Abstract
Costimulatory surface molecules and instructive cytokines expressed by dendritic cells (DCs) determine the outcome of an immune response. In malignant disease, DCs are often functionally compromised. In most tumors studied so far, the deficient induction of effective T cell responses has been associated with a blockade of DC maturation, but little has been known on DCs infiltrating malignant B cell lymphoma. Here, we investigated for the first time the phenotypic and functional status of DCs in B cell lymphoma, and we analyzed the network of DCs, tumor cells, natural killer (NK) cells and cytokines present in the tumor micromilieu. Therefor, we used an endogenous myc-transgenic mouse lymphoma model, because transplanted tumor cells foster an IFN-γ-driven Th1 antitumor response rather than an immunosuppressive environment, which is observed in autochthonous neoplasias. Lymphoma-infiltrating DCs showed a mature phenotype and a Th2-inducing cytokine pattern. This situation is in contrast to most human malignancies and mouse models described. Cellular contacts between DCs and tumor cells, which involved CD62L on the lymphoma, caused upregulation of costimulatory molecules, whereas IL-10 primarily derived from lymphoma cells induced an IL-12/IL-10 shift in DCs. Thus, alteration of costimulatory molecules and instructive cytokines was mediated by distinct mechanisms. Normal NK cells were able to additionally modulate DC maturation but this effect was absent in the lymphoma environment where IFN-γ production by NK cells was severely impaired. These data are relevant for establishing novel immunotherapeutic approaches against B cell lymphoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dendritic cells (DCs) exert a key role in the induction of adaptive immune responses [1]. DCs take up antigens and subsequently undergo a maturation process, which is associated with upregulation of major histocompatibility complex (MHC) class II and costimulatory molecules like CD80 and CD86 [1–3]. Upon maturation, DCs are capable of priming naïve T cells in an antigen-specific manner, whereas interaction of T cells with immature DCs may result in T cell anergy [4, 5]. In addition, DCs determine the outcome of an immune response by their production of instructive cytokines. Thus, the IL-12/IL-10 expression profile of DCs stipulates the bias toward Th1 or Th2 responses.
Although specific immune responses against malignant cells have been reported in numerous studies, the immune system is generally not able to counteract cancer. This may be partly due to functional impairment of tumor-infiltrating DCs (TIDCs), which are often not able to adequately stimulate T cells (review in [6]). The dysfunction of TIDCs was ascribed to factors that are present in the tumor micromilieu, such as IL-10 or TGF-β [7, 8], or to a lack of DC activation factors like microbial stimuli [9].
In most investigations conducted so far, the deficient T cell-stimulatory capacity of TIDCs was associated with blocked maturation. In several experimental mouse models using transplanted or endogenous tumors, the expression of MHC class II or costimulatory molecules like CD80 and CD86 on TIDCs was reduced [10–12]. Coculture experiments revealed that mouse tumor cell lines gave rise to DCs with low Ia expression also in vitro and that T cell activation was thereby inhibited [13]. Like in mouse models, an inhibition of DC maturation was also observed in human lung and mammary cancer [14–16]. Immaturity and reduced numbers of DCs were reported to correlate with clinical prognosis [17, 18].
Whether the number or the maturation status of DCs alone, however, is responsible for functional deficits, has been questioned in other papers [19, 20]. Some studies even revealed a mature phenotype of TIDCs in mouse and in human malignancies [21, 22]. Thus, lack of costimulatory molecules may not be the only reason for deficient T cell priming by TIDCs.
Apart from the requisite costimulatory signals, cytokines provided by DCs are also decisive for the efficacy of an antitumor response. TIDCs often show a shift from IL-12 to IL-10 expression [11, 18, review in 6]. This may also provide a mechanism impeding antitumor responses because T cell-mediated tumor rejection requires a Th1-driven response involving IFN-γ and IL-12 rather than a Th2-prone milieu [23–26].
