Abstract
The blood neutrophil-to-lymphocyte ratio (NLR) is reported to be a prognostic marker in several cancers. However, the prognostic role of NLR in patients with advanced cholangiocarcinoma on chemotherapy is unknown. A total of 221 patients with pathologically confirmed locally advanced or metastatic cholangiocarcinoma receiving first-line palliative chemotherapy were enrolled. Associations between baseline clinical and laboratory variables including NLR and survival were investigated. Patients were classified into two groups according to the NLR level (≤5 vs. >5). Median overall survival (OS) and time to progression (TTP) in patients with NLR ≤ 5 were 10.9 and 6.7 months, respectively, and 6.8 and 4.1 months in patients with NLR > 5 (P < 0.001, P = 0.002, respectively). In multivariate analysis, number of cycles of chemotherapy was a significant predictor of longer OS (HR 0.86, P < 0.001), whereas adverse prognostic factors for OS were CA 19-9 > 300 (HR 1.43, P = 0.025), CEA > 5 (HR 1.44, P = 0.029), higher stage (HR 1.69, P = 0.004), and NLR > 5 (HR 1.87, P < 0.001). NLR > 5 was also associated with reduced TTP (HR 1.66, P = 0.007). Among 50 patients with initial NLR > 5, 33 patients had NLR ≤ 5 after two cycles of chemotherapy and they had significantly better survival than the others (HR 0.48, P = 0.015). NLR independently predicts survival in patients with advanced cholangiocarcinoma undergoing chemotherapy. Considering cost-effectiveness and easy availability, NLR may be a useful biomarker for prognosis prediction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholangiocarcinoma (CCA) is a malignancy that is often fatal. More than one-third of patients are unresectable at presentation. Although several regimens including gemcitabine are available for the palliation of CCA, the response rate remains low (<30 %) [1].
It is becoming clear that the inflammatory process is critical for tumor progression. Proinflammatory cytokines and signaling molecules could lead to neoangiogenesis or lymphangiogenesis, which may potentiate neoplastic growth [2]. CCA development is possibly mediated by chronic inflammation of the bile duct [3]. Recently, the prognostic role of inflammation in CCA was reported [4–6].
The neutrophil–lymphocyte ratio (NLR) is an indicator of the systemic inflammatory response and has been shown to be associated with poor prognosis in various types of tumors [7–9]. Although NLR has been implicated as a prognostic factor in biliary tract cancer including CCA or gallbladder cancer [10–13], only one study has demonstrated the association between NLR and overall survival (OS) [4]. Moreover, no study has yet investigated the prognostic role of NLR in patients with pure advanced CCA.
The aim of this study was to assess the association of NLR with OS and progression-free survival (PFS) in patients with advanced CCA, and to evaluate whether the early change of NLR during systemic chemotherapy was predictive of survival in these patients.
Materials and methods
Study subjects
Between August, 2004 and September, 2013, 579 consecutive patients with locally advanced or metastatic CCA received systemic chemotherapy at Seoul National University Hospital. All data were entered retrospectively by a single researcher (BS Lee), after approval from the institutional review board of Seoul National University Hospital.
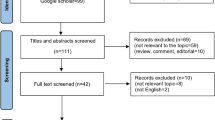
Diagnosis of all CCAs was based on pathologic confirmation. Exclusions comprised fewer than two cycles of chemotherapy (n = 21), history of another malignancy within the previous 5 years (n = 9), and prior systemic treatment (n = 10). Patients who underwent operation (n = 316) and never evaluated (n = 2) were also excluded. Finally, 221 patients were eligible for the analysis and were followed until June 30, 2014 (Fig. 1).
Data collection and definition
NLR was defined as absolute neutrophil count divided by the absolute lymphocyte count in peripheral blood. Pre- and post-treatment NLRs were checked before and after two cycles of chemotherapy, respectively.
Demographic and clinical variables collected included age, gender, Eastern Cooperative Oncology Group (ECOG) performance status, Charlson comorbidity index score [14], obesity (body mass index >25 kg/m2) [15], concomitant biliary infection (cholecystitis or cholangitis), location of tumor (intrahepatic, perihilar, distal), stage [16], and disease status (locally advanced or metastatic). Laboratory variables included total bilirubin level, prothrombin activity (%), carbohydrate antigen 19-9 (CA 19-9), carcinoembryonic antigen (CEA), and C-reactive protein (CRP) level. Parameters of treatment information included biliary decompression (endoscopic biliary drainage or percutaneous transhepatic biliary drainage), regimen of chemotherapy, and number of cycles of chemotherapy.
