Abstract
High-dose interleukin-2 (HD IL-2) is an approved immunotherapy agent for metastatic melanoma and renal cell carcinoma resulting in objective responses in 15–20 % of patients. An additional subset of patients achieves stable disease, and the natural history of these patients has not been well documented. We hypothesized that stable disease following HD IL-2 is associated with a survival advantage. To explore this hypothesis, a retrospective chart review of 305 patients diagnosed with metastatic melanoma or renal cell carcinoma treated with HD IL-2 was conducted. Patient characteristics, response based on standard RECIST criteria and overall survival were analyzed using the Kaplan–Meier method and associations with clinical response were compared using a log-rank test. Two hundred and forty-five patients had melanoma and 60 had renal cell carcinoma. Of these, 217 had complete data available for analysis. Fifty-nine percentage had progressive disease (PD), 26 % had stable disease (SD) and 15 % had an objective complete (CR) or partial response (PR). Median overall survival was 16.8 months for all patients with available survival data; patients with PD had a median survival of 7.9 months compared to 38.2 months for stable disease, while the median has not been reached for those with objective responses. This retrospective data support an association between overall survival and stable disease, suggesting that clinical benefit may be underestimated for patients treated with HD IL-2. The data further support the use of disease control rate (CR + PR + SD) as a more meaningful endpoint for future clinical studies of tumor immunotherapy, including future studies of HD IL-2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
High-dose interleukin-2 (HD IL-2) was one of the first immunotherapy agents to exhibit therapeutic efficacy in patients with advanced cancer. In phase 2 clinical trials of patients with metastatic melanoma and renal cell carcinoma, HD IL-2 demonstrated an objective response rate of 15–20 % and, based on this, was approved by the FDA for the treatment of metastatic renal cell carcinoma in 1992 and metastatic melanoma in 1996 [1–3]. Notably, between 5 and 10 % of patients treated with HD IL-2 have been reported to achieve a durable complete response, which in the majority of cases is not associated with further recurrence [4]. Another 10–15 % of patients will have an objective partial response, which has been associated with a median overall survival of 36–45 months [5]. In addition to objective responders, some patients achieve stable disease (SD) following treatment; however, there is a paucity of published data describing the frequency of this response and its prognostic significance.
Traditionally, the efficacy of HD IL-2 has been defined according to Response Evaluation Criteria in Solid Tumors (RECIST) or World Health Organization (WHO) criteria as an objective complete or partial response [6]. However, the use of upfront tumor regression as a surrogate endpoint for improved survival is based on historical experience with cytotoxic chemotherapy response patterns in which SD is generally short-lived and not associated with durable clinical benefit [6–8]. In contrast, recent studies have suggested that clinically meaningful responses to immunotherapy can occur after a delayed period and manifest with novel patterns of regression, which traditional evaluation rubrics may fail to accurately capture [9–11].
A good example of new response patterns observed with immuno-oncology agents is provided by recent experience with the T cell checkpoint inhibitor, ipilimumab, that was approved in 2011 by the US Food and Drug Administration for the treatment of patients with metastatic melanoma [12]. In clinical trials of ipilimumab, eventual tumor regression was found to follow several patterns, including an initial period of prolonged SD or disease progression [11, 13]. In some cases, radiographically apparent tumor regression did not occur for more than 12 weeks. Durable SD, with or without mild gradual regression, was a frequent outcome and associated with improved survival. More recently, immune-related response patterns have also been reported with an anti-programmed death 1 (PD-1) monoclonal antibody [14]. The kinetics of response to HD IL-2, however, has not been previously described.
Given the recent experience with ipilimumab, we sought to explore the hypothesis that patients treated with HD IL-2 who achieve SD as the initial response to therapy may experience a significant survival benefit compared to patients who have clear disease progression after HD IL-2 treatment. In order to generate data, we conducted a retrospective review of a large prospectively collected HD IL-2 database. Here, we report the long-term survival outcomes of patients with metastatic melanoma and renal cell carcinoma who achieved SD following their initial course of HD IL-2. Although this study is limited by the retrospective nature of the analysis, the data do support the underlying hypothesis and support further prospective studies to better define the full therapeutic potential of HD IL-2.
