Abstract
Adoptive transfer of genetically modified T cells to treat cancer has shown promise in several clinical trials. Two main strategies have been applied to redirect T cells against cancer: (1) introduction of a full-length T cell receptor (TCR) specific for a tumor-associated peptide—MHC, or (2) introduction of a chimeric antigen receptor, including an antibody fragment specific for a tumor cell surface antigen, linked intracellularly to T cell signaling domains. Each strategy has advantages and disadvantages for clinical applications. Here, we present data on the in vitro and in vivo effectiveness of a single-chain signaling receptor incorporating a TCR variable fragment as the targeting element (referred to as TCR-SCS). This receptor contained a single-chain TCR (Vα-linker-Vβ) from a high-affinity TCR called m33, linked to the intracellular signaling domains of CD28 and CD3ζ. This format avoided mispairing with endogenous TCR chains and mediated specific T cell activity when expressed in either CD4 or CD8 T cells. TCR-SCS-transduced CD8-negative cells showed an intriguing sensitivity, compared to full-length TCRs, to higher densities of less stable pepMHC targets. T cells that expressed this peptide-specific receptor persisted in vivo, and exhibited polyfunctional responses. Growth of metastatic antigen-positive tumors was significantly inhibited by T cells that expressed this receptor, and tumor cells that escaped were antigen-loss variants. TCR-SCS receptors represent an alternative targeting receptor strategy that combines the advantages of single-chain expression, avoidance of TCR chain mispairing, and targeting of intracellular antigens presented in complex with MHC proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adoptive transfer of genetically modified T cells to treat cancer has shown tremendous promise over the past decade (reviewed in [1]). In this process, peripheral T cells from a patient are transduced ex vivo with a vector encoding a receptor that recognizes tumor cells. The transduced cells are re-introduced into the patient, where they can mediate an anticancer immune response, sometimes resulting in impressive tumor regression. Two main types of receptors have been used for adoptive T cell treatments: (1) a full-length αβ TCR, sometimes engineered for enhanced affinity, that is specific for a tumor-associated peptide presented by an MHC molecule on the surface of tumor cells [2–6], and (2) a chimeric antigen receptor (CAR) that consists of a single-chain antibody fragment (scFv), specific for a cancer-associated cell surface epitope, fused to a transmembrane region followed by intracellular signaling domains [7–9].
Introducing a full-length, tumor-directed TCR into a patient’s T cells has several advantages. The endogenous signaling machinery associated with αβ TCRs, including the CD3 complex and co-receptors CD4 and CD8, enables reactivity with very high sensitivity to the pepMHC ligand, requiring as few as 1–10 cognate peptide–MHC complexes to stimulate T cell responses [10–13]. TCRs, unlike typical CARs, also possess the advantage of targeting intracellular antigens that can be cross-presented [14]. This feature allows tumor antigen recognition in lymph nodes and on tumor stroma [15] and may aid in extravasation, tumor penetration, and destruction. Furthermore, hundreds of MHC-restricted peptide epitopes have been characterized [16], and the ability to rapidly identify mutated peptide antigens in cancer will expand this even further [17].
One of the disadvantages of the TCR approach is that the introduced TCR chains can pair with endogenous TCR chains, thereby reducing the surface levels of the cancer-associated TCR. More concerning is that the mispairing can result in uncharacterized, potentially pathogenic, autoimmune reactivities [18]. Strategies to reduce mispairing include the introduction of cysteine residues in constant domains that result in a novel disulfide bond to facilitate pairing of the exogenous TCR chains [19–22]. There is some evidence that the cysteines do not fully eliminate mispairing [23]. Voss and colleagues addressed the issue of TCR mispairing by transducing a single-chain Vα-linker-VβCβ to pair with a free Cα domain via introduced disulfide bond, which assembled with normal CD3 chains and signaled functionally in T cells [24]. Our previous results have shown that such Cβ-containing constructs also have potential to mispair as the energy of dimer association is driven largely by C:C region interactions [23]. Finally, while there is strong evidence that TCRs with higher affinity for a class I pepMHC antigen can mediate enhanced effectiveness of CD4 T cell responses because the TCRs are “CD8 independent” [25–27], some of these TCRs also cross-react with self-peptides in a CD8-dependent process; these can lead to self-reactive CD8 T cells [28] or to complete deletion of the CD8 T cells [29, 30].
Early studies with CARs have shown significant efficacy in controlling B cell malignancies in patients [7, 8]. In these studies, use of an anti-CD19 scFv as a targeting element in CAR-transduced T cells mediated reduction, and in some cases elimination, of chronic lymphocytic leukemias. Although there is evidence that some CAR-transduced T cells are more sensitive to cell surface antigens when compared to a soluble bispecific antibody with the same scFv [31], the sensitivity may not be as important for CARs as for TCRs, as CARs typically target highly overexpressed, cell surface proteins. In addition, there are no problems associated with pairing of the variable domains in a CAR with endogenous chains of the αβ TCR. One disadvantage of typical CARs is that by not targeting pepMHC antigens, there are no opportunities for cross-presentation on tumor stroma, which could facilitate destruction of stroma in solid tumors.
Here, we have incorporated several of the advantages of these two receptor strategies by designing a novel format we refer to as the TCR-SCS (T cell receptor single-chain signaling) construct. TCR-SCS consists of a single-chain TCR variable domain fusion (Vα-linker-Vβ or Vβ-linker-Vα), linked to a transmembrane region, followed by intracellular signaling domains, in one polypeptide chain. In this work, we have used a model system involving the high-affinity TCR called m33 (K d value of 30 nM, [28]) that is specific for the SIYRYYGL (SIY) peptide bound to murine MHC H2-Kb. The TCR-SCS format has the advantage of binding to intracellular or cross-presented peptide–MHC epitopes, while eliminating possible mispairing with endogenous TCR chains [23]. We show that m33 TCR-SCS was expressed at high levels on both CD4 and CD8 T cells, and directed specific, polyfunctional responses in transduced T cells. Interestingly, while the TCR-SCS was less sensitive than full-length TCR to the agonist peptide, it was more sensitive to agonists that bind weakly to the MHC (albeit at high densities of surface antigen). Furthermore, the TCR-SCS mediated in vivo anti-tumor responses and tumor control when transduced into CD4 or CD8 T cells. The adoptively transferred T cells also greatly reduced the number of lung lesions in a metastatic B16 tumor model. Finally, the T cells persisted in vivo and responded to specific target antigen with a polyfunctional response.
Materials and Methods
Antibodies, proteins, and peptides
Anti-mouse H2-Kb antibody (B.8.24.3), anti-mouse Vbeta 8 (F23.2), and anti-mouse CD3 epsilon (145-2C11 [32]) were purified from hybridoma supernatant using protein A resin. SIY (SIYRYYGL) peptide was synthesized by the Macromolecular Core Facility of the Section of Research Sources, Penn State College of Medicine. Peptides were purified by reverse phase chromatography, and masses were confirmed by MALDI. Quantification by amino acid analysis was performed at the Molecular Structure Facility, University of California, Davis.
Soluble, single-chain TCR m67 [28] was produced as previously described [33] in E. coli as inclusion bodies, refolded, and biotinylated for specific detection of SIY/Kb complexes on the surface of tumor cells. Bound, biotinylated m67 was detected using streptavidin Alexa 647 (Molecular Probes, Life Technologies).