Using transplantable murine lymphoma models, we previously showed that natural killer (NK) cells as a major source of IFN-γ readily initiated a Th1-dependent cytotoxic T cell (CTL) response involving IL-12-producing DCs [27, 28]. In spontaneously arising lymphoma, by contrast, NK cell functions including their expression of IFN-γ were heavily impaired [29, 30]. Lack of IFN-γ was verified in the micromilieu of spontaneous lymphoma [30], but the relevance of these findings for putative DC alterations in endogenous lymphoma remained elusive.
As malignant lymphoma in humans still has a poor prognosis, new therapeutic modalities are needed. Vaccination strategies have been proposed that rely on intact antigen presentation and T cell priming by endogenous DCs of the tumor-bearing host [e.g. 31–34]. However, little is known on the functional status of TIDCs in solid lymphoma, and a systematic evaluation of the factors determining their differentiation including the role of NK cells and soluble factors has not yet been done. Therefore, we characterized TIDCs in a c-myc-transgenic model of spontaneous B cell lymphoma [35]. We show that direct cellular contacts with malignant B cells as well as soluble factors and NK cells contribute to DC alterations in a complex manner involving a differential regulation of DC maturation and DC cytokine expression.
Materials and methods
Mice
C57BL/6 wild type (wt) and BALB/c mice were purchased from Taconic (Ry, Denmark). λ-myc mice [35] were bred in our own animal facility under specific pathogen-free conditions. All animal experiments were approved by the competent authority.
Preparation of cells
DCs and NK cells were purified from tumor-infiltrated spleens or lymph nodes (LNs) or from organs of wt animals by positive or negative immunomagnetic selection using mouse CD11c microbeads (N418) or the NK Cell Isolation-Kit II (both from Miltenyi Biotec, Bergisch-Gladbach, Germany), respectively. For isolation of normal or malignant B cells ex vivo, positive selection with anti-CD19 (ID3; BD Biosciences, Heidelberg, Germany) and for isolation of T cells, the CD8+ and the CD4+ T Cell Isolation Kit, respectively, was used (Miltenyi Biotec). Purity was >95 %. 291 is a variant of 291S, which is derived from the lymphoma of a λ-myc mouse [29] and was propagated in RPMI 1640 supplemented with 10 % fetal calf serum (FCS).
To obtain supernatants from tumors, LN or spleen cells were washed and 106 cells were plated on 96-well dishes in 200 μl AIM-V medium (Invitrogen) per well. After 48 h, supernatants were harvested by centrifugation.
Flow cytometry
For phenotyping, the following directly labeled antibodies (Ab) were used: anti-CD11c (N418; BioLegend, San Diego, USA), anti-CD80 (16-10A1; BD Biosciences), anti-CD86 (GL1; BD Biosciences), anti-CD83 (Michel-19; BioLegend), anti-CD40 (3/23; BioLegend), anti-MHC class II (2G9; BD Biosciences), anti-PD-L1 (MIH6; AbD Serotec, Raleigh, USA), anti-CD62L (MEL-14; eBioscience, San Diego, USA), anti-CD19 (1D3; BD Biosciences, Heidelberg, Germany). After labeling, cells were analyzed in an LSR II flow cytometer. For intracellular cytokine staining, cells were stimulated with PMA/ionomycin for 4 h in the presence of brefeldin A, labeled with anti-CD11c and further processed using the fixation and permeabilization kit (eBioscience). IL-12 and IL-10 were detected with PE-labeled C15.6 (BD Biosciences) and APC-labeled JES5-16E3 (BD Biosciences), respectively.
