Abstract
We studied the impact of natural killer T (NKT) cell activation by alpha-galactocysylceramide (α-GalCer, α-GC) on cancer cell repopulation during chemotherapy in murine mesothelioma. The number of NKT cells was found to be increased during the development of murine mesothelioma. NKT cells specifically recognize α-GC through CD1d resulting in their activation and expansion. Tumor-bearing mice were treated with chemotherapy once weekly, and α-GC was followed after each cycle of chemotherapy. Anti-tumor effect was evaluated on wild-type (WT) and CD1d knockout (CD1dKO) mice. Cancer cell proliferation and apoptosis were evaluated by Ki67 and TUNEL immunohistochemistry. CD4+ and CD8+ T cell proportion and activation in tumor, spleen, draining lymph node and peripheral blood were determined by flow cytometry, and gene expression of activated T cell-related cytokines was quantified by reverse transcription PCR. NKT cells were identified by CD1d-α-GC-tetramer staining. In WT mice, tumor growth delay was achieved by cisplatin (Cis), and this effect was improved in combination with α-GC, but α-GC alone had little effect. Cancer cell proliferation during chemotherapy was significantly inhibited by α-GC, while cancer cell death was significantly upregulated. α-GC following chemotherapy resulted in NKT cell expansion and an increase of interferon-γ production in the draining lymph node, blood and spleen. Gene expression of immune-associated cytokines was upregulated. Strikingly, the percentage of inducible T cell co-stimulator+CD4 T cells, Th17/Tc17 cells increased in splenocytes. In CD1d KO mice, however, Cis alone was less effective and Cis + α-GC provided no additional benefit over Cis alone. α-GC alone had minimal effect in both mice. NKT activation between cycles of chemotherapy could improve the outcome of mesothelioma treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Two major issues of chemotherapy include systemic toxicity and drug resistance. Considerable evidence shows that cancer cell repopulation during the intervals of chemotherapy has been considered a neglected factor leading to drug resistance [1–3]. Anti-tumor effect can be improved if this process could be effectively inhibited. Previous studies of ours and others demonstrated that specific inhibitors and immunoenhancing agents can inhibit cancer cell repopulation in a variety of cancers in animal models [4–6]. This concept has been applied to malignant pleural mesothelioma (MPM), which is a rare but aggressive cancer with poor prognosis. It has been associated with long-term exposure to asbestos. The incidence keeps going up even though asbestos has been banned due to the long latency period after exposure [7]. The first-line chemotherapy is composed of cisplatin (Cis) and pemetrexed (alimta), and has shown clinical benefits with a prolonged median survival time of about 3 months [8]. Trimodality therapy with chemotherapy followed by radical surgery and adjuvant high-dose hemithoracic radiation has shown encouraging results for patients with N0 disease [9]. Unfortunately, the efficacy of current multimodality treatments is limited to selected patients with early stage disease and patients with advanced stage disease have limited therapeutic options. Therefore, more effective systemic therapies may offer significant improvement in survival for some MPM patients [10].
Immunotherapy provides hope for cancer patients. Previous studies demonstrated that the number of invariant natural killer T cells (iNKT, NKT) increased during the development of cancers including murine mesothelioma [11, 12]. A clinical trial indicated that NKT cells increase following the injection of α-galactosylceramide (α-GalCer, α-GC)-loaded dendritic cells (DCs) [13]. NKT cells specifically recognize the α-GC pulsed by DC through CD1d resulting in their activation and expansion [14]. NKT cell activation involves uptake of glycolipid antigen α-GC by antigen-presenting cells (APC) and presentation to NKT cells in CD1d–antigen complexes, resulting in rapid expression of proinflammatory cytokines by iNKT cells [15]. Cross talk between DC and NKT induces CD40L upregulation on DCs, their maturation and IL-12 production [16]. IL-12 activates NK and NKT cells to produce interferon-γ (IFN-γ) and modulate bystander activity of innate NK cells and adaptive CD8+ T cells [17, 18]. Mature DCs along with activated NKT cells can enhance helper T cell and B cell responses [19]. NKT cells can be mediated through cognate antigenic interactions by CD1d-expressing B cells [20]. NKT cell activation is able to mediate both cellular and humoral immune responses.
As a result, the high-affinity CD1d antigen α-GC analog activates a cascade of anti-tumor effector cells, and clinical studies have already shown promising results [14, 21, 22]. We study the impact of NKT cell activation by α-GC on cancer cell repopulation between cycles of chemotherapy in murine mesothelioma model and expect to translate this approach into MPM patients.
Materials and methods
Murine mesothelioma cells and animal model
Murine malignant mesothelioma cell line AB12, derived from an asbestos-induced tumor in Balb/c mice, has been described before [6, 13]. AB12 cells were cultured and maintained in RPMI 1640 medium supplemented with 10 % fetal bovine serum and 1 % penicillin and streptomycin. The cultures were maintained at 37 °C in an atmosphere containing 5 % CO2. AB12 cells (2 × 106) were injected subcutaneously (sc) into the right flank of female BALB/c mice which were provided by The Jackson Laboratory (Bar Harbor, Maine).