In vivo biotinylated H2-Kb H chain [33] and human β2 microglobulin were expressed separately in E. coli as inclusion bodies, solubilized in urea, and refolded with excess SIY peptide as described previously [34]. Folded SIY/Kb complexes were purified by anion exchange using HiTrap Q columns (GE Healthcare). For incorporation into SA oligomers, phycoerythrin-labeled SA (BD Biosciences) was added stepwise to biotinylated SIY/Kb in aliquots on ice over 20 min to a 1:4 (SA to MHC) final molar ratio.
Mice and cell lines
C57BL/6 and C57BL/6Rag1 −/− mice were purchased from Jackson Laboratory, and colonies were maintained in animal facilities at the University of Illinois. Mice were used at 2–5 months of age. All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Illinois. Murine melanoma B16-F10 cells were purchased from ATCC. B16-SIY was derived from B16-F10 cells engineered to express green fluorescent protein (GFP) as a fusion protein with SIYRYYGL (SIY) [35, 36]. T2-Kb is a TAP-deficient lymphoblastoid cell line transfected with mouse Kb. Cell lines were cultured at 37 °C and 5 % CO2 in complete RPMI 1640 medium (containing HEPES, 10 % FBS, l-glutamine, 2-ME, penicillin, and streptomycin).
T cell transduction
Platinum-E (Plat-E) retroviral packaging cells were transfected with m33, m33 or 2C Y46β TCR library [37], or m33 TCR-SCS genes cloned into pMP71 (or no DNA for “mock”) as described [23, 29, 30]. Viral supernatants were harvested 48 h after transfection, 0.45-μm filtered, and 50 μL Lipofectamine 2000 (Life Technologies) was added per 6 mL. For transductions, CD8, CD4, or total T splenocytes from C57BL/6 mice were negatively selected using mouse CD8α+ or CD4+ T Cell Isolation Kit II (MACS Miltenyi Biotec) or Dynabeads Untouched Mouse T Cells Kit (Dynal, Life Technologies) and activated with anti-CD3/anti-CD28 Mouse T-activator Dynabeads (Dynal, Life Technologies) and 30 U/mL of recombinant mouse IL-2 (Roche) for 24 h. Dynabeads were removed, and cells were transferred into a 24-well plate coated with Retronectin at 15 μg/mL (Takara). 106 T cells in 1 mL of T cell media were mixed with 1 mL sterile viral supernatant and 60 U of IL-2 per well. Plates were centrifuged at 2,000 rpm at 30 °C for 1 h, and cells were incubated at 37 °C and 5 % CO2.
For T cell hybridoma transductions, 58−/− T cell hybridoma cells (106) in 1 mL of T cell media were mixed with 1 mL sterile viral supernatant. Plates were centrifuged at 2,000 rpm at 30 °C for 1 h, and cells were incubated for 48 h at 37 °C and 5 % CO2. To enrich for the transduced positive population, cells were stained with SIY/Kb and sorted using a FACS Aria (BD Biosciences).
Subcutaneous tumor inoculation, treatment, and characterization
B16-SIY melanoma cells were harvested and washed twice with Hanks’ balanced salt solution (HBSS, Cellgro Mediatech Inc). Shaved, Rag1 −/− mice received 106 tumor cells subcutaneously into the right flank while under isoflurane (Baxter) inhalation anesthesia. For adoptive T cell transfer, 7–10 × 106 mock-transduced, TCR-transduced, or TCR-SCS transduced T cells harvested from 24-well plates and washed twice with HBSS were injected in the tail vein of mice 5 days after tumor inoculation. Tumor growth was monitored by measuring tumor length and width with calipers every 2 days. Tumor volume was estimated as (length × (width)2)/2.
For detection of antigen expression in subcutaneous tumors [30], implanted B16-SIY melanoma tumors were harvested, sectioned, and incubated with 300 Collagenase Digestion U/mL (Sigma-Aldrich) and 1 mg/mL Dispase (Roche) for 40 min at 37 °C. After incubation, 0.002 MU/mL DNase (Calbiochem) was added for further dissociation using gentleMACS Dissociator (Miltenyi Biotec). Cell suspensions were pipetted through 100-μm filters and were cultured for 4–6 days alongside parental B16-F10 and B16-SIY cell lines. All cell lines were treated with 10 ng/mL IFN-γ overnight prior to evaluation by flow cytometry.
Metastatic melanoma model
B16-SIY melanoma cells were harvested and washed twice with HBSS (Cellgro Mediatech Inc). Rag1 −/− mice received 1 × 106 tumor cells injected into the tail vein. For adoptive T cell transfer, 5–10 × 106 mock-transduced, TCR-transduced, or TCR-SCS transduced T cells harvested and washed twice with HBSS were injected in the tail vein of mice either at the same time, or 7 days after tumor cell injection. Mice were monitored for weight and health and were killed after 2 weeks when T cells were delivered simultaneously with tumor cells, or were killed when they exhibited severe hunching, lack of mobility, or weight loss greater than 25 % of their initial weights. Mice were dissected, and lung nodules were quantitated or characterized.
In vitro T cell stimulation and cytokine detection
Splenocytes from mice that had received adoptive T cell transfer treatment were isolated from mice and incubated overnight (18–24 h) in a 96-well plate with T cell media alone, immobilized anti-CD3 antibody, B16-SIY tumor cells, parental B16-F10 tumor cells, or 10 μM SIY peptide. After incubation, media supernatants were harvested and analyzed for IFN-γ by ELISA Ready-SET-Go! Kit (eBioscience), or for a panel of cytokines including IFN-γ, IL-2, IL-4, IL-6, IL-10, IL-15, IL-17, and TNF-α by magnetic bead-based MILLIPLEX MAP assay kit (Millipore) using a Bio-Rad Bio-Plex Cytometric Bead Analyzer.
For IL-2 release by 58−/− T cell hybridomas, cells (7.5 × 104) were incubated with SIY alanine mutants or OVA peptide loaded T2-Kb (7.5 × 104) for 24 h at 37 °C in a final volume of 200 µL in 96-well plates. For IL-2 detection, 96-well plates were coated with 2.5 µg/mL anti-murine IL-2 (BD Pharmingen) in 0.1 M Na2HPO4 (pH = 9.0), and IL-2 in supernatants was detected with 6.7 µg/mL biotinylated anti-murine IL-2 (BD Pharmingen), followed by a 1:10,000 dilution of streptavidin-HRP (BD Pharmingen), and, finally, TMB substrate (KPL). Absorbance at 450 nm was measured with an ELx800 universal plate reader (Bio-Tek Instruments).
Statistical analysis
Survival data were analyzed by a log-rank test, with a criterion of 1,000 mm3. Lung metastasis data were analyzed by Student’s t test. Graphpad Prism software was used for analyses.
Results
Expression of high-affinity TCR-SCS in primary T cells
The high-affinity (30 nM K d) TCR m33 [28], specific for H2-Kb presenting the SIY peptide (SIY/Kb), was used as the specific targeting element in two T cell-directing receptors: (1) a standard, full-length T cell receptor (TCR), consisting of Vβ-Cβ and Vα-Cα sequences separated by a self-cleaving P2A sequence and containing the extra cysteines in the C regions (Fig. 1a); and (2) a TCR-SCS, consisting of a single polypeptide chain with m33Vα and Vβ sequences joined by a flexible linker, the CD8 hinge region, the CD28 transmembrane region, and intracellular CD28 and CD3ζ signaling domains (Fig. 1b, [23]). Genes encoding each m33 construct were cloned into the retroviral vector, pMP71 [38].
