayed vertebral collapse after osteoporotic spinal fractures may cause progressive kyphosis, neurological deficits, and chronic back pain. We treated 14 consecutive patients with posterolateral decompression and posterior reconstruction and followed them over a period ranging from 24 to 54 months. The mean age was 67 (range: 62–72) years and the fracture level was T12 and L1. Seven patients were graded as Frankel stage C and seven as stage D. The mean segmental kyphotic angle was 22.6° (7–29°) preoperatively, 4.4° (1–6°) postoperatively, and 6.8° (2–15°) at the final follow-up. The pain score on a visual analogue scale improved from 9.5 preoperatively to 2.7 postoperatively, and the neurological status improved in all patients. Bone fusion was present 9 months after operation. Of four surgical complications, two were dural tears, one a superficial infection, and there was one death due to an acute adrenal insufficiency. Posterolateral decompression with posterior reconstruction is a useful treatment for patients with delayed osteoporotic vertebral collapse.
Résumé
Le collapsus vertébral secondaire, après une fracture vertébrale ostéoporotique, peut créer une cyphose progressive, un déficit neurologique et des lombalgies chroniques. Nous avons traité 14 malades consécutifs par décompression postérolaterale et reconstruction postérieure et les avons suivis de 24 à 54 mois. L'âge moyen était 67 (62–72) années. Le niveau de la fracture était T12 et L1. Sept malades ont été notés comme Frankel stade C et sept comme stade D. L'angle cyphotique segmentaire moyen était 22.6° (7–29°) avant l'opération, 4.4° (1–6°) postopératoire, et 6.8° (2–15°) au dernier examen. Le score de la douleur sur une échelle analogue visuelle s'est amélioré de 9.5 en préopératoire à 2.7 en postopératoire. Le statut neurologique a été amélioré chez tous les malades. La fusion osseuse était visible 9 mois après la chirurgie. Il y avait quatre complications chirurgicales, deux brèches duremériennes, une infection superficielle et un décés dû à une insuffisance surrénale aiguë. La décompression posterolateral avec reconstruction postérieure est une bonne option de traitement chez les malades avec collapsus vertébral différé.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is the most common cause of a spinal compression fracture, and most of these are stable and can be managed conservatively. However, vertebral collapse may occur later in a portion of the osteoporotic spine compression fracture area [1, 2, 3, 4, 5, 7, 8, 12, 13]. This delayed vertebral collapse may cause progressive kyphosis, neurological deficits, and chronic back pain necessitating surgical correction [6, 7, 9, 14]. However, most patients with osteoporotic spinal fractures have other old-age health problems, and their poor bone mass makes operative fixation difficult.
The purpose of our study was to assess the value of posterolateral decompression with posterior reconstruction after delayed osteoporotic vertebral collapse in patients with neurological deficits associated with compression spinal fractures.
Material and methods
Fourteen consecutive patients with delayed vertebral collapse and neurological deficits resulting from osteoporotic spinal fractures underwent posterolateral decompression with posterior reconstruction in order to correct kyphosis, to aid recovery from neurological deficits, and to relieve back pain. Mean follow-up was 36 (range: 24–54) months. There were 12 female and two male patients, and their mean age was 67 (range: 62–72) years . Associated medical disorders included diabetes mellitus in six patients, hypertension in five, and Cushing's syndrome in six. Levels of fracture were T12 in four patients and L1 in ten. Preoperative neurological symptoms comprised motor weakness in 14 patients, increased deep tendon reflexes in four, and voiding or defecation difficulty in seven. Preoperative neurological status using Frankel's system was grade C in seven patients and grade D in seven.