The CD1d knockout (CD1dKO) mice were also provided by Jackson Laboratory. The strain was generated from C.129S2-Cd1tmGru/J. Genotyping of CD1d wild-type (WT) and knockout mice was confirmed.
All procedures followed the animal care regulations of University Health Network after approval by the Research Ethic Board.
Genotyping of the CD1d KO mice
Genotyping was performed to confirm that the expression of CD1d gene was deleted, and WT Balb/c mice were used as controls. DNA was extracted from the ear tissue of mice with NaOH extraction method. NaOH (50 mM) was added to the ear sample and briefly vortexed. After incubation at 95 °C for 5 min, Tris–HCl (1 M, pH = 8.0) was added to neutralize the lysis solution. PCR was then carried out following the protocol. PCR product was separated by gel electrophoresis on a 1.5 % agarose gel.
Treatment of tumor-bearing mice with α-GC between cycles of chemotherapy
Wild-type mice were randomly divided into four groups as follows when tumor size reached 5 mm in diameter: (1) no treatment (NoRx); (2) NKT activation by α-GC alone, 100 µl of α-GC (20 µg/ml PBS, approximately 100 µg/kg body weight) was injected ip once weekly for three doses, i.e., on day 5, 12 and 19 after tumor cell injection. α-GC was purchased from Enzo Life Sciences (Farmingdale, NY). The first day of tumor cell injection was referred to as day 0; (3) Cis alone, 5 mg/kg body weight was injected intravenously (iv) through the tail vein once weekly for three doses, i.e., on day 4, 11 and 18 after tumor cell injection. Chemotherapy treatment with Cis was initiated on day 5 after tumor cell injection; (4) combination therapy, α-GC was given one day after each dose of Cis (Cis + α-GC), following the same schedule as in groups 2 and 3.
Tumor-bearing CD1d KO mice (KO) were included as controls of WT mice. The groups and treatment schedule remain the same as WT mice, in order to evaluate the effect on tumor growth in the WT versus CD1dKO mice.
Tumor size was measured twice weekly by a caliper, and tumor volume was estimated by a formula: V = ab 2 π/6, where a and b represent the longest and shortest maximal perpendicular diameters, respectively [23].
In vitro cytotoxicity of glycolipid α-GC on AB12 cells
Cultured AB12 cells (2 × 106/5 ml flask) were exposed to a series of doses of α-GC 0, 100, 250, 500, 1,000, 5,000 and 10,000 ng/ml or Cis 10, 100, 500, 1,000, 5,000 and 10,000 ng/ml for 24 h. All cells were collected and washed in PBS twice. Annexin V or propidium iodide (PI; BD Biosciences, Mississauga, ON) 1 µl of each was added to the tubes for staining according to the instructions provided by manufacturer. Cellular apoptosis and death was determined by flow cytometry.
Immunohistochemical staining of Ki67 and TUNEL for evaluation of cancer cell proliferation and apoptosis
When animals were killed 7 days after completion of the last dose of treatment, tumor tissues were removed and snap frozen immediately in liquid nitrogen, and then transferred to dry ice and kept at −80 °C until frozen sectioning was performed. Frozen sections were fixed in cold ethanol. Endogenous peroxidases, avidin and biotin were blocked using 1 % hydrogen peroxide and the Avidin/Biotin blocking kit (Dako, Carpinteria, CA). Sections were stained with a primary monoclonal antibody (1:50) against Ki67, and secondary antibody linked to streptavidin-HRP (Dako). For TUNEL staining, sections incubated in TdT reaction mixture for 1 h at 37 °C in a humidified chamber. After rinsing with stop wash buffer, sections were incubated with streptavidin-HRP in PBS for 15 min at room temperature. After washing in PBS, DAB (3, 3-diaminobenzidine) was added to serve as a peroxidase substrate (125 μl/section) and slides were counterstained with hematoxylin to visualize nuclei. Sections were then dehydrated and mounted with DPX (Ultramount, Scot Scientific).
Immunostained sections were quantified by using Aperio ImageScope digital scanner and Aperio ImageScope Viewer software version 9.0 (Vista, CA) under 200 magnification. Tumor cell repopulation was quantified as the proportion of Ki67 or TUNEL positive nuclear areas divided by total nuclear areas [6].