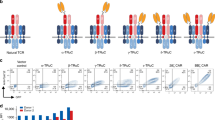
High-affinity receptor expressed as a full-length TCR or a TCR single-chain signaling construct (TCR-SCS). a, b The m33 TCR was cloned into the retroviral vector pMP71 as a full-length alpha–beta TCR (a), or a TCR-SCS containing the single-chain TCR variable domain fragment (scTv), followed in frame with a CD8 hinge region, CD28 transmembrane region, and intracellular signaling domains of CD28 and CD3zeta (b). c, d Expression of the Vβ8 TCR domain and binding of target SIY/Kb MHC tetramers after mock, m33 TCR, or m33 TCR-SCS transduction into CD4 T cells (c) or CD8 T cells (d). e, f In vitro IFN-γ released into the supernatants (measured by ELISA) from mock, m33 TCR, or m33 TCR-SCS-transduced CD4 (e) or CD8 (f) T cells stimulated with T2-Kb antigen-presenting cells and various amounts of the SIY peptide
Both the m33 TCR and the m33 TCR-SCS were transduced separately into ex vivo activated CD4 and CD8 murine T cells from C57BL/6 mice and compared with mock-transduced T cells where the retroviral vector was omitted. In mock-transduced cells, approximately 10–20 % of T cells expressed the endogenous Vβ8 TCR segment in both CD4 and CD8 populations; this proportion was increased to 60–75 % when either the m33 TCR or the m33 TCR-SCS gene was transduced (Fig. 1c, d). When the T cells were stained with SIY/Kb streptavidin-linked tetramers, no staining was observed with mock T cells, but the majority (60 to >80 %) of m33 TCR-transduced and m33 TCR-SCS-transduced T cells were stained. The enhanced affinity of the m33 TCR allows efficient staining with soluble SIY/Kb tetramer even in CD4 T cells as it does not require CD8 for binding or activity [25, 28]. The surface levels of the TCR-SCS construct were twofold to fivefold higher than the conventional αβ TCR on the transduced C57BL/6 T cells, consistent with our previous studies using the αβ-negative T cell hybridoma 58−/− [23]. This finding supports the view that expression of the TCR-SCS construct is not limited by endogenous levels of CD3 subunits, as would be the case for the αβ TCR.
TCR-SCS receptors show activity and specificity in CD4 and CD8 T cells
To evaluate the function of the m33 TCR and m33 TCR-SCS receptors, T cells were stimulated in vitro by T2-Kb cells loaded with various concentrations of the SIY peptide. T cell activation was examined by the amount of IFN-γ secreted into supernatants. CD4 T cells expressing full-length m33 TCR exhibited an SD50 of approximately 0.2 pM SIY peptide, while CD4 T cells expressing the m33 TCR-SCS required about tenfold more, or 2 pM, for equivalent IFN-γ secretion (Fig. 1e). By contrast, m33 TCR-SCS expressed in CD8 T cells could detect lower levels of SIY peptide (SD50 ~0.3 pM, Fig. 1f). CD8 T cells that expressed full-length m33 TCR were stimulated even in the absence of SIY peptide (Fig. 1f), as has been shown previously [37]. This is likely due to self-pep/Kb reactivity (T cell/T cell) that occurs with the m33 TCR, as first observed in CD8+ T cell hybridomas [28]. Importantly, the TCR-SCS construct completely avoids this self-reactivity in CD8 T cells, probably because the CD8 molecule is unable to synergize with the m33 single-chain complex in recognition of self-pep/Kb complexes.
TCR-SCS receptors trigger polyfunctional responses to SIY/Kb
As the TCR-SCS receptor differs in its signaling complex compared to a conventional full-length TCR/CD3 complex, we examined the cytokine secretion profiles of CD4+ T cells transduced with m33 TCR-SCS compared to full-length m33. T2-Kb cells were used as antigen-presenting cells at various concentrations of SIY peptide, and culture supernatants were assayed for the cytokines IFN-γ, IL-2, IL-4, IL-6, IL-10, and TNF-α. As shown in Fig. 2, all six of the cytokines were secreted by CD4+ T cells transduced with either the full-length TCR or TCR-SCS, indicating that both receptors mediated polyfunctional responses. However, as with IFN-γ, in each case, the full-length TCR was 10–50-fold more sensitive to SIY peptide. The sensitivity of the cytokine responses varied, with the order of decreasing sensitivity to SIY peptide as follows: IFN-γ > IL-4 > IL-10 > IL-2 > TNF-α > IL-6.
TCR-SCS receptor effects polyfunctional cytokine responses. CD4 T cells transduced with either m33 TCR (triangles) or m33 TCR-SCS (squares) were stimulated with T2-Kb cells and various concentrations of SIY peptide. Concentration-dependent secretion of IFN-γ, IL-2, IL-4, IL-6, IL-10, and TNF-α was measured via flow cytometry with a multiplexed bead assay
To verify that the difference in sensitivity between the full-length TCR and the TCR-SCS was not due to an unexpected difference in affinity of the m33-binding site, we titrated both forms with monomeric SIY/Kb (biotinylated) and detected the bound form with SA-PE. The binding curves for the two forms were essentially identical (Supplementary Figure 1).
Analysis of TCR-SCS fine specificity
In addition to the H2-Kb-restricted agonist peptide SIY for m33, it has been shown previously that CD8+ T cells expressing m33 are stimulated by the structurally similar self-peptide called dEV8 [28]. As with reactivity of CD8+ T cells to self-peptides on H-2b targets, reactivity to exogenously added dEV8 was not observed in the absence of CD8, for example, with CD4+ T cells [39]. Thus, our expectation was that neither full-length m33 TCR nor the m33 TCR-SCS would mediate activity to dEV8. Surprisingly, when a high concentration of dEV8 (100 μM) was co-incubated with T2-Kb and CD4+ T cells transduced with m33 TCR-SCS (open bars) but not full-length m33 (solid bars), the cells responded by secreting all the cytokines tested (IFN-γ and IL-6 shown, Fig. 3a). The level of secretion was at levels similar to the positive control stimulus, immobilized anti-CD3 antibody. It is important to note that the level of dEV8/Kb complexes on the target cells when incubated with this high concentration of exogenous dEV8 are no doubt orders of magnitude higher than the endogenous levels of dEV8/Kb.