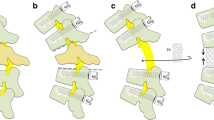
Under general endotracheal anaesthesia, patients were placed prone on the operating table, which was then 'flexed' into a reverse V position. Subperiosteal dissection exposed the posterior elements as far laterally as the transverse processes. Pedicle screws were inserted into the two or three segments above and below the pedicles to be resected. After identification, partial resection of both pedicles was carried out. Curettes were then used to remove the necrotic cancellous bone and fibrous tissue within the vertebral body, taking care to avoid injuring the endplates. With the aid of angled curettes, the cancellous bone of the body was pushed anteriorly into the body in order to create a cavity in the vertebrae. After thinning the posterior wall with curettes, the remaining posterior cortex was dissected from the adherent dura and pushed into the body so as to decompress the spinal canal. The pedicle screws were next connected to the rod and distracted in order to correct the kyphosis and restore the area of collapse. The vertebral body was filled with a mixture of autogenous and allogenous bone (Fig. 1) and reinforced by posterior fusion.
Posterolateral decompression and posterior reconstruction. A Removal of medial part of both pedicles; B Curettage of necrotic bone and fibrocartilaginous tissue; C Dissection of posterior wall of vertebral body from dura and pushing down the retropulsed bony fragments; D Impacted bone graft with autogenous and allogenous bone;E Posterior reconstruction using pedicle screw system
Spinal cord function was continuously monitored by somato-sensory-evoked potentials. A wake-up test was performed in all patients during the surgical closure.
We recorded the operation time; amount of blood loss; preoperative, postoperative, and final follow-up kyphotic angle; union time; clinical outcome; and complications. The kyphotic angle was measured between a line drawn on the upper endplate of the proximal vertebral body and a line on the lower endplate of the distal vertebral body. Homogeneous radiodensity of vertebral bodies on plain radiography with continuity of bony trabeculae was considered a sign of union. Clinical outcomes for back pain were assessed using a visual analogue scale (VAS) and neurological status using the Frankel grading system.
Results
Mean operating time was 217 (range: 150–300) min, and mean blood loss was 682 (range: 420–1,210) ml. Mean kyphotic angle was 22.6° (7–29°) on preoperative radiographs. This was corrected to 4.4° (1–6°) immediately after operation but then deteriorated very slightly to 6.8° (2–15°) at final follow-up. Mean union time of the intracorporeal bone graft was 9 months. Postoperatively, the neurological status improved in all patients and was grade D in two and grade E in 11.Mean pain score recorded on a VAS was 9.5 preoperatively, and this was improved by operation to a mean of 2.7.
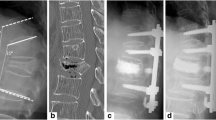
There were four complications: The dura was torn during two operations; one occurred because the anterior aspect of the conus was adherent to the posterior wall of the vertebral body and thus prevented direct repair—cover was obtained by using gel foam and fibrin glue; and there was one superficial infection that was treated with wound dressing and antibiotics. One patient died on the seventh postoperative day due to acute adrenal insufficiency, which first appeared on the third postoperative day. There was no evidence of either metal failure or vertebral body collapse at final follow-up (Fig. 2).
Plain radiography and magnetic resonance (MR) image of a 68-year-old woman with osteoporotic spine compression fracture and delayed vertebral collapse. A Lateral radiography at 2 and 4 months after trauma shows a compression fracture of T12;B Plain radiography and magnetic resonance (MR) image at 9 months after injury shows delayed vertebral collapse with vacuum cleft and low signal intensity on T1-weighted image; C Postoperative and follow-up plain radiography show good sagittal alignment and fusion state
Discussion
Most osteoporotic spine fractures are of wedge compression type, and they are therefore stable fractures with loss of height of their anterior column. Fortunately, this seldom results in instability or any neurological deficit [7, 14]. However, in 1891 Kummel reported delayed vertebral collapse after trauma to a vertebral body and called it Kummel's disease [5]. According to Parfitt and Duncan [12], an important difference between vertebral fractures due to osteoporosis and traumatic fractures of vertebral bone of normal density is the complete absence of any risk of spinal cord involvement in the latter. However, there are several accounts of paraplegia or other neurological complications in the spinal canal due to delayed posttraumatic or atraumatic vertebral collapse in osteoporotic patients, and these were published after Kempinsky's report [1, 5, 7, 8, 13, 14].