Cell preparation and analysis by flow cytometry
T cell subsets including CD4, CD8 and NKT and their activation were determined by flow cytometry. On day 7 after completing treatment, spleens and draining lymph nodes were removed from tumor-bearing mice and placed into ice-cold RPMI1640 medium containing 1 % FBS. The axillary lymph nodes from the tumor side were called draining lymph nodes. Peripheral blood was drawn from the heart of mice that were immediately euthanized by inhalation of CO2. Homogenized spleen and lymph node were passed through the cell strainer to achieve single cells. ACK lysis buffer (Invitrogen, Carlsbad, CA) was added and allowed to react for at least 15 min at reverse transcription (RT) to lyse red blood cells. For intracellular cytokine staining, splenocytes 2 × 106 cells/well in 200 µl of RPMI-1640 complete medium were stimulated for 5 h in 96-well plates with 25 ng/ml phorbol myristate acetate (PMA, Sigma) and 500 ng/ml ionomycin (Cell Signalling Technology, Inc, Danvers, MA) in the presence of 10 μg/ml Brefeldin A (Cell Signalling) at 37 °C 5 % CO2. After washing thrice with staining buffer, appropriate dilutions (1:50 ~ 100) of Abs or isotype controls were added to each tube, 15 min at RT in the dark. Staining of surface markers including CD3, CD4, CD8 and ICOS was washed thrice with staining buffer and resuspended in 1 % paraformaldehyde/PBS (v/v) (Sigma). After fixation with 1 % paraformaldehyde at 4 °C overnight, cells were permeabilized for intracellular staining of IFN-γ, perforin and granzyme B and fixed with 200 µl permeabilization/fixation solution (eBioscience), and then washed twice with permeabilization buffer. Anti-mouse antibodies against IFN-γ (1:30), perforin (1:50) and granzyme B (1:50) were added and maintained at RT for 20 min in the dark.
Single cell suspensions were stained with monoclonal antibodies conjugated with different fluorescent dyes, CD3 (clone: 17A2)-PE-Cy5, CD4 (clone: RM4-5)-FITC, CD8β (clone: H35-17.2)-APC, ICOS (clone: 7E.17G9)-PE, IFN-γ (clone: XMG1.2)-PE, perforin (clone: eBioOMAK-D)-FITC and granzyme B (clone:16G6)-PE. NKT cells were recognized by CD1d-α-GC-PE tetramer staining (ProImmune Inc, Oxford, UK).
Cultured AB12 cells were checked to see whether CD1d expression was detectable, since AB12 cells were challenged into the KO mice. Anti-mouse CD1d (clone: 1B1)-PE antibody was used 1:100 (eBiosciences).
All antibodies and isotypes were purchased from eBioscience or BioLegend (San Diego, CA). All cells from the same group were pooled together as one sample for running the flow cytometry. Becton–Dickinson LSR II Flow Cytometer (San Jose, CA) and FACSDiva™ software were used for data acquisition ™, and FlowJo™ software was used for analysis.
RNA extraction and real-time reverse transcription PCR (RT-PCR)
Total RNA was extracted from tumor and spleen using TRizol Reagent (Invitrogen), and RNeasy MinElute Cleanup kit (QIAGEN, Valencia, CA) enabled cleanup of RNA. cDNA was synthesized with High-Capacity cDNA Reverse Transcription kits (ABI, Foster City, CA) on a PTC-100™ Programmable Thermal controller (MJ research Inc, Gaithersburg, MD) following the manufacturer’s protocols. Regular PCR was carried out to establish RT-PCR standards of all target genes including CD3, CD4, CD8, ICOS, IL-2, IFN-γ, granzyme B and perforin, and housekeeping gene GAPDH. DNA fragments were obtained from regular PCR on a PTC-100™ Programmable Thermal controller. Regular PCR was performed by 10× High Fidelity PCR Buffer, Platinum® Taq polymerase High Fidelity, 50 mM MgSO4, 10 mM dNTP Mix (Invitrogen). A SYBR GREEN real-time PCR was performed on ABI PRISM 7900HT system. PCR was composed of Power SYBR® GREEN PCR 2× Master Mix (ABI), 200 nM primer and 2 μl 500 ng/μl cDNA × 40 cycles. Primers of all target genes and housekeeping gene were designed by using ABI Prism® Primer Express™ software version 2.0.
ELISA
When animals were killed 7 days after completion of last dose of treatment, the spleens from mice of different groups were removed and splenocytes were prepared as stated before. Co-culture of splenocytes (5 × 106 per well) and AB12 cells (5 × 105 per well) was performed in a 24-well plate with supplementation of IL-2 10 ng/ml in 1 ml of RPMI-1640 complete medium; 24 h later, 0.5 ml of supernatant from each well was collected to determine IFN-γ produced by T cells using ELISA kit (eBioscience).
Statistical analysis
All data are presented as the mean ± SEM. The multiple comparisons of cytokine gene expression, T cell subsets, Ki67 and TUNEL proportion of positive nuclear areas among groups were analyzed by using GraphPad Prism 5 statistical software (La Jolla, CA) with one-way ANOVA and Bonferroni’s post-test. For two group comparison, paired t test with two-tailed P values was performed. A value of P < 0.05 was considered significantly different for all comparisons. *P < 0.05; **P < 0.01; ***P < 0.001.
Results
The anti-tumor effect induced by NKT cells is CD1d-restricted in mice
In CD1d WT Balb/c mice, tumor growth delay was achieved by Cis alone, and this effect was dramatically improved by combination with α-GC following each cycle of chemotherapy, but α-GC alone had little effect (Fig. 1a). Tumor sizes in volume were compared with pre-treatment at 1, 2 and 3 weeks after the first dose of Cis treatment (Fig. 1b). Tumor growth was significantly inhibited by α-GC between cycles of Cis treatments (Fig. 1c). A representative experiment was shown on day 21 after tumor challenge (Supplementary Fig. 1).
