Abstract
Considerable evidence shows that the tumor microenvironment is an active participant in preventing immunosurveillance and limiting the efficacy of anticancer therapies. Hypoxia is a prominent characteristic of the solid tumor microenvironment. The transcription factor hypoxia-inducible factor-1α (HIF-1α) is an important mediator of hypoxic response of tumor cells that modulates the expression of specific genes involved in tumor immunosuppression. Using a 4T1 breast cancer model, we show that in vivo administration of PX-478, an inhibitor of oxygen-sensitive HIF-1α, led to reduced expression of Foxp3 and VEGF transcript and/or protein, molecules that are directly controlled by HIF-1. When combined with dendritic cell (DC)-based vaccination, HIF-1α inhibition resulted in an augmented cytotoxic T lymphocyte effector function, improved proliferation status of T cells, increased production of inflammatory cytokine IFN-γ, as well as reduced regulatory function of T cells in association with slower tumor growth. Taken together, our findings indicate that the use of HIF-1α inhibition provides an immune adjuvant activity, thereby improves the efficacy of tumor antigen-based DC vaccine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite advances in the development of immunotherapies, clinical trials of different cancer vaccines performed in recent years demonstrated a lack of clinical efficacy [1]. A major obstacle to the therapeutic efficacy of cancer immunotherapies is the immunosuppressive tumor microenvironment [2]. These immunosuppressive factors include the production of inhibitory cytokines such as IL-10 and transforming growth factor-β (TGFβ) and immunoregulatory agents such as galectins, lactic acid, adenosine, prostaglandins; the expression of negative co-stimulatory ligands such as programmed cell deathligand 1 (PDL1); and the presence of regulatory T (Treg) and myeloid-derived suppressor cells (MDSCs).
Hypoxia, a common characteristic of all solid tumors, has been proven to elicit mechanisms inhibiting anti-tumor immune cells and thereby protect cancerous tissues [3–7]. These mechanisms are operated via activating adaptive transcriptional responses that are involved in crucial aspects of cancer biology, including angiogenesis, cell survival, glucose metabolism, and invasion. The transcriptional response to hypoxia is mediated primarily by hypoxia-inducible factors (HIF) [8, 9]. HIF is a heterodimer molecule comprised of the constitutively expressed HIF-1β subunit, which partners with one of three oxygen-sensitive α-subunits: HIF-1α, HIF-2α, or HIF-3α. Alpha subunits are posttranslationally stabilized under hypoxic conditions and dimerize with HIF-1β following translocation to the nucleus, where they transactivate the hypoxia response element (HRE) present in the promoter of related genes [10].
Given its critical role in immune regulation and resistance to therapy, tumor hypoxia might be considered as a potential target in cancer therapy. In this regard, targeting HIF-1 appears to be a possible therapeutic approach to break down the immunosuppressive regulatory network of tumor microenvironment. Inhibition of HIF-1 in animal model systems leads to decrease in tumorigenesis and increase in survival [11]. A growing number of drugs have been identified as HIF-1 inhibitors and been validated as anticancer agents [10, 12]. PX-478 (S-2-amino-3-[4 V-N,N,-bis(2-chloroethyl) amino]-phenyl propionic acid N-oxide dihydrochloride) is a small-molecule shown to suppress HIF-1α translation under normoxic and hypoxic conditions, as well as hypoxia-induced HIF-1α transcriptional activity and VEGF expression, in a variety of cancer cell lines [13–15].
In this study, we explored the effects of HIF-1 inhibition using administration of PX-478 in the murine breast cancer model 4T1 and investigated the possibility of combining this drug with a DC-based tumor immunotherapy. We demonstrated for the first time that this strategy can be exploited to induce tumor regression and increase survival. In fact, this strategy can improve DC vaccines for the treatment of cancer via induction of tumor specific T cell activity.