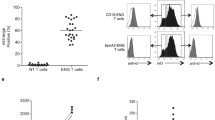
Fine specificity of m33 TCR-SCS. a CD4 T cells were transduced with m33 TCR (black bars) or m33 TCR-SCS (open bars) and incubated with either T2-Kb cells with 100 mM dEV8 peptide, immobilized anti-CD3 antibody, or no added stimulus. Cytokine secretion (shown are IFN-γ and IL-6) was measured by flow cytometry with a mulitplexed bead assay. b: Transduction of m33 TCR and m33 TCR-SCS into the TCR and co-receptor negative murine T cell hybridoma, 58−/−. Staining of untransduced (gray, filled), m33 TCR (black trace), or m33 TCR-SCS (red trace) cells is shown for expression of TCR Cβ (left panel) and SIY/Kb tetramer (right panel). c Activation of transduced 58−/− cells with various concentrations of dEV8 peptide. IL-2 secretion is shown for m33 TCR (triangles) and m33 TCR-SCS (squares). d, f Stimulation of 58−/− transductants with alanine variants of the agonist SIY peptide. d SD50 values for alanine substitutions at each position of the SIY peptide for IL-2 secretion from 58−/− with m33 TCR (black bars) and m33 TCR-SCS (open bars). e, f Stimulation of m33 TCR (triangles) and m33 TCR-SCS (squares) with titrations of e SIY Y5A and f SIY L8A. g View of the SIY peptide (orange) bound in the H2-Kb (pale green) binding groove. Residues Y5 and L8 are highlighted in blue. Structural information was obtained from PDB ID 1G6R [41]
To further investigate this unexpected behavior of m33 TCR-SCS, we utilized the TCR- and CD8-negative T cell hybridoma line, 58−/−, in which the CD8 dependence of dEV8 recognition by m33 was originally characterized [28, 39]. Both full-length m33 TCR and m33 TCR-SCS were transduced separately into this cell line (Fig. 3b). As expected, the murine TCR Cβ domain was detected only on the full-length TCR (left panel, black trace), whereas both TCR (black) and TCR-SCS (red) transduced cells were able to bind to SIY/Kb tetramers (right panel). However, the TCR-SCS was expressed at a considerably higher density (~40-fold) than the full-length TCR, consistent with previous results [23]. As with the transduced CD4+ T cells (Fig. 3a), the co-receptor negative 58−/− T cells transduced with m33 TCR-SCS were stimulated by high levels of dEV8 peptide, whereas the m33 TCR-transduced 58−/− T cells were not (Fig. 3c). The finding of dEV8 reactivity with the m33 TCR-SCS is additionally surprising given that the affinity of m33 for dEV8/Kb is quite low, over 1,000-fold below that of m33 for SIY/Kb [28].
This “reversal” of sensitivity to the strong agonist, SIY, versus the weak, CD8-dependent agonist dEV8, prompted us to further investigate the fine specificity of the two m33 receptors using an alanine scan of the SIY peptide (Fig. 3d). The specificity of the full-length m33 TCR (black bars) was similar to that observed previously with the 2C TCR and a related high-affinity TCR called m67 [40]. For seven of the alanine variants, the relative sensitivity and fine specificity of the m33 TCR-SCS was quite similar to the full-length m33. However, there were two alanine variants (Y5A and L8A) where there was a very significant difference between m33 full-length TCR and TCR-SCS. The TCR-SCS was about 10- to 100-fold more sensitive to SIY Y5A (Fig. 3e) and SIY L8A (Fig. 3f) compared to the full-length TCR. Thus, these two peptides behaved more like dEV8 in that the TCR-SCS was stimulated more effectively that the TCR.
Interestingly, both Y5 and L8 of SIY represent key Kb anchor residues and point directly into the MHC binding groove, rather than toward the TCR interface (Fig. 3g, blue residues) [41]. Accordingly, the alanine substitutions of these two residues have the most significant impact on Kb binding, yet the complexes are able to bind to the TCR as well as the wild-type SIY peptide [40]. Thus, the m33 TCR binds to dEV8/Kb with about 1,000-fold lower affinity than these two peptide/Kb complexes. Despite these wide differences in affinity, the TCR-SCS form of the receptor is stimulated more effectively than full-length TCR by each of these peptides, suggesting a distinct mechanism of action between conventional TCRs and the single-chain form inherent in the TCR-SCS (and CARs; see “Discussion”).
TCR-SCS receptors show in vivo activity against SIY-bearing tumors
Transduced m33 TCR-SCS T cells were tested for ability to control SIY/Kb+ tumors in RAG−/− mice implanted subcutaneously with the murine melanoma line B16-SIY. Five days later, mice were treated intravenously with 7–9 × 106 mock, m33 TCR, or m33 TCR-SCS transduced CD4 or CD8 T cells. Both CD4 and CD8 T cells expressing the m33 TCR-SCS were able to delay tumor outgrowth significantly, compared to mock-transduced T cells (p < 0.01, Fig. 4a).
In vivo activity of T cells transduced with m33 TCR-SCS. a B16-SIY tumor outgrowth in RAG−/− mice with 5 day established tumors. Treatment by intravenous injection with mock-transduced CD8 T cells (gray, filled circles, solid lines), mock-transduced CD4 T cells (gray, open circles, dashed line), m33 TCR-transduced CD8 T cells (black X’s, solid lines), m33 TCR-transduced CD4 T cells (black X’s, dashed lines), m33 TCR-SCS transduced CD8 T cells (black, filled squares, solid lines), or m33 TCR-SCS transduced CD4 T cells (black, open squares, dashed lines). Individual mice are shown. b For each treatment group of four mice (except the m33 TCR-transduced CD8 cells, where n = 2), the average time for the subcutaneous B16-SIY tumor to reach 1,000 mm3 is shown. Error bars represent standard error. Treatment with m33 TCR-SCS in CD8 T cells significantly delayed (p < 0.01) tumor outgrowth compared with m33 TCR in CD8 T cells. Treatment with m33 TCR-SCS transduced CD4 cells significantly delayed (p < 0.05) tumor outgrowth compared with m33 TCR-SCS in CD8 T cells. c, d Phenotype of tumor outgrowths after treatment with m33 TCR-SCS-transduced T cells. c RAG−/− mice carrying 5-day-old B16-SIY subcutaneous tumors were treated intravenously with saline (black, open symbols), mock-transduced CD4 and CD8 T cells (gray, filled symbols), or m33 TCR-SCS transduced CD4 and CD8 T cells (black, filled symbols). Individual mice are shown. Tumor outgrowth to 1,000 mm3 was significantly delayed (p < 0.01) for m33 TCR-SCS-transduced T cells compared with mock-transduced T cells. c The tumors from three mice treated with m33 TCR-SCS-transduced T cells were re-isolated, briefly adapted to culture, and compared with the stock B16-SIY and parental B16-F10 cell lines. All cells were incubated overnight with 10 ng/mL IFN-γ. Cells were stained for total H2-Kb levels (left panels) and for the specific complex SIY/Kb (right panels)
To compare the extent of tumor control, the times required for tumors to reach 1,000 mm3 were determined for each treatment regimen (Fig. 4b). In CD8 T cells, the m33 TCR-SCS significantly delayed the time that it took tumors to reach 1,000 mm3 (p < 0.01), while in CD4 T cells, time to outgrowth was delayed even longer, significantly longer than CD8 T cells (p < 0.05). By contrast, CD8 T cells expressing full-length m33 TCR do not significantly delay B16-SIY tumor outgrowth (Fig. 4a, b) as these high-affinity CD8 T cells are deleted rapidly in the periphery [29, 30, 37]. A significant advantage of TCR-SCS, compared to full-length TCR, is that both CD8 and CD4 T cells maintained specificity, activity, and persistence. One animal that received mock-transduced CD8 T cells was able to effect late control of the B16-SIY tumor (Fig. 4a), and temporary regression has been seen previously in about 50 % of mock-transduced CD8 T cell recipients [30]. As suggested previously, we believe some of the mock-transduced T cell preparations contain a sufficient repertoire of T cells with endogenous TCRs that are able to generate a class I-restricted tumor response.