Anti-tumor effect induced by NKT cell activator α-GalCer (α-GC) combined with chemotherapy in murine mesothelioma AB12. a Tumor growth on individual mouse after treatment with cisplatin (Cis) 5 mg/kg, iv, once weekly for three doses, α-GC 100 µg/kg, ip, once weekly for three doses, and α-GC following each cycle of cisplatin (Cis + α-GC), untreated tumor-bearing mice were used as controls (NoRx). Treatment was initiated on day 5 after tumor cell injection. The first day of tumor challenge was referred to as day 0; this experiment was repeated three times; b comparisons of tumor volumes at weekly time points after treatment; c treatment schema as indicated in the text
Genotyping of the CD1d KO mice was performed to confirm that the expression of cd1d gene was not detectable (Supplementary Fig. 2A). Lack of NKT cells in the spleen or peripheral blood of CD1d KO mice was determined by flow cytometry with α-GC-tetramer staining (Supplementary Fig. 2B). Furthermore, after AB12 cells were challenged into the KO mice, cultured AB12 cells were checked and no CD1d expression was detected (Supplementary Fig. 2C).
Comparing tumor growth in CD1d WT versus KO mice, tumor progression in untreated mice was faster in CD1d KO than in the WT mice beyond 11 days after tumor challenge. The administration of α-GC during the intervals of chemotherapy was not effective in CD1d KO mice compared to WT mice, and chemotherapy alone appeared to work better in WT mice than in KO mice (Fig. 2a, b). Overall, there was no significant difference between Cis alone and Cis + α-GC in KO mice (Fig. 2c). α-GC alone had little effect on tumor growth in either KO or WT mice in this model.
Comparisons of tumor growth in CD1dWT and KO mice after treatment. The growth curves of tumors in CD1d WT mice versus KO mice plotted as a function over time after sc implantation (a, b). Statistical analysis was done between the groups of Cis alone and Cis combined with α-GC (Cis + α-GC) in WT vs KO mice (c). The experiment was repeated at least twice, n> or =15 animals/group. *P < 0.05; **P < 0.01; ***P < 0.001; *WT Cis + α-GC vs WT Cis. Hash symbol WT Cis + α-GC vs KO Cis; WT Cis vs KO Cis. Dollar symbol WT Cis + α-GC vs KO Cis + α-GC; WT Cis vs KO Cis + α-GC
In vitro cytotoxicity of α-GC on cultured AB12 cells
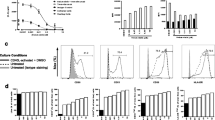
α-GC, known as an immunoenhancing agent, had no cytotoxicity observed even though the concentrations reached doses as high as 10 µg/ml. Cis, used as positive control, displayed cytotoxicity to AB12 cells in a dose-dependent manner. Doses higher than 1 µg/ml resulted in significant increase of early apoptotic cells (Annexin V+/PI−) and dead or necrotic cells (Annexin V+/PI+), while viable and non-necrotic cells (double negative) decreased (Supplementary Fig. 3).
The impact of α-GC on cancer cell proliferation and apoptosis during the intervals of chemotherapy
Cancer cell proliferation and apoptosis were quantified by Ki67 and TUNEL nuclear staining, respectively (Fig. 3a). The proportion of positive nuclear area occupied by Ki67 or TUNEL divided by total nuclear area was referred to as Ki67 or TUNEL labeling index. Cancer cell repopulation (Ki67) was significantly inhibited by α-GC when given weekly after each cycle of Cis; on the contrary, cancer cell apoptosis increased significantly when compared to Cis alone, and the untreated tumor had lowest TUNEL labeling index. Chemotherapy alone resulted in significant inhibition of tumor cell proliferation and rise of apoptosis. α-GC alone had little effect on tumor cell proliferation, but appeared to have some effect on tumor cell apoptosis (Fig. 3b, c).
Effect on cancer cell proliferation and apoptosis induced by α-GC between cycles of chemotherapy. Treatment was initiated when tumor size reached approximately 5 mm in diameter, about 5 days after tumor cell injection. a Histology of tumors: H&E (top panel); Ki67 immunostaining (mid panel); TUNEL immunostaining (bottom panel), ×200; b, c Proportions of Ki67 and TUNEL were quantified by the positive nuclear areas occupied by proliferating or apoptotic cells divided by total nuclear areas, respectively. This experiment was repeated twice. *P < 0.05; **P < 0.01; ***P < 0.001
Enhancement of anti-tumor immune responses induced by NKT cell activation
ELISA results indicated that administration of α-GC following each cycle of chemotherapy resulted in a significant increase in IFN-γ produced by splenocytes independently of the administration of Cis. Granzyme B tended to increase in mice receiving Cis and α-GC, but the difference did not reach significance. Immunosuppressive cytokines such as TGF-β were not different between groups, and IL-10 tended to decrease in mice receiving Cis and α-GC but the difference did not reach significance (Fig. 4a).