Coculture experiments
DCs (106) freshly isolated ex vivo were cultivated for 16 h together with 106 lymphoma cells (tumor suspensions or 291 cells) in RPMI 1640 medium supplemented with 10 % FCS or in the presence of tumor supernatants in a volume of 2 ml in 24-well plates. Optionally, trans-well plates (Corning, New York, USA) were used. In some experiments, 106 NK cells enriched from λ-myc or wt organs were additionally included. When used in trans-well plates, NK cells were kept separately from DCs and tumor cells. For blocking cellular receptors or cytokines, mAbs against CD40 (1C10; BioLegend), CD40L (MR1; BioLegend), CD62L (MEL-14; BioLegend), IL-10 (JES5-16E3; BioLegend) or IFN-γ (XMG1.2; eBioscience) in a concentration of 5 μg/ml were used. Alternately, 5 ng/ml recombinant IFN-γ (Peprotech, Rocky Hill, USA) were added.
For T cell stimulation experiments, DCs derived from wt or from tumor-bearing λ-myc mice were pulsed with OT-I (OVA257–264) or OT-II (OVA323–339) peptide at a concentration of 1 μg/ml for 3 h. In 96-well plates, 5 × 104 DCs were then incubated with 105 T cells that were derived from OT-I or OT-II mice (kindly provided by S. Edelmann, München). After 3 or 5 days, supernatants were harvested and tested for cytokine concentrations. Allostimulation was done according to the same protocol using 104 normal DCs or TIDCs and 2 × 105 T cells isolated from BALB/c mice.
Cytokines
For the measurement of cytokine secretion, 48-h supernatants of tumor suspensions or supernatants of T cell stimulations were assayed by Bioplex analysis (Bio-Rad, München, Germany) using the Mouse Cytokine Th1/Th2 panel 8-plex.
Total RNA from enriched cells was prepared by using 200 μl TRIzol reagent (Invitrogen, Karlsruhe, Germany) per 1 × 106 cells and by extracting with 1-bromo-3-chloro-propane (Sigma, Deisenhofen, Germany) followed by precipitation with isopropanol. RNA was reverse transcribed with First Strand cDNA Synthesis Kit (Roche Diagnostics, Mannheim, Germany) and quantified with primer pairs (Search-LC, Heidelberg, Germany) specific for IL-6, IL-10, TGF-β, or the housekeeping gene hypoxanthine guanine phosphoribosyl transferase (hprt) in a LightCycler 2.0 Real-Time PCR system (Roche Diagnostics). The specific signals were normalized to those obtained for hprt and for normal cells.
Results
TIDCs in λ-myc lymphoma show signs of maturation but suppression of IL-12
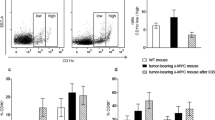
λ-myc mice harbor the oncogene c-myc under the control of an Igλ enhancer sequence. Constitutive expression in B cells leads to development of B cell lymphomas by week 15 after birth. The tumors share multiple features of human Burkitt lymphoma, e.g. the primary location in cervical LNs and the characteristic histologic starry sky appearance [35]. As the tumors are growing very aggressively, mice have to be killed and analyzed immediately after onset of disease. At this time point, numbers of CD11c+ DCs were strongly increased in malignant LNs and spleens as compared to the respective organs from wild type (wt) animals, which might be due to an increased recruitment to the tumors (Fig. 1a). In lymphoid organs of transgenic mice that did not yet show clinical signs of lymphoma growth, DC numbers were also augmented albeit to a lesser extent. This may be ascribed to incipient, yet undetectable tumors [29].