Tumor outgrowths from m33 TCR-SCS-treated mice are antigen-loss variants
Since complete elimination of tumors after treatment with T cells expressing the m33 TCR-SCS did not occur, we examined whether tumors from treated mice had lost the antigen SIY/Kb. In this experiment, B16-SIY tumors were inoculated in RAG−/− mice, and 5 days later, mice were treated with saline only, 8 × 106 mock-transduced total T cells (CD4 and CD8), or 8 × 106 m33 TCR-SCS-transduced total T cells. In saline-only-treated mice, tumors rapidly grew to the size where mice had to be killed (Fig. 4c). When mice were treated with mock-transduced T cells, one mouse experienced essentially unchanged tumor growth compared with saline only, while one mouse experienced a brief, late regression of the tumor, followed by continued outgrowth. As indicated above, this is consistent with previous results where mock-transduced CD8 T cells can effect this temporary, late regression of B16-SIY tumors in about 50 % of treated mice [30]. As with mice treated with separated CD4 or CD8 T cells, mice treated with total T cells transduced with m33 TCR-SCS exhibited a significant delay in tumor outgrowth, in which tumors reached 1,000 mm3 26–30 days after the control tumors (p < 0.01 compared to mice treated with mock-transfected T cells, Fig. 4c).
Tumors that recurred in the three mice treated with m33 TCR-SCS T cells were isolated and re-adapted to culture. Once sufficient numbers of cells were reached (4–6 days after isolation, prior to passaging), cells were incubated overnight with 10 ng/mL IFN-γ and then analyzed by flow cytometry. H2-Kb levels were examined with an anti-Kb antibody, and nearly identical levels were found for each tumor, and the B16-SIY and parental B16-F10 (SIY-negative) cells (Fig. 4d, left panels). The levels of specific antigen, SIY/Kb, in tumor outgrowths were measured with a soluble, high-affinity single-chain TCR m67 [33]. In contrast to total Kb levels, SIY/Kb was undetectable on any of the three tumor outgrowths, which resembled the parental, SIY-negative B16-F10 cells, whereas significant levels were detected on the original B16-SIY line that was implanted (Fig. 4d, right panels). Tumors that developed in the saline and mock-treated mice were also removed, adapted to culture, and examined for levels of SIY/Kb. Consistent with the absence of immune-driven selection, SIY/Kb was detected on these tumors (Supplementary Figure 2). This is also consistent with previous analysis of B16-SIY tumors from mice treated with saline or mock-transduced T cells that showed retention of the SIY epitope in the absence of SIY-specific T cells [42]. Thus, outgrowth of antigen-loss variant tumors accounted for the re-occurrence of tumors in m33 TCR-SCS-treated mice.
TCR-SCS receptors are an effective treatment in a model of metastatic melanoma
Given the effectiveness of m33 TCR-SCS receptor in destroying antigen-bearing solid tumors, we further tested the approach in a model of metastatic melanoma. In initial experiments, 1 × 106 B16-SIY cells were co-injected intravenously with saline, 8 × 106 mock-transduced T cells, 9 × 106 T cells transduced with full-length 2C or m33 TCR libraries [37], or 7 × 106 m33 TCR-SCS-transduced T cells. Total T cells (CD4 and CD8) were transduced with each construct. The full-length TCR libraries were used for transduction instead of unmodified, full-length m33 TCR because CD8 T cells do not persist with the high-affinity TCR, whereas CD8 T cells persist and control subcutaneous tumors with most of the TCRs in the libraries [37]. After 14 days, mice were killed, and lungs were examined for metastatic nodules. An example of the appearance of lungs from controls and m33 TCR-SCS-treated mice is shown in Fig. 5a. Nodules on the lungs of each mouse (two per group) were quantitated, showing an average of over 200/mouse in saline-only mice, 100/mouse when treated with mock-transduced cells, and under 10/mouse when treated with full-length TCRs or m33 TCR-SCS-transduced T cells (p < 0.05 for treated compared to controls, Fig. 5b). The result indicated that specific, transduced T cells were able to dramatically reduce the number of melanoma nodules when co-injected with B16-SIY tumor cells.
High-affinity m33 TCR-SCS-transduced T cells are effective at reducing lung tumors in a model of metastatic melanoma. a RAG−/− mice (2 per group) were co-injected intravenously with 1 × 106 B16-SIY cells and treatment consisting of saline, mock-transduced T cells, 2C TCR Yβ46 library-transduced T cells, m33 TCR Yβ46 library-transduced T cells, or m33 TCR-SCS-transduced T cells. Mice were killed 14 days later, and lungs were evaluated for metastatic nodules. Representative lung images from the saline, mock, and m33 TCR-SCS group are shown. b Lung nodules were enumerated from each group. Error bars represent standard error. A number of nodules on lungs treated with TCR or m33 TCR-SCS transduced T cells were significantly reduced compared with those treated with mock-transduced T cells (p < 0.05). c RAG−/− mice were injected intravenously with 1 × 106 B16-SIY cells on day 0, and treatment consisting of saline, mock-transduced T cells, or m33 TCR-SCS-transduced T cells was delivered on day 7 via tail vein. Weights were monitored for each mouse over time. d Kaplan–Meier survival plot for mice with treatment on day 7. Survival was significantly extended for mice treated with m33 TCR-SCS transduced T cells (p < 0.01 for m33 TCR-SCS compared to mock)
As a more stringent test of efficacy, we waited seven days after tumor cell injection to treat mice with T cells. By this time, tumor cells have already migrated to the lung where they have begun to form nodules. In this experiment, 1 × 106 B16-SIY melanoma cells were injected intravenously, and treatments with saline (n = 4), mock-transduced (n = 4), or m33 TCR-SCS-transduced (n = 5) total T cells were administered seven days later. Mice were monitored for weight and general health and killed when they exhibited severe hunching, lack of mobility, or weight loss greater than 25 % of initial weight. The weights of mice were plotted over the time course of the experiment (Fig. 5c), and overall survival was determined (Fig. 5d). Average survival times for different treatment groups were 24 ± 1 days for saline-treated, 26 ± 4 days for mock-transduced T cell-treated, and 55 ± 5 days for m33 TCR-SCS T cell-treated mice (p < 0.01 for m33 TCR-SCS compared to mock). All mice contained metastatic nodules in their lungs at kill, although m33 TCR-SCS-treated mice had fewer, large nodules as opposed to numerous smaller nodules observed in saline- and mock-treated mice. This finding is consistent with a rapid destruction of the majority of tumor cells in m33 TCR-SCS-treated mice, allowing a fewer number of antigen-loss variant tumors to persist and develop into lung nodules.
Functional, antigen-specific TCR-SCS cells persist in treated mice
While antigen-loss variants appear to be responsible for outgrowths of subcutaneous tumors, and possibly lung nodules, it is also possible that m33 TCR-SCS T cells had acted rapidly and then either disappeared, or somehow become tolerized. To evaluate this, specific functional responses were tested in splenocytes of treated, tumor-bearing mice. First, splenocytes were isolated from mock- and m33 TCR-SCS-treated mice killed on day 14 (shown in Fig. 5a, b). Splenocytes were incubated overnight in culture with media alone, immobilized anti-CD3 antibody, or 10 μM SIY peptide. Supernatants were then assayed for various cytokines using Luminex beads (Fig. 6a). There was no significant cytokine release without added stimuli (gray bars), whereas splenocytes from both mock and m33 TCR-SCS mice secreted a variety of cytokines in response to immobilized anti-CD3 (white bars). However, only the m33 TCR-SCS-transduced cells, and not mock-transduced cells, secreted cytokines in response to SIY peptide (Fig. 6a, red bars). Significant levels of IFN-γ, IL-2, IL-4, IL-6, IL-17, and TNF-α were observed, constituting a substantial polyfunctional response.