Cytokine profile and the expression of the immune-associated genes. a Cytokine production from the splenocytes of mice untreated (NoRx), and treated with α-GC, Cis, and Cis + α-GC was determined by ELISA. When animals were killed 7 days after completion of last dose of treatment, the spleens from mice of different groups were removed and splenocytes were prepared as stated before. Co-culture of splenocytes (5 × 106 per well) and AB12 cells (5 × 105 per well) was performed in a 24-well plate with supplementation of IL-2 10 ng/ml in 1 ml of RPMI-1640 complete medium. 24 h later, 0.5 ml of supernatant from each well was collected to determine cytokine production. This experiment was repeated twice; b the expression of the immune-associated genes and cytolytic enzymes in the spleen were quantified by reverse transcription PCR; c TGF-β expression in both spleen and tumors, and IL-12 and IL-10 expressions in spleens were quantified as well; d gene expression of cytokines IFN-γ, granzyme B, IL-17 and ICOS in tumor tissues of WT mice was quantified by RT-PCR. When animals were killed 7 days after completion of last dose of treatment, tumor tissues were collected for RNA extraction as stated in Methods. This experiment was repeated once
Gene profile of the immune-associated cytokines and cytolytic enzymes determined by RT-PCR showed that the gene expression of the immune-associated cytokines (IFN-γ and ICOS) was significantly upregulated in mice receiving Cis and α-GC. Cytolytic enzymes (granzyme B and perforin) tended to be upregulated after chemotherapy and α-GC in spleen but did not reach significance (Fig. 4b). IL-12 expression in the spleen was also remarkably upregulated after treatment with α-GC and Cis (Fig. 4c). On the other hand, gene expression of the immunosuppressive molecules such as IL-10 in spleen and TGF-β in spleen or tumor did not change. IFN-γ, granzyme B, ICOS and IL-17 significantly increased in the tumor after treatment combining Cis + α-GC (Fig. 4d).
Activation of T cell subsets induced by α-GC was observed in the group of combination treatment with Cis + α-GC. Compared with Cis alone and α-GC alone, the number of NKT cells (CD1d–α-GC-tetramer+CD3+) increased in the peripheral blood and tumor of WT mice in the combination group with Cis + α-GC (Fig. 5a). In contrast, the number of NKT cells showed little difference in spleen and draining lymph nodes (data not shown). There were more IFN-γ-producing cytotoxic CD8 T cells after 5 h culture of lymphocytes with supplementation of IL-2, especially from the peripheral blood and the draining lymph nodes of mice treated with Cis + α-GC (Fig. 5b). The number of NKT cells in tumor and blood increased dramatically after treatment with Cis alone and in combination with α-GC. We found that the proportion of NKT cells is approximately 6 % in WT tumor-bearing mice whereas it was much lower in CD1dKO mice. These could possibly be type I NKT cells after Cis, since both types are CD1d-restricted.
Determination of NKT cells in tumor, peripheral blood and lymphoid tissues. The number of NKT cells was identified by α-GalCer-tetramer and CD3 double positive cells in peripheral blood (top panel) and in tumor (bottom panel) (a); and IFN-γ produced by CD8 T cells in peripheral blood, draining lymph node and spleen of mice treated with chemotherapy alone versus chemotherapy followed by NKT cell activation with α-GC (b). When animals were killed 7 days after completion of the last dose of Cis, the spleens from mice of different groups were removed and splenocytes were prepared as stated in Methods. For intracellular staining, splenocytes were stimulated with 25 ng/ml PMA, 500 ng/ml ionomycin and 10 μg/ml BFA for 5 h at 37 °C 5 % CO2. ICOS expression (c) and IL-17 intracellular staining in CD4 and CD8 T cells in the spleen was compared in WT versus KO mice (d). This experiment was repeated once
The number of activated conventional cytotoxic CD8 T cells and helper CD4 T cells both increased in the spleen of mice treated with Cis + α-GC, compared with Cis alone (Fig. 5c). Interestingly, the proportion of IL-17-producing CD8 T cells (Tc17) and CD4 T cells (Th17) rose in the splenocytes of WT mice treated with combination of Cis and α-GC rather than in KO mice (Fig. 5d).
Discussion
Mesothelioma treatment remains challenging even though various novel approaches have been developed. Cancer immunotherapy has shown promising results when combined with conventional therapies [24–26]. The present study demonstrated for the first time that NKT cell activation by glycolipid analog α-GC resulted in significant inhibition of cancer cell repopulation between cycles of chemotherapy. Several clinical trials using α-GC are currently ongoing for different types of cancers worldwide [21, 27–29]. However, there have been limited studies on α-GC in pre-clinical models or in patients with mesothelioma. Our results suggest that α-GC given during the intervals of Cis treatments can improve the efficacy of chemotherapy through an enhanced activation of the immune system against the tumor. α-GC could therefore be expected to achieve clinical benefits if it could be combined with conventional chemotherapy for MPM patients in the future.
It has been well identified that the glycolipid α-GC analog α-GC is able to activate NKT cells through CD1d molecule expressed on APC. However, the mechanisms of α-GC on other subsets of lymphocytes or cell types remain not fully understood. First of all, it is necessary to know whether α-GC had direct cytotoxicity on tumor cells. Our in vitro data indicated that AB12 cells after exposure to this agent up to 2,000 ng/ml did not induce cell apoptosis or death. As expected, when Cis concentration reached levels as high as 1,000 ng/ml, AB12 cells became apoptotic and died, demonstrating that the effect of α-GC on cancer cells was to enhance the immunity against tumor rather than through non-specific cytotoxicity.