All patients with cholangitis or jaundice received biliary drainage before chemotherapy, and no patient had clinical signs of sepsis at the time of blood sampling for NLR. Nevertheless, in order to adjust for potential confounding effect, data on biliary infection at admission and biliary decompression were also collected, and these variables were included in the analyses.
Statistical analyses
NLR > 5 was selected as the cutoff level based on previous investigations [7, 9, 17]. Primary end points were OS and time to progression (TTP).
Survival was measured from the time of initiation of first-line therapy until death or last contact. Dates of death were obtained from the Korean Central Cancer Registry or final medical records. Treatment response or tumor progression was assessed using Response Evaluation Criteria in Solid Tumors version 1.1 [18]. TTP was defined as the time from the initiation of chemotherapy until disease progression ascertained by radiologic evaluation. Univariate survival analysis was performed with Kaplan–Meier method and log-rank test. All variables with P < 0.1 in univariate analysis were included in the multivariate model. Hazard ratios (HRs) and 95 % confidence intervals (CIs) were estimated from forward stepwise Cox proportional analysis.
To assess the prognostic role of change in NLR during early stage of treatment, patients were categorized using NLR values estimated before and after initial two cycles of chemotherapy, as follows: (a) subjects with NLR ≤ 5 consistently, (b) subjects with NLR change from >5 to ≤5, (c) subjects with NLR change from ≤5 to >5, and (d) subjects with NLR > 5 consistently. Survival curves of these groups were compared by use of the log-rank test.
Two-sided P values <0.05 were considered statistically significant. All statistical analyses were performed with SPSS 21.0 (SPSS, Chicago, IL, USA).
Results
Clinical characteristics of the patients
Baseline patient characteristics are summarized in Table 1. Intrahepatic CCA was the most common subtype (n = 124, 56.1 %). One hundred and thirty-seven (62 %) patients had CCA with distant metastasis, and 84 (38 %) had locally advanced CCA. The majority of patients received gemcitabine-based treatment (n = 179, 81 %). Median NLR was 3.3 (interquartile range 2.2–4.9), and 50 patients (22.6 %) had NLR > 5. Median TTP and OS were 5.1 and 9.5 months, respectively. At the time of final data analysis, 197 patients (89.1 %) had died.
OS according to clinical characteristics and NLR
In the univariate analysis, median OS in patients with NLR > 5 was 6.8 months (95 % CI 5.1–8.4 months) versus 10.9 months (9.5–12.4 months) in patients with NLR ≤ 5 (P < 0.001). Other prognostic factors significantly associated with survival in univariate analysis were CA 19-9 > 300 (HR 1.57, P = 0.002), CEA > 5 (HR 1.39, P = 0.030), CRP > 2.5 (HR 1.38, P = 0.023), intrahepatic location (HR 2.13, P = 0.002), metastatic disease status (HR 1.51, P = 0.007), and the number of cycles of chemotherapy (HR 0.88, P < 0.001; Table 2).
To identify the independent prognostic significance of the NLR for OS, multivariate Cox analysis was performed including covariates with P < 0.1 from univariate analysis. NLR > 5 was significantly associated with poor survival (HR 1.87, P < 0.001). Other independent prognostic factors for poor prognosis were CA 19-9 > 300 (HR 1.43, P = 0.025), CEA > 5 (HR 1.44, P = 0.029), and higher stage (HR 1.69, P = 0.004). Number of cycles of chemotherapy was significantly associated with longer OS (HR 0.86, P < 0.001) (Table 4).
Subgroup analysis was performed, including only patients with intrahepatic CCA. NLR > 5 was still a significant prognostic factor for poor survival in the multivariate analysis (HR 1.97, P = 0.002) (Supplementary Table 1).
TTP according to clinical characteristics and NLR
NLR > 5 and number of cycles of chemotherapy were significantly associated with TTP in univariate and multivariate analyses. Median TTP in subjects with NLR > 5 was 4.1 months (95 % CI 2.4–5.8 months) and 6.7 months (95 % CI 5.0–8.4 months) in patients with NLR ≤ 5. CRP > 2.5 was also predictive of shorter TTP in univariate analysis (P = 0.043). However, the associations did not reach statistical significance in multivariate analysis (Tables 3, 4).
Survival differences according to the change in NLR after chemotherapy
NLR exceeded 5 in 50 patients at baseline, and 171 patients had NLR ≤ 5. Among the 171 patients, 24 had increased NLR (>5) after two cycles of treatment (C). However, the others still had NLR ≤ 5 after chemotherapy (A). Likewise, among the 50 subjects who had NLR > 5 at baseline, 33 had decreased NLR (≤5) after two cycles of chemotherapy (B), whereas NLR was still higher than 5 in 17 patients (D) (Fig. 2).