Adoptive cell therapy with m33 TCR-SCS T cells leads to long-term persistence of specific, functional T cells. a RAG−/− mice (2 per group) were co-injected intravenously with 1 × 106 B16-SIY cells and either mock-transduced T cells, or m33 TCR-SCS-transduced T cells. Mice were killed 14 days later, and splenocytes were incubated overnight in culture with media alone (gray bars), immobilized anti-CD3 antibody (white bars), or 10 mM SIY peptide (red bars). Culture supernatants were analyzed for the indicated cytokines by multiplexed cytokine bead assay. b RAG−/− mice carrying 5-day-old B16-SIY subcutaneous tumors were treated with m33 TCR-SCS-transduced CD4 and CD8 T cells and injected intravenously. Splenocytes were isolated 42 days after T cell injection and incubated overnight with media alone, immobilized anti-CD3 antibody, parental B16-F10 cells, or B16-SIY cells. Supernatants were analyzed for IFN-γ release by ELISA
In order to assess even longer-term persistence of functional m33 TCR-SCS T cells, splenocytes were isolated from two of the long-term surviving, subcutaneous B16-SIY tumor-bearing mice 42 days after T cell injection (Fig. 4c, filled, black symbols). Splenocytes from these mice were incubated overnight in culture with media alone, immobilized anti-CD3, or cultured B16-F10 parental or B16-SIY melanoma cells. Significant, specific responses were observed against B16-SIY, but not B16-F10 cells, as measured by IFN-γ released into the supernatant (Fig. 6b). These results suggest that m33 TCR-SCS cells persisted and could continue surveillance for an extended period in vivo.
Discussion
The results presented here show that the T cell receptor single-chain signaling (TCR-SCS) format, with engineered, high-affinity TCRs as a single-chain, is effective in vitro and in vivo. The TCR-SCS, which combines some of the advantages of both TCRs and CARs, was functional and specific in both CD4 and CD8 T cells. This contrasts with high-affinity, full-length m33 TCR, which in CD8 T cells exhibited SIY/Kb-independent activity in vitro, leading to rapid deletion in vivo [29, 30, 37]. It is thought that the latter is due to cooperation between CD8 co-receptor and m33 TCR, which binds in a CD8-dependent manner to a self-peptide called dEV8 that is restricted by H2-Kb and expressed by most mouse cells [28, 43, 44]. Interestingly, in the present report, CD4 T cells with the m33 TCR-SCS were stimulated by high concentrations of exogenous dEV8, yet T cells expressing this receptor were not stimulated in vitro by endogenous self-peptides and the cells were able to persist in vivo, with no evidence of graft-versus-host-type reactions. Thus, the m33 TCR-SCS form retained the ability of m33 to drive activity of CD4 T cells, but also allowed CD8 T cells to persist and function in mice.
The targeting element used in this construct is derived from the high-affinity m33 TCR formatted as a single-chain linked variable domain. It has been engineered by directed evolution from the 2C TCR for increased affinity (Kd 30 nM) for its ligand, SIY/Kb [28]. It is unlikely that a wild-type affinity TCR (with Kd values of 1–300 μM or more, [45]) would be as effective since we have shown that the wild-type TCR 2C exhibited much lower activity in the TCR-SCS format [23]. Most TCR variable domains are difficult to express in this single-chain format [46] (although three-domain single-chain TCRs containing a constant domain have been used [24]), but human TCRs containing the Valpha2 region are particularly stable and amenable to engineering as single-chain fragments (Vα-linker-Vβ or Vβ-linker-Vα) [47, 48].
The TCR-SCS construct presented here includes CD28 and CD3ζ intracellular signaling domains (Fig. 1a, [23]), analogous to some second-generation CARs [49]. Other second-generation CARs with intracellular CD3ζ and 4-1BB signaling domains have been used clinically [7] and shown improved persistence compared to the CD28-containing CARs [50]. Although the TCR-SCS with CD28 and CD3ζ signaling domains used here showed persistence beyond one month (Fig. 6), it remains to be seen if other constructs will have improved activities and persistence. For example, future studies can incorporate the 4-1BB signaling domain in place of or in addition to the CD28 signaling domain into the TCR-SCS format. It will also be useful to test the TCR-SCS in a system that can avoid antigen-loss variants (Fig. 4d) and thus be able to more fully address the functional correlates of TCR-SCS persistence.
The m33 full-length TCR, with an affinity of 30 nM for SIY/Kb [28, 34], responds to its target in CD4 or co-receptor negative T cells [25]. Likewise, m33 TCR-SCS was functional in CD4 or CD8 T cells. However, while CARs have been suggested to be co-receptor independent, there does appear to be a slight sensitivity difference between our m33 TCR-SCS receptor expressed in CD4 and CD8 T cells, even when assaying the same cytokine as the activation marker (Fig. 1e,f). Since T2-Kb cells in our assay are murine class I MHC positive, but class II MHC negative, they could be engaged by CD8, but not by CD4. It is possible that CD8 binding to class I MHC acted as a simple adhesion interaction. If so, one would expect to see the same effects with CARs, but to the best of our knowledge this has not been reported. Alternatively, it is possible that ex vivo activated and transduced CD4 T cells are inherently less sensitive to clustering of the TCR-SCS signaling domains than are CD8 T cells [11, 12].
While the m33 TCR-SCS receptor was slightly less sensitive to the agonist peptide SIY than full-length m33 TCR, the TCR-SCS unexpectedly showed a greater level of sensitivity to exogenous self-peptide dEV8 and the alanine substituted variants Y5A and L8A of SIY that represent modifications of the peptide anchor residues (Fig. 3). The importance of stable peptide binding to the MHC in order to allow efficient TCR recognition is well known [51, 52]. It has been shown that dEV8 binding to Kb is much weaker than SIY [39, 53]. Similarly, the SIY alanine variants Y5A and L8A were 10- to 100-fold less capable of stabilizing Kb [40].
Thus, in the absence of CD8, there is a distinct divergence with regard to sensitivity of the conventional full-length TCR versus the TCR-SCS format (which is analogous to CARs). It appears that the former exhibited greater sensitivity when the number of peptide/MHC complexes was low and the TCR affinity was above a threshold (e.g., about 1 μM, [34]), However, the TCR-SCS exhibited greater sensitivity when the density of some peptide/MHC complexes was high. We suggest that this apparent conundrum is associated with the considerably higher levels of the TCR-SCS that can be achieved on T cells compared to the levels of the conventional TCR/CD3. In fact, the lower levels of TCR/CD3 may have in part evolved to maintain exquisite selectivity.