Characterization of TIDCs in λ-myc lymphomas. a Increase in DC numbers in tumor LNs and spleens. The absolute numbers of CD11c+ cells per mg LN tissue or spleen were determined by FACS analysis and related to the numbers detected in organs of wt mice. DC numbers in spleens are lower because spleens are infiltrated with malignant cells later during disease development. b, c Ratio of CD11clow and CD11chigh DCs in organs derived from normal or λ-myc mice. λ-myc animals that do not yet show clinical symptoms yield variable results because the stage of tumor development is not predictable in these mice. In the example shown in c, the ratio CD11clow/CD11chigh is 7.6 (wt mouse) and 11.6 (tumor mouse). d Expression of maturation markers on DCs derived from tumor LNs. Mean fluorescence intensities (MFIs) were measured in TIDCs and related to normal DCs (broken line). Tumor spleens showed similar results. e Histograms exemplifying altered maturation markers. In panels a, b and d, the columns show means and SD; the differences between tumor-bearing and wt mice are significant with at least p < 0.05 (Mann–Whitney) with the exception of LNs in panel b. All results are compiled from at least 5 mice
Phenotypic characterization showed that the ratio of CD11chigh to CD11clow cells was reduced in comparison with normal DC populations although this difference was significant only in the spleen (Fig. 1b, c). As CD11clow cells have been described as a T cell-suppressing DC population expressing reduced levels of MHC class II [13], we next examined the expression of costimulatory and MHC class II molecules on the λ-myc lymphoma-infiltrating DCs. Surprisingly, TIDCs showed a more mature phenotype than DCs from normal wt LNs because the level of all molecules tested (CD80, CD86, CD83, CD40, MHC class II) was strongly increased (Fig. 1d, e). This upregulation was equally seen in the CD11chigh and the CD11clow subpopulation.
Given the lack of IFN-γ in spontaneous lymphoma [25, 26], we anticipated a cytokine profile in TIDCs that favors Th2 rather than Th1 responses. Indeed, a reduced production of IL-12 (Fig. 2a and [26]) and augmented levels of IL-10 (Fig. 2b) was observed in TIDCs upon stimulation ex vivo. IL-10 was also increased in supernatants of tumor suspensions, which was also found for IL-4 and IL-5, whereas IL-12, IL-2 and IFN-γ were at background levels (not shown). This is in line with a Th2-inducing milieu.
Distinct pathways regulate expression of costimulatory molecules and cytokines in DCs exposed to a lymphoma microenvironment
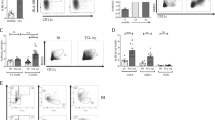
To elucidate the mechanisms underlying these unexpected alterations, we asked whether the “TIDC phenotype” could also be induced in DCs from normal wt mice by coculture with lymphoma cells. To this end, we used tumor suspensions freshly prepared from λ-myc mice or 291 cells, which are indefinitely growing malignant B cells established from a λ-myc mouse. The presence of either tumor suspensions or 291 cells equally caused an upregulation of CD80, CD86, CD83, CD40 and MHC class II on wt DCs (Fig. 3a, b). This suggests that the presence of malignant B cells alone is sufficient for this upregulation and that other cellular components included in the tumor microenvironment are not relevant. Since such alterations were not seen in experiments using trans-well systems or cell-free supernatants generated from λ-myc lymphomas (Fig. 3a, b), we concluded that cellular contacts between tumor cells and DCs are necessary to generate the mature phenotype of TIDCs. Blocking experiments using monoclonal antibodies (mAbs) were done to identify molecules involved in this interaction. While the inclusion of anti-CD40 or anti-CD40L mAbs showed no effect (not shown), blocking of CD62L, which was shown to be expressed by λ-myc lymphoma cells, at least partially inhibited the upregulation of maturation markers on DCs (shown for CD80 in Fig. 3c).
Induction of a TIDC phenotype in normal DCs in vitro. a CD11c+ cells freshly isolated from wt spleens were incubated with λ-myc tumor suspensions or tumor supernatants for 16 h. MFIs were determined in the CD11c+ population and related to DCs that were cultivated with wt B cells or in medium only (broken line). The effects of fresh tumor cells and 291 cells were identical, and no difference was seen between tumor supernatants and tumor cells that were kept apart from DCs in trans-well chambers. Compilation of five independent experiments. The differences between the control and the values obtained with tumor cells are significant with p < 0.05 (Mann–Whitney). The degree of upregulation in vitro cannot be compared to the upregulation found in vivo because the duration of cell contacts that happened in vivo is unknown. b Exemplary histogram showing levels of CD86 after incubation with tumor cells or tumor cell supernatant in vitro. c The inclusion of an anti-CD62L Ab in the coculture of normal DCs and lymphoma cells partly abrogates upregulation of CD80 (reduction in MFI by about 55 % in the presence of the mAb). The other maturation markers behaved similarly. Representative result from four experiments
The shift from IL-12 to IL-10 expression observed in TIDCs ex vivo was also seen in vitro when wt DCs were coincubated with tumor cells (Fig. 4a, b). In contrast to the alteration of maturation markers, however, the cytokine shift was mediated by soluble factors because the same results were obtained when cocultures were performed in trans-well plates (Fig. 4a, b). The data indicate that different mechanisms contribute to upregulation of costimulatory molecules and to altered cytokine expression, respectively, in the lymphoma microenvironment.