In this study, we found that α-GC following chemotherapy worked more efficiently on tumor growth in WT mice than in KO mice, indicating that α-GC activity is highly CD1d-restricted. Interestingly, chemotherapy alone appeared to work better in WT mice than KO mice as well, implicating that NKT cell activation might take place following chemotherapy and this effect might be partly CD1d-dependent. As shown before, NKT cells kill target cells either through direct cytotoxicity by producing cytolytic enzymes such as granzyme B and perforin, or through indirect cytotoxicity by producing type I cytokines such as IFN-γ to activate helper T and cytotoxic effector T cells [30]. IFN-γ is also able to activate NK cells leading to lysis of tumor cells, and NK cells themselves also produce some amount of IFN-γ [31]. Evidence showed that in a Colon26 adenocarcinoma murine model, not only NKT cells but also liver-associated NK cells play an important role in the anti-tumor activity induced by α-GC [32]. The effect of α-GC on NK cells need to be investigated in this model.
Since previous experiments demonstrated that liver NKT cells provided the most anti-tumor effector functions [33], we evaluated NKT cell infiltration into the tumor after treatment with Cis and α-GC. Although the percentage was quite small, the proportion of tumor-infiltrating NKT cells increased dramatically in the Cis + α-GC-treated group, compared with other groups (Fig. 5), suggesting that accumulation of NKT cells in tumor microenvironment may play an important role in anti-tumor immunity.
Gene expression of cytokine TGF-β did not change significantly after NKT cell activation, suggesting that α-GC-induced immune response may not be modulated through TGF-β pathway, since TGF-β is known as an immunosuppressive cytokine by inhibiting DC maturation and inducing T cell dysfunction [34, 35].
The number of Th17 and Tc17 cells increased in WT mice after treatment with α-GC during chemotherapy, but did not change in CD1dKO mice. The effect of Th17/Tc17 subsets is not fully characterized so far, yet some studies have reported that Th17 cells are a highly plastic population and promote cytotoxic T cell activation in tumor immunity [36–38]. IFN-γ played a critical role by creating a microenvironment to promote Tc17-mediated anti-tumor immunity which was independent of the direct effects of IFN-γ on the tumor [39]. Further studies need to be conducted to clarify the association of Th17/Tc17 and NKT cell activation in this model.
Both RT-PCR and flow cytometry results showed that the inducible co-stimulator ICOS had high levels of expression on T cells in tumor and lymphatic organs. ICOS and its ligand interaction activate T cells that are primed under Th17 polarizing conditions and convert into IFN-γ-producing effector cells [40]. Expression of ICOS on Th1 and Tc1 cells could partly explain why α-GC enhanced the anti-tumor immunity by activating NKT cells and cytotoxic effector T cells. Based on the cytokine release, CD4+ T cells were predominantly Th1. However, activated helper T cells might also include some Th2 cells since anti-inflammatory cytokines such as IL-10 is also upregulated. As shown before, upon recognition of α-GC/CD1d complex, NKT cells rapidly release large amounts of a broad range of cytokines including pro-inflammatory Th1 cytokines, such as IFN-γ and TNF-α, and anti-inflammatory Th2 cytokines, such as IL-10. Our RT-PCR results indicated that the gene expression of IFN-γ, TNF-α and IL-10 was upregulated in tumor and spleen after treatment with Cis + α-GC compared with Cis alone, α-GC alone or untreated group (Fig. 4).
IL-12 has been shown to induce anti-tumor immunity against malignant mesothelioma [41, 42]. Effect on tumor growth occurred when IL-12 was released within the tumor by co-administration of IL-12 gene transfected mesothelioma cells. This effect was dependent on the involvement of both CD4 and CD8 T cells but not NK cells [41]. The upregulation of IL-12 gene expression in tumor might be associated with the anti-tumor effect induced by NKT cell activation in our model (Fig. 4). It has been well studied that α-GC analogs stimulated production of cytokines IFN-γ and IL-12, and its therapeutic activity required the presence of IL-12 [43]. IFN-γ produced by activated iNKT cells after in vitro stimulation or infection was overwhelmingly dependent on toll-like receptor-driven IL-12 [44].
In conclusion, the anti-tumor immunity induced by NKT cell activation might trigger a series of immune signal cascade and orchestrate the immune responses against tumor. As shown in Fig. 1, α-GC alone had little effect, and Cis alone had some anti-tumor effect. However, strikingly, α-GC in combination with Cis had synergistic effect against tumor growth without increasing systemic toxicity. NKT cells may play a critical role in shaping both innate and adaptive immune responses against cancer [45]. The functional roles of NKT cells in tumor microenvironment rely on multiple factors, including cytokines, T cell subsets and the APC with which NKT cells interact. The contribution of these factors is likely to be context dependent (Fig. 6) [46]. Therefore, profound understanding of NKT cell biology and the working mechanisms will be helpful to target NKT cells successfully for cancer immunotherapy.