Patients and methods
Patients and response assessment
A retrospective chart review was conducted on all patients treated with HD IL-2 by a single physician between 2000 and 2012. The data were collected after acknowledgment by the Institutional Review Board (IRB), and written informed consent was obtained by all participating subjects. Patient characteristics, including age, sex, primary diagnosis, stage, location of metastasis and LDH were all collected and are reported based on all available data. Response to IL-2 was based on RECIST criteria and was determined following the initial course (two cycles) of therapy. The RECIST criteria defined progressive disease (PD) as more than a 20 % increase in area of disease based on radiographic imaging. Stable/mixed (SD) response was described as less than a 20 % increase in disease but not more than a 30 % decrease in disease. A partial response was defined as at least a 30 % reduction in tumor burden, while a complete response indicated that no tumor was visible on radiographic studies [15]. For the purposes of this analysis, an objective response included all patients with a complete or partial response to therapy, as this has been the metric applied to prior reports of clinical response to HD IL-2. A disease control rate (DCR), where reported, includes all patients who did not progress following their initial course of therapy (CR + PR + SD).
Treatment regimen
IL-2 was administered as a 600,000 IU/kg intravenous bolus every 8 h for up to 15 doses. Patients were admitted to an inpatient unit for physiologic monitoring and clinical management. Therapy was stopped after 15 doses or when the patient had Grade III/IV adverse events, as described in the Common Toxicity Criteria for Adverse Events (CTCAE) put forth by the National Cancer Institute, that did not resolve within 24 h. Patients typically received two cycles of therapy with a 1–2 weeks interval between cycles. All patients underwent full-body imaging, including computed tomography (CT) scans of the chest, abdomen and pelvis and a magnetic resonance imaging (MRI) scan of the brain prior to and 4–6 weeks after completion of a course of therapy. Patients with stable or partial response were offered subsequent courses of treatment.
Statistical analyses
Descriptive statistics were obtained and reported. Overall survival was the primary endpoint and was calculated from the date when HD IL-2 was initiated to the last date of known follow-up. Final status was determined at the last date of follow-up, and patients were noted to be alive, deceased or lost to follow-up. Differences in overall survival were calculated using the log-rank test, and Kaplan–Meier curves were used to estimate median overall survival. Statistical analyses were completed using the statistical software R (R Foundation). We also conducted landmark analyses at 1-year, 2-year and 3-year time points. Landmark analysis was conducted by calculating percent of patients who remained alive and not lost to follow-up at the first, second and third year following initial treatment date with HD IL-2.
Results
Patient characteristics
The total study population consisted of 305 consecutive patients with unresectable stage III or IV melanoma or stage IV renal cell carcinoma treated with HD IL-2. Initial response data were missing on 12 patients, 19 patients had unavailable survival data and another 57 patients were missing both response and survival data after complete chart review. Final survival analysis was conducted on 217 patients for whom all necessary response and survival data were available (Fig. 1). Demographics and response to therapy are presented in Table 1. The average age among patients in this cohort was 54.9 years, and the median age was 57 years. In this series, 147 (67.7 %) were male and 70 (32.3 %) were female. One hundred and seventy-two (79.3 %) of the patients carried a diagnosis of metastatic melanoma, and 45 (20.7 %) had metastatic renal cell carcinoma.
Progressive disease was noted in 128 (59.0 %) of patients. Another 57 (26.3 %) patients were categorized as stable or mixed response based on initial assessment after one course of HD IL-2. A total of 24 (11.1 %) patients had a partial response, while 8 (3.7 %) patients had a documented complete response after one course of HD IL-2. The objective response rate (CR + PR) was 32/217 (14.7 %). The DCR was 89/217 (41.0 %). Among patients who had a stable response to therapy, 18 patients had extent of disease limited to distant lymph nodes, skin and soft tissue (Stage IV MIA), 29 patients had metastatic disease in the lungs (Stage IV MIB) and 14 patients had disease involving the central nervous system or other viscera (Stage IV M1C). Of note, many patients in this analysis with metastatic disease to viscera or the central nervous system also had skin, soft tissue, distant nodal or pulmonary disease at the time of treatment. A summary of metastatic sites is described in Table 2.