The cause of this posttraumatic vertebral collapse remains uncertain. There are some reports of associated bone ischaemia, and others mention vertebral pseudarthrosis as a possible pathological mechanism resulting in posttraumatic vertebral collapse [2, 3, 4, 5]. Kaneda et al. [7] reported that the collapsed portion of the resected vertebral bodies was always less 'bloody', or ischaemic, and that the histology of the collapsed portion revealed necrosis, which might be secondary to ischaemia. They also suggested that the collapse could be caused by fractures of osteoporotic and fragile trabecular bone due to repeated micro-traumas and to ischaemia.
Hasegawa et al. [4] examined histologically the 'intervertebral vacuum cleft' and found that the cleft surface was covered with smooth white tissue that grossly resembled fibrocartilage. Microscopically, they found that there were chondrocytes buried within the fibrous stromas, and that this tissue also contained many flattened fibroblastic cells. There was very little evidence of fracture healing. Maldague et al. [10] reported a case with an intravertebral cleft, which initially probably contained gas. Subsequent examination a month later revealed replacement of the 'gaseous density' in the cleft by a 'water density', and that this was associated with progressive collapse of the vertebra. Malghem et al. [11] showed the progressive disappearance of a 'gas-like' area of radiolucency on stress radiographs and the concurrent appearance of an area of 'fluid-like' signal intensity on magnetic resonance (MR) imaging. Hasegawa et al. [4] aspirated the fluid and found its composition similar to plasma, except for low levels of protein and albumin. They concluded, therefore, that the cleft was a false joint.
In our study, we also found these intervertebral vacuum clefts on plain radiography (Fig. 2b). They were revealed by a low signal intensity on T1-weighted MR images (Fig. 2b) and a high signal intensity on T2-weighted MR images. At operation, the vacuum cleft was filled with a serous liquid resembling synovial fluid. Tissue removed from the surrounding bone was a mixture of fibrous granulation tissue, necrotic bone, and newly formed woven bone on pre-existing lamellar bone. These findings support both ischaemic and pseudarthrosis theories.
Kaneda et al. [7] reported 22 cases of osteoporotic posttraumatic vertebral collapse. Their patients were neurologically intact immediately after the injury but developed neurological deficits1–12 months after the injury.
In the current study, 11 of our patients had mild compression fractures and three gave no history of any injury. All were neurologically intact when first seen but developed neurological problems at a mean of 9.5 months after the original event. Therefore, close observation is recommended, even with a mild compression fracture in an osteoporotic patient. With regard to the treatment of patients with neurological deficits due to delayed vertebral collapse after osteoporotic spinal fractures, decompressive laminectomy in Kempinsky's series failed due to kyphosis progression and further neurological deterioration after this operation [8]. In 1988, Salomon et al. [13] first stabilised the spine posteriorly using the Cortrel-Dubousset instrumentation and then proceeded to an anterior decompression at the same operative session. In 1989, Arciero et al. [1] emphasised the importance of anterior decompression combined with fusion as the best method of treatment. They used methylmethacrylate alone with a single Knot rod for anterior stabilisation after decompression. In 1992, Kaneda et al. [7] performed anterior decompression and anterior stabilisation with bioactive ceramic and the Kaneda device. By contrast, Shikata et al. [14] performed posterior decompression and posterior stabilisation. They performed a wide laminectomy, removed the retropulsed bone fragments through the pedicle, and stabilised posteriorly using Harrington distraction rods and sublaminar wires.
In our study, we considered several factors when deciding which treatment to use. All 14 patients had neurological deficits, progressive kyphosis, and back pain and therefore needed decompression and stabilisation. With regard to methods for decompression, an anterior approach may be easier and safer, as the spinal cord is compressed by the 'retropulsed' bone fragments. However, an anterior approach for problems at the thoracolumbar junction in this patient group was considered too aggressive because of factors such as old age (mean 67 years) and the presence of other medical disorders. In contrast, posterior decompression avoids the need for sectioning the diaphragm or for opening either the thoracic cavity or the retroperitoneal space, both of which are inevitable with an anterior thoracolumbar approach for T12 or L1. When methods for stabilisation are considered, anterior fixation is not very secure in the osteoporotic spine because the vertebral body consists mostly of cancellous bone and the cortical bone of the vertebral body is also very thin. In contrast, posterior fixation using the pedicle screw system provides relatively stable fixation even in the osteoporotic spine because the pedicle remains a strong part of the vertebra. On account of these factors, the authors decided to perform posterior decompression through the pedicles and posterior stabilisation using a pedicle screw system.