Materials and methods
Mice and cell lines
Female BALB/c mice, 6–8 weeks old, were obtained from the Lab Animal Center, Pasteur Institute of Iran. Animal protocols were approved by the Institutional Animal Care and Use Committee of Tehran University of Medical Sciences. The 4T1 carcinoma cell line which is of BALB/c origin was cultured in complete media [CM; RPMI-1640 (Biosera, UK) supplemented with 100 units/mL penicillin, 100 µg/ml streptomycin, 10 mmol/L l-glutamine (Biosera, UK), and 10 % heat-inactivated FBS (Gibco, Grand Island, USA)] in a humidified incubator at 37 ͦ C and 5 % CO2. Tumors were established by injection of 7 × 105 4T1 tumor cells s.c. into the right flank of syngeneic mice, with tumor size (in mm3) assessed every 2 days thereafter. Mice were sacrificed when tumors became ulcerated, or they reached a size of 400 mm2.
Dendritic cell vaccine
Bone marrow-derived dendritic cells (BMDCs) were generated according to Inaba protocol [16] with some modifications. In brief, bone marrow cells were flushed from BALB/c femur and tibia and cultured for 6 days in the presence of 20 ng/ml recombinant murine GM-CSF (PeproTech, London, UK) and 10 ng/ml recombinant murine IL-4 (PeproTech, London, UK). The complete culture medium included RPMI 1640 medium (Gibco, USA) supplemented with 10 % heat-inactivated fetal bovine serum (Gibco, USA), 2 mM l-glutamine (Biosera, UK), 100 µg/ml streptomycin, and 100 U/ml penicillin (Biosera, UK). The culture medium was replaced with fresh media in days 3 and 5. In all steps, cells were incubated in a 5 % CO2 incubator. Immature DCs were pulsed on day 6 with 100 µg/ml tumor cell lysate (for 18 h) prepared by subjecting 4 × 107 4T1 cells/ml in PBS to six cycles of rapid freezing in liquid nitrogen and thawing at 37 °C. The lysates were spun at 10,000 rpm for 10 min to remove particulate cellular debris. Dendritic cells were then stimulated by adding 1 µg/ml LPS (Sigma-Aldrich) 14 h before harvesting cells.
PX-478-based therapy
HIF-1α inhibitor, PX-478 purchased from MedKoo Biosciences. For in vivo use, tumor-bearing mice were i.p. administered PX-478 at 40 mg/kg or normal saline in a total volume of 200 µl, three times a week for 2 weeks, beginning approximately 6 days after tumor inoculation, when tumors were ~25 mm2 in the area.
Flow cytometry
Single-cell suspensions were stained using the following fluorescently labeled antibodies: APC-conjugated anti-CD3 and anti-CD107a or FITC-conjugated anti-CD8 (Biolegend, USA); or matched, fluorochrome-labeled isotype control monoclonal antibody (mAb). Flow cytometry was conducted using a FACSCalibur flow cytometer (Becton-Dickenson, Mountain View, CA, USA) and analyzed with FlowJo software.
RNA isolation and real-time quantitative PCR
RNA was extracted from frozen tissues with Hybrid-R RNA purification kit (GeneAll Biotechnology, Korea). 1 µg of RNA was reverse transcribed into complementary DNA (cDNA) using QuantiTect Reverse Transcription kit (Qiagen). cDNAs were quantified by real-time PCR using a SYBR Green real-time PCR master mix (Primer design, UK) on an ABI 7500 detection system (Applied biosystems). Relative mRNA levels were determined using the ∆Ct method. Values were expressed relative to endogenous β-actin. The following PCR primers were used: HIF-1α forward, 5′- AGCTTCTGTTATGAGGCTCACC-3′; HIF-1α reverse, 5′- TGACTTGATGTTCATCGTCCTC-3′ [17]; VEGF forward, 5′- GCGGAGAAAGCATTTGTTTG-3′; VEGF reverse, 5′-TCTTTCCGGTGAGAGGTCTG-3′; Foxp3 forward, 5′-GCAGGGCAGCTAGGTATCTGTAG-3′; Foxp3 revers, 5′-TCGGAGATCCCCTTTGTCTTATC-3′; β-actin forward, 5′-GGTCATCACTATTGGCAACG-3′; and β-actin reverse, 5′-ACGGATGTCAACGTCACACT-3′.