Overall survival analyses
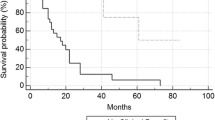
The median overall survival of all 226 patients with available follow-up data was 16.8 months (Fig. 2a). Patients with an objective response had a better overall survival compared to patients who did not achieve an objective response (median survival not reached vs. 13.8 months, p = 0.001; Fig. 2b). Patients with SD exhibited better overall survival compared to patients with PD (38.2 vs. 7.2 months, p = 0.001; Fig. 3). We next sought to determine whether DCR was associated with better overall survival compared to patients with PD. We found that DCR was associated with a 50.4 month overall survival compared to patients with PD experiencing an overall survival of 7.2 months (p = 0.001; Fig. 4).
Overall survival of patients treated with high-dose IL-2. a Kaplan–Meier analysis of overall survival of all patients (n = 226) treated with HD IL-2 was 16.8 months for whom survival data were available Note: Not all patients had response data available, and these subjects are excluded from subsequent analyses; b Traditional Kaplan–Meier analysis of overall survival based on RECIST response to one course of IL-2 treatment. Objective responders (blue line) do not reach a median survival, whereas the median survival among those without an objective response (green line) is 13.9 months (Log-rank test; p = 0.001)
Patients with stable disease following high-dose IL-2 have improved survival compared to non-responders. Patients with stable disease (green) have a median survival of 38.2 months, compared to just 7.2 months among patients with progressive disease (purple; p = 0.001). Patients with an objective response (blue) have even better survival with no median reached (p = 0.001)
Landmark analysis demonstrated that the 1-, 2-, and 3-year survival rates were greatest for an objective response (92.0, 87.5 and 78.3 %, respectively). The 1-year survival for patients with SD was 92.5 % equivalent to those patients who had an objective response and better than patients with PD who had a 1-year survival of 36.2 %. The 2-year survival was 9.9 % in patients with PD and was 70.5 % in those with SD. A similar trend was seen at the 3-year landmark survival with 78.3 % of objective responders alive, 47.5 % of SD patients alive and only 3.6 % of the PD patients still alive. Overall, those patients with SD had a better survival at each of these time points compared to patients with PD (Table 3).
Discussion
In this retrospective analysis of a large cohort of patients with advanced melanoma and renal cell carcinoma who were treated with HD IL-2, 26.3 % achieved stable disease by RECIST criteria at initial response assessment following one course of treatment (Table 1), and this was associated with a median survival over fivefold longer than non-responders (38.2 vs. 7.2 months, p = 0.001; Fig. 3). Importantly, the majority of melanoma patients with SD presented with distant visceral metastases, suggesting that these outcomes are not merely attributable to early substage, which is independently associated with a better prognosis (Table 2) [16]. While the retrospective nature of this study precludes definitive conclusions, the significant association between overall survival and SD suggests that the clinical benefit of HD IL-2 may be underestimated by reporting objective response rates alone. When taking SD into account, HD IL-2 had a DCR of 41 % and this was associated with a survival advantage compared to patients who had PD at first assessment (Fig. 4). The clinical benefit was further supported by a 1-, 2- and 3-year landmark analysis that confirmed patients who achieved SD as the initial response to HD IL-2 had a durable improvement in overall survival compared to patients who progressed at 1, 2 and 3 years (Table 3).
Although historical data surrounding the long-term outcomes of patients with SD are limited, our results are consistent with recent reports in the literature. In a single-center review of 500 patients treated with HD IL-2, 21 % achieved SD, and this was associated with a median OS of 32.6 months among those with metastatic melanoma and 57.2 months for those with renal cell carcinoma [17]. A recent analysis of a multi-institution observational registry similarly reported a median OS for melanoma patients with SD to be 36.6 months [18]. It should be noted that the overall objective response rate in our study of 13.6 % is slightly lower than what is reported elsewhere in the literature. This is likely due to our strict use of initial response after one course of therapy as opposed to best overall response after further courses of treatment. Additionally, the use of newer imaging technology, especially in the last few years, may increase the sensitivity of our assessment protocols, resulting in a higher false positive rate of tumor detection.