Due to the fact that the superior endplate is located very near to the pedicles, caution must be taken to avoid any injury to the upper endplate during curettage while performing the posterior decompression through the pedicles. In fact, in our series, the upper endplate and the disc were injured only once during curettage and, in this patient, the upper endplate and disc were removed and an interbody fusion using a 'cage' was then performed.
In the series, there were two dural tears. The dura is frequently adherent to the posterior wall of the vertebral body and therefore careful dissection between the dura and the posterior wall is essential.
One patient with Cushing's syndrome died on the seventh day after surgery due to acute adrenal insufficiency. All patients were elderly and had associated medical disorders (hypertension, diabetes mellitus, Cushing syndrome). Therefore, proper perioperative management for these problems is crucial.
In conclusion, we performed posterolateral decompression and posterior reconstruction as the treatment for delayed vertebral collapse in patients with neurological deficits after an osteoporotic spine fracture. Neurological deficits improved satisfactorily, kyphosis was corrected, and back pain was relieved after surgery in all patients.
Based on the results of this study, posterolateral decompression with posterior reconstruction is a useful treatment for delayed vertebral collapse with neurological deficits after osteoporotic spinal fracture.
References
Arciero RA, Leung KY, Pierce JH (1989) Spontaneous unstable burst fracture of the thoracolumbar spine in osteoporosis. Spine 14: 114–117
Brower AC, Downey EF Jr (1981) Kummel disease: Report of a case with serial radiographs. Radiology 1341: 363–364
Chou LH, Knight RQ (1997) Idiopathic avascular necrosis of a vertebral body. Spine 22: 1928–1932
Hasegawa K, Homma T, Uchiyama S, Takahashi H (1988) Vertebral pseudarthrosis in the osteoporotic spine. Spine 23: 2201–2206
Hermann G, Goldblatt J, Desnick RJ (1984) Case reports, Kummell disease: delayed collapse of the traumatized spine in a patient with Gaucher Type 1 disease. Br J Radiol 57: 833–835
Hu SS (1997) Internal fixation in the osteoporotic spine. Spine 22: 43–48
Kaneda K, Asano S, Hashimoto T, Satoh S, Fujiya M (1992) The treatment of osteoporotic-posttraumatic vertebral collapse using the Kaneda device and bioactive ceramic vertebral prosthesis. Spine 17: 295–303
Kempinsky WH (1958) Osteoporotic kyphosis with paraplegia. Neurology 8: 181–186
Kim SW, Chung YK (2001) The surgical reconstruction of osteoporotic vertebral fractures. J Kor Soc Fracture 14: 30–36
Maldague BE, Noel HM, Malgehm JJ (1978) The intravertebral vacuum cleft. Radiology 129: 23–29
Malghem J, Maldague B, Labaisse MA, Dooms G, Duprez T, Devogelaer JP, Vande Berg B (1993) Intravertebral vacuum cleft: changes in content after supine position. Radiology 187: 483–487
Parfitt AM, Duncan H (1982) Metabolic bone disease affecting the spine. In: Rothman RH , Simone FA (eds) The spine, vol 2, 2nd edn. Saunders, Philadelphia, pp 828–830
Salomon C, Chopin D, Benoist M (1988) Spinal cord compression: An exceptional complication of spinal osteoporosis. Spine 13: 222–224
Shikata J, Yamamuro T, Ida H, Shimizu K, Yoshikawa J (1990) Surgical treatment for paraplegia resulting from vertebral fractures in senile osteoporosis. Spine 5: 485–489
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, KT., Suk, KS., Kim, JM. et al. Delayed vertebral collapse with neurological deficits secondary to osteoporosis. International Orthopaedics (SICOT) 27, 65–69 (2003). https://doi.org/10.1007/s00264-002-0418-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-002-0418-5