Western blot
Tumors were homogenized in whole-cell extraction buffer (20 mM Tris–HCl, pH = 7.4; 150 mM NaCl; 2 mM ethylenediaminetetraacetic acid; 1 % Triton X100; 0.1 % Sodium Dodecyl Sulfate and complete protease inhibitor cocktail). After being centrifuged at 13,000 rpm, the supernatant was collected, and the protein concentration was quantified with Thermo Scientific BCA protein assay using bovine serum albumin (BSA) as standard. Forty micrograms of total proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride (PVDF) membrane (GE Healthcare Life Sciences) and probed with primary Abs specific for HIF-1α (eBiosciences Technology, CA), Foxp3 (Biolegend), and actin (Sigma-Aldrich), followed by respective HRP-conjugated secondary antibodies. Reactive bands were visualized using an ECL Western blotting detection kit (Thermo Scientific).
HIF-1α immunohistochemistry
Paraffin-embedded tumor sections were heated at 60º C for 30 min and rehydrated through xylene and graded alcohols. Slides were subjected to heat-induced epitope retrieval using 0.01 M citrate buffer for 10 min in a microwave oven. The slides were blocked with 0.1 % PBS-triton buffer containing 2 % BSA for 2 h. Endogenous peroxidase activity was quenched using PBS containing 0.3 % hydrogen peroxide for 10 min. The sections underwent an overnight incubation with mouse anti-mouse HIF-1α mAb (Biolegend). After washing with PBS, sections were incubated with HRP-conjugated secondary antibody (Biolegend) for 1 h to detect antigen–antibody complexes. The sections were then developed with DAB (diamino-benzidine tetrachloride) solution. Finally, sections were counterstained with hematoxylin, dehydrated, mounted, and observed.
CD107 cytotoxicity assays
106 splenic cells were cocultured with 2 × 105 4T1 tumor cells (derived from culture) for 6 h in the presence of anti-CD107 antibody and 10 µmol/l monensin (Biolegend). Cultures were allowed to incubate at 37 °C for 6 h. After the incubation, cells were washed and stained with anti-CD8 followed by flow cytometric analysis as described above.
Proliferation assays
Splenic cells were labeled with 5 mmol/L CFSE, according to the manufacturer’s instructions (Invitrogen). 2 × 105 CFSE-labeled splenic cells per well were incubated in the presence of 80 μg/ml tumor lysate in complete medium. After 5 days of incubation, cells were washed and stained with anti-CD3 followed by analysis by flow cytometry. Cell proliferation was determined by measuring the dilution of CFSE by flow cytometry after gating on the CD3+ cell populations.
Suppression assays
Naïve mice were injected subcutaneously by 100 µg tumor lysate, after 2 weeks, the spleens were excised, dissociated, and cells were incubated on nylon wool columns (37 °C, 45 min) after red blood cell lysis. More than 80 % of the eluted cells were T lymphocytes based on T cell receptor (TCR)αβ expression. Isolated T cells were labeled with 5 mmol/L CFSE, and 5 × 104 cells were plated in 96-well plates with 5 × 104 nylon wool-isolated splenic T cells from 4T1 tumor-bearing mice from each treatment state, as the source of Treg cells. 5 × 104 tumor antigen pulsed- or un-pulsed DCs were then added to the culture. After 5 days, cells were harvested and stained with anti-CD3, (Biolegend) and cell proliferation was determined through measuring the dilution of CFSE by flow cytometry after gating on the CD3+ cell populations. The division index (DI) was reported as the indicator of cell proliferation. Total proliferation was calculated as (DI+) − (DI−), with DI+ is the division index of the T cells stimulated with pulsed DCs in the presence of Tregs; DI− is division index of T cells stimulated with un-pulsed DCs in the presence of Tregs.
ELISA
To assess the production of cytokines and Granzyme B (GrB), splenocytes from BALB/c mice bearing 4T1 tumors were depleted of red blood cells. Cells were incubated at 106cells/well with 80 µg/ml tumor antigen lysate, in the complete culture medium at 37 °C, 5 % CO2 for 72 h (cytokines) or 78 h (GrB) in a total volume of 1 ml/well. Supernatants were evaluated using ELISA kits for GrB (eBiosciences), IFN-γ, IL-10, VEGF, and IL-17 (R&D Systems).
Statistical analysis
Two-group comparisons were assessed with a Mann–Whitney U test. Comparisons between groups were done using one-way ANOVA with Tukey post hoc analysis. Kaplan–Meier survival curves were analyzed using log-rank tests. GraphPad Prism software (version 5.0) was used for graphs and statistical analysis. Differences with a p < 0.05 were considered as significant.