The assumption that stable disease is not indicative of a meaningful therapeutic response originates from historical experience with cytotoxic chemotherapy in which SD is generally neither durable nor associated with improved survival [6–8]. Immunotherapy, however, represents a major paradigm shift in the mechanism of anti-tumor activity for the treatment of neoplastic disease. Whereas chemotherapy, when effective, acts directly on cancer cells to induce cytotoxic changes that typically result in objective regression within weeks, tumor immunotherapy works indirectly by priming and expanding host anti-tumor immune responses. The process of inducing host lymphocytes to recognize, infiltrate, and ultimately mediate the regression of clinically apparent tumors can take a considerable amount of time to occur depending on the therapeutic agent, host immune response kinetics, and specific features of the tumor and tumor microenvironment. While lack of tumor regression within weeks of chemotherapy may result from a variety of different tumor-specific resistance mechanisms [19], SD following immunotherapy may still represent a mounting anti-tumor immune response, the kinetics of which can be delayed, manifest heterogeneously, and include novel patterns of regression [10, 20].
The concept that immunotherapy can be associated with unique patterns of response which fundamentally differ from cytotoxic chemotherapy has been well described in melanoma patients treated with ipilimumab [11, 20]. Careful analyses of clinical trials with ipilimumab revealed that eventual reductions in overall tumor burden may be delayed and can follow an initial period of SD, a transient increase in tumor size, or the appearance of new lesions. PD followed by later regression may be attributable to continued tumor growth pending the development of a sufficiently robust anti-tumor immune response. Alternatively, early radiographic tumor enlargement may be the result of infiltration of the tumor by lymphocytes, inflammatory cells, and accompanying edema, as opposed to an actual increase in the number of tumor cells, a so-called tumor “flare” [21]. Similarly, small, previously undetected lesions can initially enlarge due to immune cell infiltration and, as such, give a misleading radiographic impression of PD. In addition, a significant proportion of patients treated with ipilimumab have been reported to achieve disease stabilization that is durable and associated with prolonged overall survival [12, 13].
These observations with ipilimumab have led to the development of a new set of immunotherapy-specific response guidelines, termed immune-related response criteria (irRC), designed to account for nonconventional but clinically beneficial responses which would otherwise be overlooked with RECIST and WHO criteria [11]. Whereas RECIST and WHO criteria define the appearance of new lesions as PD, irRC evaluates treatment response according to changes in total tumor burden, defined as the sum of the product of the largest biperpendicular diameters of all index lesions as well as any new measurable lesions that emerge after treatment. By incorporating new lesions into measurements of total tumor burden, this model is able to account for eventual regression which is preceded by tumor enlargement and/or accompanied by the appearance of new lesions while still capturing traditional WHO-defined response categories. To accommodate for delayed kinetics of response, establishing PD according to irRC requires measurement of total tumor burden at two separate points in time at least 4 weeks apart. Retrospectively applying irRC to a large phase 2 clinical trial data set suggested that an additional 9.7 % of patients had in fact achieved a therapeutic response which according to WHO criteria was categorized as PD; the majority were categorized as a having immune-related SD, and survival among this subset was noted to be similar to those with WHO-defined disease control [11]. The development of irRC represents a promising stride toward a refined immunotherapy treatment response metric but still requires prospective validation before it can be applied in the clinical setting.
In our study, it is unclear whether patients with SD after a single course subsequently experienced delayed regression, prolonged stabilization (with or without mild gradual regression), or simply indolent progression. Similar to ipilimumab, however, it may be useful to consider confirmatory imaging studies prior to defining responses in patients treated with HD IL-2. The data would also support continuing treatment with HD IL-2 in those patients who do have SD at initial assessment. Future studies may seek to incorporate radiographic data from multiple time points to better characterize response kinetics and account for immune-related response patterns.
Given the association observed between SD and overall survival following HD IL-2 treatment in this study, a more accurate measure of clinical impact may be captured by the DCR, which incorporates the objective response with SD patients (CR + PR + SD), as has been reported with ipilimumab [12, 13]. HD IL-2 in this study had DCR of 37.7 % and patients with in the DCR group also had improved overall survival compared to patients with PD (Fig. 4), suggesting that the clinically meaningful effects of treatment may actually extend to far more patients than reported in the initial studies of IL-2. While HD IL-2 is not a new drug, clinicians should be aware that HD IL-2 is a valuable treatment options for many patients with metastatic melanoma and renal cell carcinoma, and like other immunotherapy regimens, is associated with durable clinical responses in a significant number of patients.