The mechanistic basis of these findings remains to be determined, but it is possible that the signaling domains contained within a single polypeptide chain in the TCR-SCS yield distinct kinetics of triggering that may be more rapid than for a full-length TCR. Triggering through conventional TCR/CD3 complexes may require more extensive co-localization of co-receptors and/or co-stimulatory molecules for productive signaling. This signaling may be more efficient at low levels of agonist peptide/MHC as long as the affinity of the TCR is above a particular threshold. In contrast, the TCR-SCS stimulation was observed even with low-affinity interactions with the ligand (e.g., m33 TCR has an affinity of about 60 μM for dEV8/Kb). This ability of the TCR-SCS to be stimulated by highly abundant, but more transient or unstable targets than TCRs, may well extend to traditional CARs. To our knowledge, this behavior has not been reported in CARs (and, in fact, the behavior was only observed here in a direct comparison between full-length TCR and the related TCR-SCS specific for the same ligands). Thus, unexpected, low-affinity cross-reactive epitopes of some targets may be worth considering in CAR-based therapies. In the present study, we postulate that there were no in vitro or in vivo effects of the endogenous dEV8/Kb complexes because the cell surface levels of these are well below that required to stimulate T cells bearing the TCR-SCS (i.e., well below the levels achieved with exogenous, synthetic dEV8 peptide at 10–100 μM; Fig. 3c).
A primary advantage of the TCR-SCS construct, compared to conventional CARs, is the ability to recognize intracellular antigens presented as peptide–MHC complexes in the same way as αβ TCR on normal CD8 T cells. This advantage could extend of course to CARs with scFv fragments from TCR-like antibodies. Although there are some challenges to isolating bonafide peptide-specific antibodies against pepMHC ligands, recent advances, including immunizations of MHC-transgenic mice, and phage display techniques have improved these opportunities (reviewed in [54]). TCR-like antibodies are not specifically evolved to recognize peptide–MHC complexes and can dock in either similar [55] or dissimilar [56] orientations to the canonical diagonal docking angle of a TCR binding to an MHC [57]. It remains to be seen whether this will impact the peptide specificity of this class of antibodies and whether this will pose a potential problem given the enhanced sensitivity of CAR formats [58], as well as full-length TCRs [2, 3], compared to standard antibody-based drugs. In any case, isolation of TCRs against many different pep/HLA complexes, including the use of humanized TCR transgenic mice [59], should provide a rich source of leads for engineering improved affinities and formats of the TCR-SCS approach.
Abbreviations
- CAR:
-
Chimeric antigen receptor
- MHC:
-
Major histocompatability complex
- scFv:
-
Single-chain variable fragment of an antibody
- TCR:
-
T cell receptor
- TCR-SCS:
-
TCR single-chain signaling receptor
References
Hinrichs CS, Rosenberg SA (2014) Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev 257(1):56–71
Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ, Binder-Scholl GK, Smethurst DP, Gerry AB, Pumphrey NJ, Bennett AD, Brewer JE, Dukes J, Harper J, Tayton-Martin HK, Jakobsen BK, Hassan NJ, Kalos M, June CH (2013) Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122(6):863–871
Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM, Phan GQ, Hughes MS, Kammula US, Miller AD, Hessman CJ, Stewart AA, Restifo NP, Quezado MM, Alimchandani M, Rosenberg AZ, Nath A, Wang T, Bielekova B, Wuest SC, Akula N, McMahon FJ, Wilde S, Mosetter B, Schendel DJ, Laurencot CM, Rosenberg SA (2013) Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 36(2):133–151
Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA (2006) Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314(5796):126–129
Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA (2010) T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 19(3):620–626
Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA (2011) Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 29(7):917–924
Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH (2011) T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3(95):95ra73
Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, Hakim FT, Halverson DC, Fowler DH, Hardy NM, Mato AR, Hickstein DD, Gea-Banacloche JC, Pavletic SZ, Sportes C, Maric I, Feldman SA, Hansen BG, Wilder JS, Blacklock-Schuver B, Jena B, Bishop MR, Gress RE, Rosenberg SA (2013) Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 122(25):4129–4139
Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E (2006) Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol 24(13):e20–e22
Huang J, Brameshuber M, Zeng X, Xie J, Li QJ, Chien YH, Valitutti S, Davis MM (2013) A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells. Immunity 39(5):846–857
Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM (2002) Direct observation of ligand recognition by T cells. Nature 419(6909):845–849
Purbhoo MA, Irvine DJ, Huppa JB, Davis MM (2004) T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol 5(5):524–530
Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN (1996) Evidence that a single peptide–MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 4(6):565–571
Nair-Gupta P, Blander JM (2013) An updated view of the intracellular mechanisms regulating cross-presentation. Front Immunol 4:401
Engels B, Rowley DA, Schreiber H (2012) Targeting stroma to treat cancers. Semin Cancer Biol 22(1):41–49
Vigneron N, Stroobant V, Van den Eynde BJ, van der Bruggen P (2013) Database of T cell-defined human tumor antigens: the 2013 update. Cancer Immun 13:15
Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, Samuels Y, Rosenberg SA (2013) Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med 19(6):747–752
Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, Kaiser AD, Pouw N, Debets R, Kieback E, Uckert W, Song JY, Haanen JB, Schumacher TN (2010) Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med 16(5):565–570, 561 p following 570
Boulter JM, Glick M, Todorov PT, Baston E, Sami M, Rizkallah P, Jakobsen BK (2003) Stable, soluble T-cell receptor molecules for crystallization and therapeutics. Protein Eng 16(9):707–711
Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA (2006) Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res 66(17):8878–8886
Kuball J, Dossett ML, Wolfl M, Ho WY, Voss RH, Fowler C, Greenberg PD (2007) Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood 109(6):2331–2338
Sebestyen Z, Schooten E, Sals T, Zaldivar I, San Jose E, Alarcon B, Bobisse S, Rosato A, Szollosi J, Gratama JW, Willemsen RA, Debets R (2008) Human TCR that incorporate CD3zeta induce highly preferred pairing between TCRalpha and beta chains following gene transfer. J Immunol 180(11):7736–7746
Aggen DH, Chervin AS, Schmitt TM, Engels B, Stone JD, Richman SA, Piepenbrink KH, Baker BM, Greenberg PD, Schreiber H, Kranz DM (2012) Single-chain ValphaVbeta T-cell receptors function without mispairing with endogenous TCR chains. Gene Ther 19(4):365–374
Voss RH, Thomas S, Pfirschke C, Hauptrock B, Klobuch S, Kuball J, Grabowski M, Engel R, Guillaume P, Romero P, Huber C, Beckhove P, Theobald M (2010) Coexpression of the T-cell receptor constant alpha domain triggers tumor reactivity of single-chain TCR-transduced human T cells. Blood 115(25):5154–5163
Chervin AS, Stone JD, Bowerman NA, Kranz DM (2009) Cutting edge: inhibitory effects of CD4 and CD8 on T cell activation induced by high-affinity noncognate ligands. J Immunol 183(12):7639–7643