Results
HIF-1α inhibition interferes with Foxp3 and VEGF expression in tumor
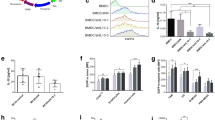
Previous studies indicated that HIF-1-dependent induction of VEGF and Foxp3 is critical in attenuating hypoxia-driven vascular leakage or inflammation [18–20]. To determine whether the HIF-1α inhibition involves VEGF and Foxp3 expression, the tumors and lymph nodes of PX-478-treated and untreated mice were analyzed for transcript and/or protein expression of these molecules via real-time RT-PCR and Western blotting or immunohistochemistry, respectively. These studies revealed that HIF-1α blockade led to the diminished tumoral expression of Foxp3 at both mRNA and protein level. VEGF expression evaluated at mRNA level also showed a remarkable decrease in tumors. Analysis of lymph nodes showed a significant decrease in Foxp3 mRNA level, while no alteration in HIF-1α and VEGF expression (Fig. 1).
Effect of HIF-1α inhibition on the expression of HIF-1 downstream genes. BALB/c mice bearing 4T1 tumors were left untreated or administered PX-478 (i.p at a dose of 40 mg/kg, every other day for 2 weeks). Tumors and lymph nodes were excised on day 12 after initiating treatment. a HIF-1α, VEGF, and Foxp3 transcript levels in tumors and lymph nodes were measured by real-time RT-PCR, calculated relative to housekeeping gene β-actin. Data are expressed as the mean ± SEM of four mice per group. *p < 0.05 by Mann–Whitney U test. b HIF-1α and Foxp3 protein synthesis of tumors was determined by western blotting as described in Materials and Methods. β-actin was used as a loading control. c Immunohistochemical analyses of representative sections obtained from untreated and PX-478 treated mice stained with an antibody directed against mouse HIF-1α (inset, negative staining control)
Combination of vaccination and HIF-1α inhibition promotes tumor regression and improved survival of tumor-bearing mice
Given the crucial role of hypoxia in tumor progression and resistance to therapy, we hypothesized that a combination protocol based on active vaccination against whole-tumor antigens along with targeting HIF-1α would provide superior efficacy against tumors via breaking down the immunosuppressive regulatory network. As shown in Fig. 2, PX-478 treatment alone, served to slow 4T1 tumor growth, whereas DC vaccination had no significant effect on tumor growth. Interestingly, administration of PX-478, significantly delayed tumor growth and increased survival in DC vaccine-treated mice. Moreover, it was observed that all treatment groups were capable of complete tumor regression (in one of five cases; i.e., 20 % in single therapies and three of six cases; i.e., 50 % in combination immunotherapy group); while the combination therapy was the only treatment in which cured animals were capable of preventing tumor growth after rechallenge with 4T1 tumor cells.
Delay of tumor growth and enhanced survival in mice treated with DC and PX-478. a schematic of the experimental design followed to evaluate the effects of HIF-1α inhibition combined with vaccination in the 4T1 breast cancer model. BALB/c mice bearing 4T1 tumors were left untreated or administered PX-478 (as described), DC vaccine (106 cells, subcutaneously around the tumor site, day 7 of tumor inoculation), or PX-478 plus vaccine, with b tumor size (mean ± SEM) reported in mm3 followed for up to 25 days (left) and tumor growth rate reported in mm3 per 48 h (right). *p < 0.05; **p < 0.01; ***p < 0.001 (ANOVA). c Survival of the animals in each group was monitored and the respective Kaplan–Maier curves are given; (n = 6 mice for control and combination therapy group, n = 5 for single therapies)
Combination therapy enhances T cell proliferation and CTL activation in tumor-bearing mice
To determine further the mechanism by which combination of HIF-1α blockade with vaccine causes tumor regression, the effect of treatment on cytolytic capacity of the splenocytes was tested via measurement of CD107 translocation and Granzyme B secretion by these cells. It was observed that GrB secretion level was significantly increased in the combination therapy group compared with untreated and other treatment modalities (Fig. 3a). Analysis of CD107 translocation on CD8+ T cells revealed that PX-478 treatment was capable of improving CTL function in the context of DC vaccine immunotherapy as CD8+ T cells showed significantly higher levels of CD107 translocation in mice received combination therapy, compared to mice treated with DC vaccine alone (Fig. 3b). Consistent with these results, the proliferation status of splenic T cells, evidenced by dilution of the CFSE dye, was augmented in animals received both treatments (Fig. 3c). VEGF secretion by antigen stimulated splenocytes was also evaluated and showed a significant decrease in mice treated with both vaccine and PX-478 (Fig. 3d).