Induction of a cytokine shift in normal DCs in vitro. a IL-12 suppression and IL-10 induction in normal DCs incubated with lymphoma cells or tumor supernatants. MFIs were related to DCs that were kept in medium (broken line). Incubation with 291 cells and with tumor suspensions gave identical results. b Histogram exemplifying IL-12 expression in normal DCs after incubation with tumor. c Relative IL-10 mRNA levels in λ-myc lymphoma cells and in normal B cells detected by RT-PCR. The difference between normal and malignant B cells is significant with p < 0.005 (Mann–Whitney). d IL-10 produced by normal and malignant B cells detected at the protein level in cell supernatants. e Including an anti-IL-10 Ab in cocultures of normal DCs with lymphoma cells abrogates IL-12 suppression. Mean value from three independent experiments showing relative MFIs
As observed for the alteration of DC surface molecules, the cytokine shift induced in DCs in vitro was also independent of whether primary tumors or a malignant B cell line were used. This indicated that the factor(s) encompassing the altered IL-12/IL-10 balance in TIDCs was (were) primarily derived from malignant B cells. Therefore, we characterized purified malignant B cells in terms of their cytokine expression by RT-PCR. Compared to normal B cells, lymphoma cells produced enhanced amounts of IL-10 (Fig. 4c, d), while expression of all other cytokines tested was identical in normal and malignant B cells. To address the question whether IL-10 produced by lymphoma cells was necessary for suppressing IL-12 in wt DCs, coincubation experiments were repeated under mAb-mediated blocking of IL-10. Indeed, the suppression of IL-12 affected by lymphoma cells or supernatants in wt DCs was reversed under IL-10 ablation (Fig. 4e).
The role of normal and λ-myc lymphoma-derived NK cells for DC activation
It is well established that, in healthy tissues, close interactions between DCs and NK cells give rise to reciprocal activation of either cell population. As in our coculture system, malignant B cells alone did not differ from whole tumor cell suspensions with regard to inducing DC maturation (vide supra), we anticipated that NK cells in the λ-myc lymphoma microenvironment did not contribute to DC differentiation. To confirm this hypothesis, we first tested the effect of NK cells freshly isolated from spleens of wt mice in coculture experiments using wt DCs and lymphoma cells. As shown in Fig. 5a, the costimulatory molecules became even more upregulated when wt NK cells were present in addition to the tumor cells.