Survival was compared between these (A–D) groups. Group A (or patients with NLR ≤ 5 both before and after chemotherapy) displayed the highest survival (median 11.2 months). Among subjects with high NLR at baseline, patients with improved NLR (group B) after treatment had better survival than the others (group D) (median 7.9 vs. 4.3 months, P = 0.015). In patients with low NLR before the treatment, subjects with post-treatment NLR > 5 (group C) had significantly worse survival than other patients (Group A) (median 4.9 vs. 11.2 months, P = 0.004).
Of the 199 assessable subjects, 29 (14.6 %) patients had a partial response (PR), 106 (53.3 %) had stable disease (SD), and 64 (32.2 %) had progressive disease (PD). There was no patient achieved complete response (CR) in this investigation. Clinical response (CR + PR) was not observed in group C (0 %), whereas 16.2 % of the patients in group A had a clinical response (P = 0.046). There was a trend toward a higher clinical response rate in group B (21.4 %) than in group D (7.1 %). However, it did not reach statistical significance (P = 0.392).
Discussion
NLR has been known to be a prognosticator in various types of tumors. However, the prognostic role of NLR in advanced CCA remains unclear. The present study revealed that baseline NLR > 5 was significantly associated with reduced TTP and worse survival in patients with advanced CCA undergoing chemotherapy, and also showed that decreased NLR after treatment was linked with better survival.
Previously, only one study focused on the prognostic role of NLR in advanced biliary tract cancer [4]. The study population included patients with gallbladder cancer as well as CCA, which was one of the limitations of the study. Although the authors demonstrated that NLR ≥ 3 was predictive for worse survival in subjects with advanced biliary tract cancer, the study failed to show a prognostic value of NLR in patients with advanced CCA, except perihilar CCA. Indeed, the present study is the first to identify an association between NLR and survival in a pure population of advanced CCA, and demonstrates the predictive value of higher NLR for worse survival in advanced CCA.
Several explanations are possible for the association between higher NLR and poor prognosis of malignancy. Neutrophils secrete vascular endothelial growth factor, which is a proangiogenic mediator involved in tumor development and proliferation [19]. In addition, elevated neutrophils stimulate up-regulation of cytokines and chemokines, such as interleukin (IL)-1, IL-6, or tumor necrosis factor, and the tumor microenvironment induced by the process may contribute to a progression of malignancy [20]. On the contrary, lymphocytes are crucial in tumor defense. Lymphocytes induce cytotoxic cell death through the immune response, and so a decrease in lymphocyte production may lead to a weaker immune reaction against tumor cells [2, 21]. Cancer myelopoiesis and consequent defective myeloid-cell differentiation are also associated with recruitment of immunosuppressor cells [22].
As the prognostic role of cancer-associated inflammation was identified, there has been a growing interest on the manipulation of cancer-related inflammation for therapeutic benefit [22]. Aspirin and NSAIDs were suggested to have a role in the enhancement of cytotoxic T cell activity, possibly leading to a prevention of cancer-related immunosuppression [23]. Several drugs targeted to cytokines [24], chemokines [25], transcription factors [26], and inflammasomes [27] also showed promising results for the control of cancer-associated inflammation in previous investigations. Given the association between NLR and cancer-related inflammation, further study is warranted to focusing on the role of NLR in identifying patients that may benefit from anti-inflammatory mediators or immunocompetence mediation.
Presently, post-treatment improvement in the NLR status was related with better survival. Previously, it was suggested that a high NLR may be a possible reflection of greater tumor burden. Higher rate of clinical response in patients without increased NLR during treatment than in subjects with increased NLR in this investigation would support this explanation. Another possible explanation is the close association between inflammation and chemoresistance [28]. It has been shown that up-regulation of proinflammatory cytokines bestows cancer cells acquired resistance to chemotherapeutic drugs [29, 30].
In this study, 10 % of patients were not assessable for radiologic response. Biliary stenting for palliation or desmoplastic reaction in tumor sometimes precludes assessment for response. Given the association between change in NLR and radiologic response during chemotherapy, it is possible that NLR has adjuvant activity in early discrimination of patients who would benefit from continued treatment.
Recently, a derived neutrophil–lymphocyte ratio (neutrophil count/white cell count minus neutrophil count; dNLR) was introduced for prognostication and as a surrogate marker for the classical NLR [31–34]. This score was developed for the further widespread validation of the NLR using existing clinical trials databases, where only white cell and neutrophil counts are commonly recorded. When this score (instead of NLR) was assessed in our cohort, significant association between dNLR > 2 and worse survival was also investigated in multivariate analysis (HR 1.44, P = 0.016) (Supplementary Table 2), which would suggest that the dNLR may be further exploited in databases pertaining to this tumor type.