DC vaccination accompanied by HIF-1α blockade enhances T cell effector functions. The ability of CD8+ T cells to recognize 4T1 tumor cells evaluated by a GrB production (ELISA) and b CD107 translocation assay (described in Materials and Methods). c 2 × 106 CFSE-labeled splenocytes isolated from tumor-bearing mice were treated in vitro with tumor lysate in CM (80 μg/ml, 5 days) and the proliferation ability of CD3 stained cells (reported as division index) was evaluated by flow cytometry. d VEGF production measured in the supernatant of antigen stimulated splenocytes (four mice per group) by ELISA. e Splenic T cells isolated from tumor-bearing mice were cocultured with CFSE-labeled splenic T cells from a tumor antigen immunized mouse and tumor antigen pulsed or un-pulsed DCs. Reduction in proliferative activity indicated by CFSE dilution, was considered as suppressor activity of splenic T cells. A representative out of three independent experiments is depicted;*p < 0.05; **p < 0.01 and ***p < 0.001; n = 4
In concert with the improved effector function of tumor antigen specific T cells, we also observed that PX-478 treatment of mice led to a significant reduction in tumor-associated regulatory capacity of T cells in mice that received DC vaccination (Fig. 3d).
DC therapy combined with HIF-1α inhibition favors a Th1 inflammatory cytokine profile
We then tested if HIF-1α blockade via alteration in T helper cell subsets would affect DC vaccine outcome. The data showed that the cells isolated from PX-478-treated mice alone or in combination with vaccine were capable of producing higher amounts of IFN-γ in response to in vitro stimulation with tumor lysate antigen compared to cells isolated from control and DC vaccine-administered mice. It was also shown that, despite unchanged secretion of anti-inflammatory IL-10, the ratio of IFN-γ/IL-10 was elevated in mice received combined therapy. IL-17 secretion by splenocytes was measured as an indicator of Th17 function; and a significant decrease was observed in mice treated with PX-478 alone and in combination with vaccination (Fig. 4).
Impact of HIF-1α inhibition in combination with DC vaccine on T helper cell subsets. IFN-γ, IL10 and IL-17 production measured in the supernatant of tumor lysate antigen stimulated splenocytes (80 µg/ml) by ELISA. A representative out of three independent experiments is depicted; *p < 0.05; **p < 0.01 and ***p < 0.001; n = 4
Discussion
It has become increasingly clear that the occurrence and persistence of an immunosuppressive environment play a crucial role in the development of malignant tumors. Hypoxia is a frequent feature of a wide range of solid tumors and may contribute significantly to the induction and maintenance of the regulatory network [3–7]. HIF-1, the main mediator of hypoxic response, coordinates a transcriptional program that contributes to a multitude of pathways associated with cancer progression and poor treatment outcome [8, 9].
Of note, during hypoxia, HIF-dependent transcription can activate multiple anti-inflammatory mechanisms. Such as the induction of VEGF that plays an important role in tumor angiogenesis and immune escape [19, 20], and coordinated induction of extracellular adenosine pathway that promotes Treg differentiation and function [21–23]. The enhanced suppressor effect of tumor MDSCs is another mechanism that involves HIF-1α-mediated induction of PD-L1 on these cells [24].
In this study, we tested the effects of HIF-1α blockade, by PX-478, on the immune efficacy of tumor vaccine and defined the underlining mechanisms responsible for these effects. The major finding in this report is that the HIF-1α inhibition has an immune adjuvant effect in the context of tumor antigen-based DC vaccination in the treatment of 4T1 murine breast cancer in vivo. Indeed, combination protocol resulted in the regression of established tumors and led to complete tumor regression in 50 % of mice, with significant prolongation of survival.
After investigating the underlying mechanisms of the treatment, we assumed that this vaccine-potentiating function can be performed via at least three mechanisms, by (i) enhancing effector function of T cells, (ii) limiting suppressor activity of T cells, and (iii) reducing production of immunoregulatory molecules such as VEGF.