Working diagram of NKT cell activation by α-GC between cycles of chemotherapy associated with enhanced immunity against tumor in murine mesothelioma model. NKT cell activation requires antigen α-GalCer (α-GC) presentation through MHC-like molecule CD1d expressed on DC. Our data suggest that NKT cells activated by α-GC may enhance the immune response against cancer in this working model. Dead or dying tumor cells release tumor-associated antigens (TAA) which are co-presented by APC on MHC class I or II molecules to CD8+ and CD4+ T cells. NKT cell and APC cross talk, DC maturation and CD4+ T cell help leads to the generation and activation of potent tumor-specific effector CD8+ T cells. The production of IL-12 from activated APCs also results in NK cell mobilization and systemic production of IFN-γ by NKT cells and NK cells. AB12 tumor cells express high levels of MHC class I molecules, which are further upregulated upon exposure to IFN-γ. Therefore, it is plausible that anti-tumor activity of CD8+ T cells is stimulated by NKT cell activation due to increased expression of MHC class I molecules by tumor cells as a result of IFN-γ signaling. IFN-γ might play a critical role by creating a microenvironment to promote Th17/Tc17-mediated anti-tumor immunity. Antigen-educated T cells including NKT cells will travel into tumor and attempt to eradicate the targets
Abbreviations
- α-GC:
-
α-GalCer, alpha-galactocysylceramide
- APC:
-
Antigen-presenting cell
- Cis:
-
Cisplatin
- DC:
-
Dendritic cell
- ICOS:
-
Inducible T cell co-stimulator
- IFN:
-
Interferon
- KO:
-
Knockout
- MPM:
-
Malignant pleural mesothelioma
- NKT:
-
Natural killer T cell
- sc:
-
Subcutaneously
- WT:
-
Wild type
References
Kreso A, O’Brien CA, van Galen P, Gan OI, Notta F, Brown AM et al (2013) Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science 339:543–548
Telleria CM (2013) Repopulation of ovarian cancer cells after chemotherapy. Cancer Growth Metastasis 6:15–21
Kim JJ, Tannock IF (2005) Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer 5:516–525
Gamarra-Luques CD, Goyeneche AA, Hapon MB, Telleria CM (2012) Mifepristone prevents repopulation of ovarian cancer cells escaping cisplatin–paclitaxel therapy. BMC Cancer 12:200
Wu L, Birle DC, Tannock IF (2005) Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res 65:2825–2831
Wu L, Yun Z, Tagawa T, Rey-McIntyre K, de Perrot M (2012) CTLA-4 blockade expands infiltrating T cells and inhibits cancer cell repopulation during the intervals of chemotherapy in murine mesothelioma. Mol Cancer Ther 11:1809–1819
Stayner L, Welch LS, Lemen R (2013) The worldwide pandemic of asbestos-related diseases. Annu Rev Public Health 34:205–216
Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P et al (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636–2644
de Perrot M, Feld R, Cho BC, Bezjak A, Anraku M, Burkes R et al (2009) Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 27:1413–1418
Pasello G, Ceresoli GL, Favaretto A (2013) An overview of neoadjuvant chemotherapy in the multimodality treatment of malignant pleural mesothelioma. Cancer Treat Rev 39:10–17
Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B et al (2001) Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol 167:3114–3122
Tagawa T, Wu L, Anraku M, Yun Z, Rey-McIntyre K, de Perrot M (2013) Antitumor impact of interferon-gamma producing CD1d-restricted NKT cells in murine malignant mesothelioma. J Immunother 36:391–399
Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M et al (2005) Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med 201:1503–1517
Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M et al (2005) A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res 11:1910–1917
Godfrey DI, Stankovic S, Baxter AG (2010) Raising the NKT cell family. Nat Immunol 11:197–206
Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y et al (1999) The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med 189:1121–1128
Carnaud C, Gombert J, Donnars O, Garchon H, Herbelin A (2001) Protection against diabetes and improved NK/NKT cell performance in NOD.NK1.1 mice congenic at the NK complex. J Immunol 166:2404–2411
Nakagawa R, Nagafune I, Tazunoki Y, Ehara H, Tomura H, Iijima R et al (2001) Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by alpha-galactosylceramide in mice. J Immunol 166:6578–6584
Tonti E, Galli G, Malzone C, Abrignani S, Casorati G, Dellabona P (2009) NKT-cell help to B lymphocytes can occur independently of cognate interaction. Blood 113:370–376
Leadbetter EA, Brigl M, Illarionov P, Cohen N, Luteran MC, Pillai S et al (2008) NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA 105:8339–8344
Schneiders FL, Scheper RJ, von Blomberg BM, Woltman AM, Janssen HL, van den Eertwegh AJ et al (2011) Clinical experience with alpha-galactosylceramide (KRN7000) in patients with advanced cancer and chronic hepatitis B/C infection. Clin Immunol 140:130–141
Motohashi S, Okamoto Y, Yoshino I, Nakayama T (2011) Anti-tumor immune responses induced by iNKT cell-based immunotherapy for lung cancer and head and neck cancer. Clin Immunol 140:167–176