This study has several limitations. First, it is based on a collection of retrospective data from a single center. However, the sample size was sufficient to demonstrate prognostic significance of NLR for survival. Second, NLR could be affected by concomitant medications, which were not accounted for in our study.
Despite these limitations, our study has several important strengths that outweigh the weakness. First, this is the first analysis that demonstrated the prognostic value of NLR for survival in advanced CCA. Second, the study provides useful findings for clinician in practice. NLR is easily assessable, inexpensive, and less harmful than radiologic examination. Checking NLR gives an independent prognostic hint and may be a support for decision-making regarding therapeutic plans. Finally, the results would be useful for prognostication and stratification of subjects in clinical trials. Further studies need to clarify optimal cutoff value of NLR for the prediction of better or worse survival in CCA.
In conclusion, NLR independently predicts survival as well as time to progression in patients with advanced CCA undergoing chemotherapy. Considering the cost-effectiveness and easy availability of NLR, it may be a useful biomarker for prognosis prediction.
Abbreviations
- CA 19-9:
-
Carbohydrate antigen 19-9
- CCA:
-
Cholangiocarcinoma
- CEA:
-
Carcinoembryonic antigen
- CI:
-
Confidence interval
- CR:
-
Complete response
- CRP:
-
C-reactive protein
- dNLR:
-
Derived neutrophil–lymphocyte ratio
- ECOG:
-
Eastern Cooperative Oncology Group
- HR:
-
Hazard ratio
- NLR:
-
Neutrophil–lymphocyte ratio
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- SD:
-
Stable disease
- TTP:
-
Time to progression
References
Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Wasan H, British Society of G (2012) Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 61(12):1657–1669. doi:10.1136/gutjnl-2011-301748
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867. doi:10.1038/nature01322
Rizvi S, Gores GJ (2013) Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 145(6):1215–1229. doi:10.1053/j.gastro.2013.10.013
McNamara MG, Templeton AJ, Maganti M, Walter T, Horgan AM, McKeever L, Min T, Amir E, Knox JJ (2014) Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur J Cancer 50(9):1581–1589. doi:10.1016/j.ejca.2014.02.015
Gu FM, Gao Q, Shi GM, Zhang X, Wang J, Jiang JH, Wang XY, Shi YH, Ding ZB, Fan J, Zhou J (2012) Intratumoral IL-17(+) cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann Surg Oncol 19(8):2506–2514. doi:10.1245/s10434-012-2268-8
Goeppert B, Frauenschuh L, Zucknick M, Stenzinger A, Andrulis M, Klauschen F, Joehrens K, Warth A, Renner M, Mehrabi A, Hafezi M, Thelen A, Schirmacher P, Weichert W (2013) Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer 109(10):2665–2674. doi:10.1038/bjc.2013.610
Chua W, Charles KA, Baracos VE, Clarke SJ (2011) Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer 104(8):1288–1295. doi:10.1038/bjc.2011.100
Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, Altorki NK, Abrams JA (2011) Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 18(12):3362–3369. doi:10.1245/s10434-011-1754-8
Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner C, Stojakovic T, Samonigg H, Hoefler G, Pichler M (2013) Increased neutrophil–lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 109(2):416–421. doi:10.1038/bjc.2013.332
Hakeem AR, Marangoni G, Chapman SJ, Young RS, Nair A, Hidalgo EL, Toogood GJ, Wyatt JI, Lodge PA, Prasad KR (2014) Does the extent of lymphadenectomy, number of lymph nodes, positive lymph node ratio and neutrophil–lymphocyte ratio impact surgical outcome of perihilar cholangiocarcinoma? Eur J Gastroenterol Hepatol 26(9):1047–1054. doi:10.1097/MEG.0000000000000162
Dumitrascu T, Chirita D, Ionescu M, Popescu I (2013) Resection for hilar cholangiocarcinoma: analysis of prognostic factors and the impact of systemic inflammation on long-term outcome. J Gastrointest Surg 17(5):913–924. doi:10.1007/s11605-013-2144-2
Gomez D, Morris-Stiff G, Toogood GJ, Lodge JP, Prasad KR (2008) Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol 97(6):513–518. doi:10.1002/jso.21001
Wu XS, Shi LB, Li ML, Ding Q, Weng H, Wu WG, Cao Y, Bao RF, Shu YJ, Ding QC, Mu JS, Gu J, Dong P, Liu YB (2014) Evaluation of two inflammation-based prognostic scores in patients with resectable gallbladder carcinoma. Ann Surg Oncol 21(2):449–457. doi:10.1245/s10434-013-3292-z