Suppression of effector function of T cells is a significant obstacle to tumor immunotherapy. It has been shown that the sensitivity of tumor cells to CTL-mediated killing is diminished in hypoxic conditions by several mechanisms involving HIF-1. Caldwell et al. reported an altered pattern of CTL cytokine secretion in response to hypoxia (2.5 % O2) [25]. The regulatory effects of HIF-1 expression on CTL function might be attributed to elevated levels of CTLA-4 on CD8+ T cells and increased PD-L1 expression in hypoxic cancer cells [26, 27]. Furthermore, HIF-1-dependent induction of micro RNA (miR)-210, in tumor cells, decreases tumor cell susceptibility to CTL [28].
Here, we show that control of immunosuppressive factors via HIF-1α inhibition has profound effects on T cell proliferative capacity and responsiveness to stimulation. Importantly, PX-478 administration combined with active vaccination had superior potential to induce anti-tumor CTL function in vivo, due to elevated GrB secretion, compared to any single-component modality. Measurement of CD107 mobilization also showed that PX-478 treatment alone or in combination with DC vaccine improved activation of CD8+ T cells. This observation is consistent with previous studies demonstrating elevated CTL function in response to HIF-1 inhibition. The data show that DC vaccination alone, although was unable to elicit an effective CTL response due to the immunoregulatory network of tumors, when accompanied by PX-478 treatment, showed an improved potential to mount CTL activity.
It was also observed evidence of a protective memory CD8+ T cell responses because of the rapid rejection of a subsequent tumor rechallenge in cured mice.
We also observed decreased secretion of VEGF by splenocytes in response to tumor antigen stimulation in mice treated with PX-478. As shown in a previous study, VEGF neutralization results in the attenuation of hypoxic resistance of tumor cells to CTL-mediated killing. They also provided evidence indicating that HIF-1 induces phosphorylation of STAT3 in tumor cells by a mechanism involving at least in part, VEGF secretion. Following translocation to the nucleus, HIF-1 and pSTAT3 cooperate to alter the susceptibility of tumor cells to CTL-mediated killing [20].
The primary function of hypoxia and its mediator, HIF-1, in T cells evidenced by early studies was to suppress T cell-mediated inflammation [29, 30]. Since then, investigation of the role of HIF-1 in T cell differentiation by different researchers has revealed divergent roles for HIF-1α. These observations, combined with other reports defining a proinflammatory role for HIF-1 in myeloid lineages as well as the suppressor effect of macrophages on localized T cell responses, suggest a determinant role for microenvironmental factors specific to infected tissues, to drive T cell fate and differentiation.
Moreover, the challenge of the ongoing clinical studies might be to optimize the clinical efficacy of DC-based immunotherapy by improving in vitro DC-generating protocols. As in a recent study, it has been shown that CD11c+MHCII+ bone marrow-derived DCs generated in vitro by GM-CSF are in fact a heterogeneous group of cells that comprises conventional DCs and monocyte-derived macrophages [31]. Hence, refinement of in vitro DC culture systems along with the study of the function of DCs and macrophages might be helpful for the design of more effective DC-based vaccines.
Given the potential toxicity resulting from elimination of physiological hypoxia in normal tissues and lack of a durable anticancer efficacy, (like many chemotherapeutic agents), for single administration of HIF-1α inhibitors, as well as moderate efficacy for antigen-based vaccination in the cancer setting, our data suggest that persistent benefits can be achieved by combining a short course of PX-478 treatment along with a whole-tumor antigen vaccine. Although HIF-1 is the most important mediator of hypoxia regulating the effects of hypoxic conditions on tumor cell survival, other pathways in this regard, such as hypoxia-adenosinergic pathway, are also of great importance. Protocols combining these pathways with DC vaccine and other immunotherapy methods will be the focus of future investigations.