Impact of normal and λ-myc NK cells on DC maturation in the presence of lymphoma cells. a Normal but not λ-myc NK cells foster DC maturation in addition to the effect of lymphoma cells. Coincubation of normal DCs and lymphoma cells was done exactly as described in Fig. 3a, whereby NK cells isolated from spleens of wt mice or tumor-bearing λ-myc animals were added. Summary of three independent experiments. The values obtained with wt NK cells show a significant difference (p < 0.05; Mann–Whitney) compared to the other situations. b Recombinant IFN-γ included in the coculture of lymphoma cells and normal DCs shows a similar effect as IFN-γ-producing NK cells. Blocking of IFN-γ partly reverses the “bonus effect” provided by normal NK cells
As NK cells from tumor-bearing λ-myc mice are functionally compromised as compared with normal NK cells [29], we then tested NK cells derived from λ-myc lymphomas. Indeed, the “bonus effect” exerted by wt NK cells was not seen in this setting (Fig. 5a). Since this “bonus effect” was not dependent on cellular contacts between NK cells and DCs (not shown) and a hallmark of λ-myc NK cells was their deficient IFN-γ production [29, 30], we hypothesized that the “bonus effect” provided by normal NK cells might be related to this cytokine. This assumption could be confirmed because recombinant IFN-γ provided a similar effect as IFN-γ-producing NK cells (Fig. 5b). Furthermore, we cultivated normal NK cells expressing high amounts of IFN-γ together with wt DCs and lymphoma cells in the presence of an IFN-γ-neutralizing mAb. In this setting, the “bonus effect” of NK cells in terms of fostering DC maturation was partly reversed (Fig. 5b).
T cell-stimulatory capacity of λ-myc TIDCs
The ability of λ-myc TIDCs to stimulate naïve T cells was examined by testing the cytokine secretion of OT-I CD8+ and OT-II CD4+ T cells, which harbor transgenic OVA-specific T cell receptors (TCRs), after incubation with wt DCs or λ-myc TIDCs that had been loaded with the respective OVA peptide. In terms of IFN-γ secretion, T cells were far less activated by λ-myc TIDCs than by normal wt DCs (shown for OT-II peptide-specific stimulation of CD4+ T cells in Fig. 6). IL-4 production was also diminished albeit to a small extent (Fig. 6b). OT-I peptide-specific and alloreactive stimulation experiments yielded identical results.
T cell-stimulatory capacity of λ-myc TIDCs. OVA peptide-loaded DCs from wt mice or tumor-bearing λ-myc animals were used for stimulation of OT-II-specific CD4+ T cells. Concentrations of a IFN-γ and b IL-4 were determined in supernatants after 3 days. Only the reduction in IFN-γ is significant with p < 0.005 (Mann–Whitney)
Discussion
For characterizing TIDCs in B cell lymphoma, it is pivotal to use an autochthonous tumor model because transplantable lymphomas showed an IFN-γ-driven activation of DCs along with an IL-12 bias [27, 30], which was not seen in the endogenous λ-myc lymphoma used in our study [30]. As λ-myc lymphoma grows very aggressively, mice had to be killed as soon as manifest tumors became visible. Therefore, dynamics of immune responses could not be analyzed in this system, although some alterations were occasionally observed in transgenic animals already before clinical signs of disease were apparent (Fig. 1a).
Compared to normal DCs, λ-myc lymphoma-derived TIDCs showed a more mature phenotype and a reduced IL-12/IL-10 ratio. The same alterations could be provoked in normal DCs when these were exposed to a lymphoma micromilieu in vitro. The in vitro system enabled us to dissect the components present in the tumor environment that are responsible for inducing the “TIDC phenotype” (Fig. 7). The upregulation of costimulatory molecules was dependent on cellular contacts between DCs and lymphoma cells and was at least partly due to CD62L (Fig. 3c), which was expressed on B lymphoma cells. The altered cytokine profile of TIDC, in contrast, was caused by IL-10, which was primarily produced by the malignant B cells. Thus, different pathways seem to be involved in the regulation of the costimulatory molecules and the cytokine profile, respectively. The “TIDC phenotype” induced in vitro could be further modulated by NK cells provided the NK cells were isolated from normal wt mice and were capable of producing IFN-γ (Fig. 7).