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S (2005) Criteria and classification of obesity in Japan and Asia-Oceania. World Rev Nutr Diet 94:1–12. doi:10.1159/000088200
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474. doi:10.1245/s10434-010-0985-4
Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN (2009) Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol 16(3):614–622. doi:10.1245/s10434-008-0267-6
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH (2003) Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 6(4):283–287. doi:10.1023/B:AGEN.0000029415.62384.ba
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357(9255):539–545. doi:10.1016/S0140-6736(00)04046-0
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444. doi:10.1038/nature07205
Diakos CI, Charles KA, McMillan DC, Clarke SJ (2014) Cancer-related inflammation and treatment effectiveness. Lancet Oncol 15(11):e493–e503. doi:10.1016/S1470-2045(14)70263-3
Lönnroth C, Andersson M, Arvidsson A, Nordgren S, Brevinge H, Lagerstedt K, Lundholm K (2008) Preoperative treatment with a non-steroidal anti-inflammatory drug (NSAID) increases tumour tissue infiltration of seemingly activated immune cells in colorectal cancer. Cancer Immun 8:5–15
Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA, Thompson R, Schioppa T, Nemeth J, Vermeulen J, Singh N, Avril N, Cummings J, Rexhepaj E, Jirstrom K, Gallagher WM, Brennan DJ, McNeish IA, Balkwill FR (2011) Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res 17(18):6083–6096. doi:10.1158/1078-0432.CCR-11-0945
Germano G, Mantovani A, Allavena P (2011) Targeting of the innate immunity/inflammation as complementary anti-tumour therapies. Ann Med 43(8):581–593. doi:10.3109/07853890.2011.595732
Tam CS, Verstovsek S (2013) Investigational janus kinase inhibitors. Expert Opin Investig Drugs 22(6):687–699. doi:10.1517/13543784.2013.774373
Henao-Mejia J, Elinav E, Strowig T, Flavell RA (2012) Inflammasomes: far beyond inflammation. Nat Immunol 13(4):321–324. doi:10.1038/ni.2257
Chen R, Alvero AB, Silasi DA, Mor G (2007) Inflammation, cancer and chemoresistance: taking advantage of the toll-like receptor signaling pathway. Am J Reprod Immunol 57(2):93–107. doi:10.1111/j.1600-0897.2006.00441.x
Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G (2006) TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res 66(7):3859–3868. doi:10.1158/0008-5472.CAN-05-3948
Nakanishi C, Toi M (2005) Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer 5(4):297–309. doi:10.1038/nrc1588
Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ (2012) A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer 107(4):695–699. doi:10.1038/bjc.2012.292
Szkandera J, Gerger A, Liegl-Atzwanger B, Stotz M, Samonigg H, Friesenbichler J, Stojakovic T, Leithner A, Pichler M (2015) The derived neutrophil/lymphocyte ratio predicts poor clinical outcome in soft tissue sarcoma patients. Am J Surg 210(1):111–116. doi:10.1016/j.amjsurg.2014.10.021
Szkandera J, Stotz M, Eisner F, Absenger G, Stojakovic T, Samonigg H, Kornprat P, Schaberl-Moser R, Alzoughbi W, Ress AL, Seggewies FS, Gerger A, Hoefler G, Pichler M (2013) External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PLoS ONE 8(11):e78225. doi:10.1371/journal.pone.0078225
Absenger G, Szkandera J, Pichler M, Stotz M, Arminger F, Weissmueller M, Schaberl-Moser R, Samonigg H, Stojakovic T, Gerger A (2013) A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer 109(2):395–400. doi:10.1038/bjc.2013.346
Author contributions
Sang Hyub Lee involved in conception and design; Ban Seok Lee, Dong Kee Jang, Kwang Hyun Chung, Jun Hyuk Son collected and assembled of data; Sang Hyub Lee, Ji Kon Ryu, Yong-Tae Kim provided study materials or patients; Ban Seok Lee, Woo Hyun Paik involved in data analysis and interpretation; Ban Seok Lee, Sang Hyub Lee, Woo Hyun Paik wrote the manuscript; All authors finally approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, B.S., Lee, S.H., Son, J.H. et al. Neutrophil–lymphocyte ratio predicts survival in patients with advanced cholangiocarcinoma on chemotherapy. Cancer Immunol Immunother 65, 141–150 (2016). https://doi.org/10.1007/s00262-015-1780-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1780-7