Abbreviations
- BMDC:
-
Bone marrow-derived dendritic cell
- DI:
-
Division index
- GrB:
-
Granzyme B
- HIF-1:
-
Hypoxia-inducible factor
- HRE:
-
Hypoxia response element
- PVDF:
-
Polyvinylidene difluoride
- Treg:
-
Regulatory T cell
- VEGF:
-
Vascular endothelial growth factor
References
Brown JM, Wilson WR (2004) Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 4:437–447
Rabinovich GA, Gabrilovich D, Sotomayor EM (2007) Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 25:267–296
Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP et al (2011) Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 475:226–230
Sitkovsky M, Lukashev D (2005) Regulation of immune cells by local-tissue oxygen tension: HIF1α and adenosine receptors. Nat Rev Immunol 5:712–721
Noman MZ, Benlalam H, Hasmim M, Chouaib S (2011) Cytotoxic T cells–stroma interactions. Bull Cancer 98:E19–E24
Noman MZ, Janji B, Kaminska B, Van Moer K, Pierson S, Przanowski P et al (2011) Blocking hypoxia-induced autophagy in tumors restores cytotoxic T-cell activity and promotes regression. Cancer Res 71:5976–5986
Noman MZ, Messai Y, Carre T, Akalay I, Meron M, Janji B et al (2011) Microenvironmental hypoxia orchestrating the cell stroma cross talk, tumor progression and antitumor response. Crit Rev Immunol 31:357–377
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3:721–732
Shi YH, Fang WG (2004) Hypoxia-inducible factor-1 in tumour angiogenesis. World J Gastroenterol 10:1082–1087
Semenza GL (2010) Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29:625–634
Kim JW, Gao P, Dang CV (2007) Effects of hypoxia on tumor metabolism. Cancer Metastasis Rev 26:291–298
Wilson WR, Hay MP (2011) Targeting hypoxia in cancer therapy. Nat Rev Cancer 11:393–410
Koh MY, Spivak-Kroizman T, Venturini S, Welsh S, Williams RR, Kirkpatrick DL et al (2008) Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1alpha. Mol Cancer Ther 7:90–100
Macpherson GR, Figg WD (2004) Small molecule-mediated anti-cancer therapy via hypoxia-inducible factor-1 blockade. Cancer Biol Ther 3:503–504
Welsh S, Williams R, Kirkpatrick L, Paine-Murrieta G, Powis G (2004) Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Mol Cancer Ther 3:233–244
Inaba K, Inaba M, Naito M, Steinman RM (1993) Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med 178:479–488
Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR et al (2011) HIF1α–dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of T H 17 and T reg cells. J Exp Med 208:1367–1376
Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P et al (2012) Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A 109:E2784–E2793
Takenaga K (2011) Angiogenic signaling aberrantly induced by tumor hypoxia. Front Biosci (Landmark Ed) 16:31–48
Noman MZ, Buart S, Van Pelt J, Richon C, Hasmim M, Leleu N et al (2009) The cooperative induction of hypoxia-inducible factor-1 alpha and STAT3 ring hypoxia induced an impairment of tumor susceptibility to CTL-mediated cell lysis. J Immunol 182:3510–3521
Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K et al (2003) Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med 198:783–796
Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A et al (2007) Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204:1257–1265
Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J et al (2008) A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 111:251–259
Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P et al (2014) PD-L1 is a novel direct target of HIF-1, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 211:781–790
Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG et al (2001) Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol 167:6140–6149
Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E et al (2013) Hypoxia-inducible factors enhance the effector responses of CD8 + T cells to persistent antigen. Nat Immunol 14:1173–1182
Barsoum IB, Smallwood CA, Siemens DR, Graham CH (2014) A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 74:665–674
Noman MZ, Buart S, Romero P, Ketari S, Janji B, Mari B et al (2012) Hypoxia-inducible miR-210 regulates the susceptibility of tumor cells to lysis by cytotoxic T cells. Cancer Res 72:4629–4641
Thiel M, Caldwell CC, Kreth S, Kuboki S, Chen P, Smith P et al (2007) Targeted deletion of HIF-1alpha gene in T cells prevents their inhibition in hypoxic inflamed tissues and improves septic mice survival. PLoS ONE 2:e853
Sitkovsky MV (2009) T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol 30:102–108
Helft J, Böttcher J, Chakravarty P, Zelenay S, Huotari J et al (2015) GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c + MHCII + Macrophages and Dendritic Cells. Immunity 42:1197–1211
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kheshtchin, N., Arab, S., Ajami, M. et al. Inhibition of HIF-1α enhances anti-tumor effects of dendritic cell-based vaccination in a mouse model of breast cancer. Cancer Immunol Immunother 65, 1159–1167 (2016). https://doi.org/10.1007/s00262-016-1879-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-016-1879-5