Robbins PF, Li YF, El-Gamil M, Zhao Y, Wargo JA, Zheng Z, Xu H, Morgan RA, Feldman SA, Johnson LA, Bennett AD, Dunn SM, Mahon TM, Jakobsen BK, Rosenberg SA (2008) Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol 180(9):6116–6131
Zhao Y, Bennett AD, Zheng Z, Wang QJ, Robbins PF, Yu LY, Li Y, Molloy PE, Dunn SM, Jakobsen BK, Rosenberg SA, Morgan RA (2007) High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J Immunol 179(9):5845–5854
Holler PD, Chlewicki LK, Kranz DM (2003) TCRs with high affinity for foreign pMHC show self-reactivity. Nat Immunol 4(1):55–62
Engels B, Chervin AS, Sant AJ, Kranz DM, Schreiber H (2012) Long-term persistence of CD4(+) but rapid disappearance of CD8(+) T cells expressing an MHC class I-restricted TCR of nanomolar affinity. Mol Ther 20(3):652–660
Soto CM, Stone JD, Chervin AS, Engels B, Schreiber H, Roy EJ, Kranz DM (2012) MHC-class I-restricted CD4 T cells: a nanomolar affinity TCR has improved anti-tumor efficacy in vivo compared to the micromolar wild-type TCR. Cancer Immunol Immunother 62(2):359–369
Stone JD, Aggen DH, Schietinger A, Schreiber H, Kranz DM (2012) A sensitivity scale for targeting T cells with chimeric antigen receptors (CARs) and bispecific T-cell Engagers (BiTEs). Oncoimmunology 1(6):863–873
Leo O, Foo M, Sachs DH, Samelson LE, Bluestone JA (1987) Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci USA 84(5):1374–1378
Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, Yu P, Fu YX, Weichselbaum RR, Rowley DA, Kranz DM, Schreiber H (2007) Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med 204(1):49–55
Chervin AS, Stone JD, Holler PD, Bai A, Chen J, Eisen HN, Kranz DM (2009) The impact of TCR-binding properties and antigen presentation format on T cell responsiveness. J Immunol 183(2):1166–1178
Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF (2004) PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res 64(3):1140–1145
Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, Fu YX, Schreiber H (2002) Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity 17(6):737–747
Chervin AS, Stone JD, Soto CM, Engels B, Schreiber H, Roy EJ, Kranz DM (2012) Design of T-cell receptor libraries with diverse binding properties to examine adoptive T-cell responses. Gene Ther 20(6):634–644
Engels B, Cam H, Schuler T, Indraccolo S, Gladow M, Baum C, Blankenstein T, Uckert W (2003) Retroviral vectors for high-level transgene expression in T lymphocytes. Hum Gene Ther 14(12):1155–1168
Holler PD, Kranz DM (2003) Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity 18(2):255–264
Bowerman NA, Colf LA, Garcia KC, Kranz DM (2009) Different strategies adopted by K(b) and L(d) to generate T cell specificity directed against their respective bound peptides. J Biol Chem 284(47):32551–32561
Degano M, Garcia KC, Apostolopoulos V, Rudolph MG, Teyton L, Wilson IA (2000) A functional hot spot for antigen recognition in a superagonist TCR/MHC complex. Immunity 12(3):251–261
Soto CM, Stone JD, Chervin AS, Engels B, Schreiber H, Roy EJ, Kranz DM (2013) MHC-class I-restricted CD4 T cells: a nanomolar affinity TCR has improved anti-tumor efficacy in vivo compared to the micromolar wild-type TCR. Cancer Immunol Immunother 62(2):359–369
Santori FR, Kieper WC, Brown SM, Lu Y, Neubert TA, Johnson KL, Naylor S, Vukmanovic S, Hogquist KA, Jameson SC (2002) Rare, structurally homologous self-peptides promote thymocyte positive selection. Immunity 17(2):131–142
Tallquist MD, Yun TJ, Pease LR (1996) A single T cell receptor recognizes structurally distinct MHC/peptide complexes with high specificity. J Exp Med 184(3):1017–1026
Stone JD, Chervin AS, Kranz DM (2009) T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology 126(2):165–176
Richman SA, Aggen DH, Dossett ML, Donermeyer DL, Allen PM, Greenberg PD, Kranz DM (2009) Structural features of T cell receptor variable regions that enhance domain stability and enable expression as single-chain ValphaVbeta fragments. Mol Immunol 46(5):902–916
Aggen DH, Chervin AS, Insaidoo FK, Piepenbrink KH, Baker BM, Kranz DM (2011) Identification and engineering of human variable regions that allow expression of stable single-chain T cell receptors. Protein Eng Des Sel 24(4):361–372
Smith SN, Sommermeyer D, Piepenbrink KH, Blevins SJ, Bernhard H, Uckert W, Baker BM, Kranz DM (2013) Plasticity in the contribution of T cell receptor variable region residues to binding of peptide–HLA-A2 complexes. J Mol Biol 425(22):4496–4507
Sadelain M, Brentjens R, Riviere I (2013) The basic principles of chimeric antigen receptor design. Cancer Discov 3(4):388–398
Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, Samanta M, Lakhal M, Gloss B, Danet-Desnoyers G, Campana D, Riley JL, Grupp SA, June CH (2009) Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 17(8):1453–1464
Engels B, Engelhard VH, Sidney J, Sette A, Binder DC, Liu RB, Kranz DM, Meredith SC, Rowley DA, Schreiber H (2013) Relapse or eradication of cancer is predicted by peptide-major histocompatibility complex affinity. Cancer Cell 23(4):516–526
Yewdell JW, Bennink JR (1999) Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol 17:51–88
Tallquist MD, Weaver AJ, Pease LR (1998) Degenerate recognition of alloantigenic peptides on a positive-selecting class I molecule. J Immunol 160(2):802–809
Dahan R, Reiter Y (2012) T-cell-receptor-like antibodies—generation, function and applications. Expert Rev Mol Med 14:e6
Mareeva T, Martinez-Hackert E, Sykulev Y (2008) How a T cell receptor-like antibody recognizes major histocompatibility complex-bound peptide. J Biol Chem 283(43):29053–29059
Kumar P, Vahedi-Faridi A, Saenger W, Ziegler A, Uchanska-Ziegler B (2009) Conformational changes within the HLA-A1:MAGE-A1 complex induced by binding of a recombinant antibody fragment with TCR-like specificity. Protein Sci 18(1):37–49
Rudolph MG, Stanfield RL, Wilson IA (2006) How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol 24:419–466
Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA (2010) Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18(4):843–851
Li LP, Lampert JC, Chen X, Leitao C, Popovic J, Muller W, Blankenstein T (2010) Transgenic mice with a diverse human T cell antigen receptor repertoire. Nat Med 16(9):1029–1034
Acknowledgments
We thank Hans Schreiber and his colleagues from the University of Chicago for helpful discussions, Barbara Pilas from the University of Illinois Flow Cytometry Facility for flow cytometry support, and Wendy Woods for experimental assistance. This work was supported by a grant from the Melanoma Research Alliance (to David M. Kranz) and by National Institutes of Health grant R01 CA178844 (to David M. Kranz). Jennifer D. Stone was supported by the Samuel and Ruth Engelberg/Irvington Institute Fellowship of the Cancer Research Institute. Daniel T. Harris was supported by a Ruth L. Kirschstein National Research Service Award Predoctoral Fellowship from the National Institutes of Health FCA180723A.
Conflict of interest
David M. Kranz co-founded a company called ImmuVen that has acquired rights from the University of Illinois for some T cell receptor-based technologies. Jennifer D. Stone, Daniel T. Harris, Carolina M. Soto, Adam S. Chervin, David H. Aggen, and Edward J. Roy declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stone, J.D., Harris, D.T., Soto, C.M. et al. A novel T cell receptor single-chain signaling complex mediates antigen-specific T cell activity and tumor control. Cancer Immunol Immunother 63, 1163–1176 (2014). https://doi.org/10.1007/s00262-014-1586-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-014-1586-z