Wu L, Yun Z, Tagawa T, Rey-McIntyre K, Anraku M, de Perrot M (2011) Tumor cell repopulation between cycles of chemotherapy is inhibited by regulatory T-cell depletion in a murine mesothelioma model. J Thorac Oncol 6:1578–1586
Barbieri PG, Marinaccio A, Ferrante P, Scarselli A, Pinelli V, Tassi G (2012) Effects of combined therapies on the survival of pleural mesothelioma patients treated in Brescia, 1982–2006. Tumori 98:215–219
Kelly RJ, Sharon E, Hassan R (2011) Chemotherapy and targeted therapies for unresectable malignant mesothelioma. Lung Cancer 73:256–263
Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL et al (2010) Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res 70:9053–9061
Richter J, Neparidze N, Zhang L, Nair S, Monesmith T, Sundaram R et al (2013) Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood 121:423–430
Nicol AJ, Tazbirkova A, Nieda M (2011) Comparison of clinical and immunological effects of intravenous and intradermal administration of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells. Clin Cancer Res 17:5140–5151
Yamasaki K, Horiguchi S, Kurosaki M, Kunii N, Nagato K, Hanaoka H et al (2011) Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol 138:255–265
Peng Y, Zhao L, Shekhar S, Liu L, Wang H, Chen Q et al (2012) The glycolipid exoantigen derived from Chlamydia muridarum activates invariant natural killer T cells. Cell Mol Immunol 9:361–366
Jahn T, Zuther M, Friedrichs B, Heuser C, Guhlke S, Abken H et al (2012) An IL12-IL2-antibody fusion protein targeting Hodgkin’s lymphoma cells potentiates activation of NK and T cells for an anti-tumor attack. PLoS One 7:e44482
Nakagawa R, Motoki K, Ueno H, Iijima R, Nakamura H, Kobayashi E et al (1998) Treatment of hepatic metastasis of the colon26 adenocarcinoma with an alpha-galactosylceramide, KRN7000. Cancer Res 58:1202–1207
Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG et al (2005) Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med 202:1279–1288
Sere K, Felker P, Hieronymus T, Zenke M (2013) TGFbeta1 microenvironment determines dendritic cell development. Oncoimmunology 2:e23083
Donkor MK, Sarkar A, Savage PA, Franklin RA, Johnson LK, Jungbluth AA et al (2011) T cell surveillance of oncogene-induced prostate cancer is impeded by T cell-derived TGF-beta1 cytokine. Immunity 35:123–134
Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S et al (2009) T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31:787–798
Prabhala RH, Pelluru D, Fulciniti M, Prabhala HK, Nanjappa P, Song W et al (2010) Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood 115:5385–5392
Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S et al (2009) Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114:1141–1149
Yu Y, Cho HI, Wang D, Kaosaard K, Anasetti C, Celis E et al (2013) Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J Immunol 190:1873–1881
Hamai A, Pignon P, Raimbaud I, Duperrier-Amouriaux K, Senellart H, Hiret S et al (2012) Human T(H)17 immune cells specific for the tumor antigen MAGE-A3 convert to IFN-gamma-secreting cells as they differentiate into effector T cells in vivo. Cancer Res 72:1059–1063
Caminschi I, Venetsanakos E, Leong CC, Garlepp MJ, Scott B, Robinson BW (1998) Interleukin-12 induces an effective antitumor response in malignant mesothelioma. Am J Respir Cell Mol Biol 19:738–746
Jackaman C, Nelson DJ (2010) Cytokine-armed vaccinia virus infects the mesothelioma tumor microenvironment to overcome immune tolerance and mediate tumor resolution. Cancer Gene Ther 17:429–440
Schmieg J, Yang G, Franck RW, Tsuji M (2003) Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med 198:1631–1641
Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N et al (2011) Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med 208:1163–1177
Brennan PJ, Brigl M, Brenner MB (2013) Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 13:101–117
Mattarollo SR, Smyth MJ (2013) NKT cell adjuvants in therapeutic vaccines against hematological cancers. Oncoimmunology 2:e22615
Acknowledgments
This work was partly supported by the Mesothelioma Foundation at Princess Margaret Hospital, Canada, and the research grant from the Mesothelioma Applied Research Foundation, USA. Dr. Marc de Perrot is the recipient of the grants and the Head of Toronto Mesothelioma Research Program, Canada. We would like to thank the Flow Cytometry Facility of the Hospital for Sick Children and Animal Resources Centre, University Health Network, Toronto, Canada.
Conflict of interest
No potential conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Some preliminary results were presented at the 15th World Conference on Lung Cancer (WCLC2013), Sydney, Australia (abstract published in the Journal of Thoracic Oncology, Volume 8, Supplement 2, November 2013).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, L., Yun, Z., Tagawa, T. et al. Activation of CD1d-restricted natural killer T cells can inhibit cancer cell proliferation during chemotherapy by promoting the immune responses in murine mesothelioma. Cancer Immunol Immunother 63, 1285–1296 (2014). https://doi.org/10.1007/s00262-014-1597-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-014-1597-9