Conclusions
In this retrospective analysis, nearly a quarter of patients with metastatic melanoma and renal cell carcinoma treated with HD IL-2 achieved SD after one course of treatment and this was associated with better overall survival compared to those patients with PD. Taking SD into account, treatment with HD IL-2 had a DCR of 37.7 %, suggesting that the traditionally reported response rate of 15–20 % may underestimate the true clinical impact of HD IL-2. Although a prospective clinical trial is needed to further validate this finding, the data suggest that DCR may be a more appropriate measure of clinical benefit with HD IL-2 and should be considered in future clinical studies of HD IL-2 immunotherapy.
Abbreviations
- CR:
-
Complete response
- CT:
-
Computed tomography
- CTCAE:
-
Common toxicity criteria for adverse events
- DCR:
-
Disease control rate
- HD:
-
High-dose
- IL:
-
Interleukin
- irRC:
-
Immune-related response criteria
- IU:
-
International units
- IRB:
-
Institutional review board
- kg:
-
Kilograms
- LDH:
-
Lactate dehydrogenase
- MRI:
-
Magnetic resonance imaging
- PD:
-
Progressive disease
- PD-1:
-
Programmed death 1
- RECIST:
-
Response endpoint criteria in solid tumors
- SD:
-
Stable disease
- WHO:
-
World health organization
References
Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE (1994) Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 271(12):907–913
Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA (1999) High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 17:2105–2116
Atkins MB, Kunkel L, Sznol M, Rosenberg SA (2000) High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am 6:S11–S14
Rosenberg SA (2012) Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med 4(127):127ps8
Spanknebel K, Cheung KY, Stoutenburg J, Hurst-Wicker K, Hesdorffer C, DeRaffele G, Kaufman HL (2005) Initial clinical response predicts outcome and is associated with dose schedule in metastatic melanoma and renal cell carcinoma patients treated with high-dose interleukin-2. Ann Surg Oncol 12:381–390
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Michaelis LC, Ratain MJ (2006) Measuring response in a post-RECIST world: from black and white to shades of grey. Nat Rev Cancer 6(5):409–414
Hoos A (2012) Evolution of end points for cancer immunotherapy trials. Ann Oncol 23(Suppl 8):viii47–viii52
Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L, Wolchok J (2010) Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 102(18):1388–1397
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Wolchok JD, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, Cykowski L, de Pril V, Humphrey R, Lebbé C (2013) Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol 24:2174–2180
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Eisenhauer JK, Terenziani CM, Mudal VD et al (1999) Measuring response in solid tumors: unidimensional versus bidimensional measurement. J Natl Cancer Inst 91:523–528
Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer EA, Parulekar W, Markovic SN, Saxman S, Kirkwood JM (2008) Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 26(4):527–534
Payne R, Glenn L, Hoen H, Richards B, Smith JW 2nd, Lufkin R, Crocenzi TS, Urba WJ, Curti BD (2014) Durable responses and reversible toxicity of high-dose interleukin-2 treatment of melanoma and renal cancer in a Community Hospital Biotherapy Program. J Immunother Cancer 2:13
Daniels GA, Morse M, Wong MK, Kaufman HL, McDermott DF, Aung S, Lowder JN (2014) Improved median overall survival (OS) in patients with metastatic melanoma (mM) treated with high-dose (HD) IL-2: analysis of the PROCLAIM 2007–2012 national registry. J Clin Oncol 32:5s (suppl; abstr 9054)
Gatti L, Zunino F (2005) Overview of tumor cell chemoresistance mechanisms. Methods Mol Med 111:127–148
Saenger YM, Wolchok JD (2008) The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun 8:1
Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, Korman A, Lautz D, Russell S, Jaklitsch MT, Ramaiya N, Chen TC, Neuberg D, Allison JP, Mihm MC, Dranoff G (2008) Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA 105(8):3005–3010
Conflict of interest
Dr. Howard Kaufman has received compensation from Prometheus for advisory boards and consulting services. The other authors have no conflicts to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hughes, T., Klairmont, M., Broucek, J. et al. The prognostic significance of stable disease following high-dose interleukin-2 (IL-2) treatment in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother 64, 459–465 (2015). https://doi.org/10.1007/s00262-014-1652-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-014-1652-6