Impact of λ-myc lymphoma cells and IFN-γ on inducing a “TIDC phenotype” as detected in vitro. Cellular interactions with tumor cells upregulate costimulatory molecules on TIDCs, while IL-10 primarily produced by the lymphoma cells causes a decrease in the IL-12/IL-10 ratio in TIDCs. Maturation-associated surface molecules can be positively affected by IFN-γ, which is expressed by normal NK cells but not by lymphoma-infiltrating NK cells
Impaired IL-12 production of TIDCs is in accordance with previous studies, which were done in mouse and human [11, 18], but the expression of costimulatory molecules is a matter of controversy. Studies using transplantable mouse tumors or in vitro cultures showed that DC maturation was blocked [10, 12, 13]. In some of these studies, however, bone marrow-derived DCs differentiated in vitro were used. These might not be comparable to DCs freshly isolated ex vivo, which were exclusively used in our investigation. An immature phenotype was also found in mouse models of endogenously arising neoplasias [11, 12] as well as in several human malignancies [14–18]. By contrast, an upregulation of costimulatory molecules, as detected in our λ-myc lymphoma model, was reported for another endogenous mouse tumor [21] and for human renal cell carcinoma [22]. Thus, the maturation status of TIDCs might depend on the tumor entity.
Tumor-derived soluble factors that were described to suppress DC function include IL-6, which causes a switch of monocyte differentiation toward macrophages [36], VEGF, which inhibits DC maturation [37], TGF-β, IL-10, lactic acid and PGE2 (review in [6]). Among these factors, we only found IL-10 expression in λ-myc lymphoma cells. Why IL-10 and lactic acid, which was found in our lymphoma supernatants (manuscript in preparation), did not block upregulation of costimulatory signals, as described elsewhere [6], may be explained by the before-mentioned cell contact-dependent mechanism, which might be specific for B cell lymphoma and may overcome the maturation-inhibiting effect of IL-10 and lactic acid.
An immature phenotype of TIDCs was mostly considered to account for deficient T cell-stimulatory capacities [12] because DCs with reduced costimulatory signals may render T cells anergic. However, the mature phenotype of TIDCs in λ-myc tumors argues against induction of T cell anergy. Moreover, upregulated levels of PD-1 on T cells in tumor-bearing mice indicated that T cells became indeed activated and subsequently exhausted (manuscript in preparation). Interestingly, TIDCs isolated ex vivo expressed PD-L1 and interaction of normal DCs with lymphoma cells in vitro caused upregulation on DCs of PD-L1, which is the ligand of PD-1. Thus, TIDCs might be able to inhibit TCR-dependent signals via the PD-1 pathway. We also expect that IL-10, which is provided by the lymphoma cells as well as by the altered DCs, might exert a T cell-mediated suppressive effect. It is well established that IL-10 gives rise to differentiation of T cells that also produce high amounts of IL-10, thereby suppressing T effector cells [38]. Such “Tr1 cells” expressing IL-10 along with PD-1 [39] were indeed detected in high frequencies in λ-myc mice (manuscript in preparation). Induction of IL-10 in T cells might also be related to a counter-regulatory mechanism initiated in T effector cells [40]. Since a prerequisite for effective antitumor responses is the induction of a Th1 milieu [23–26], the bias toward Th2 cytokines in λ-myc tumors may be an additional mechanism that hampers T cell-mediated tumor suppression. Finally, it cannot be precluded that other mechanisms such as an altered arginine metabolism [13, 21] contribute to functional deficits of DC/T cell interactions in the λ-myc model.
The findings presented in this paper are relevant with regard to cancer immunotherapy. Since costimulatory molecules were readily expressed by TIDCs, triggering maturation of TIDCs in B cell lymphoma will not be an adequate therapeutic option. However, it may be beneficial to obviate the lack of IL-12 in TIDCs. As outlined in Fig. 7, this could be done by Ab-mediated blocking of IL-10.
References
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Boussiotis VA, Barber DL, Nakarai T, Freeman GJ, Gribben JG, Bernstein GM, D’Andrea AD, Ritz J, Nadler LM (1994) Prevention of T cell anergy by signaling through the γc chain of the IL-2 receptor. Science 266:1039–1042
Linsley PS, Ledbetter JA (1993) The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol 11:191–212
Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC (1996) Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol 157:1406–1414
Heath WR, Carbone FR (2001) Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol 19:47–64
Gabrilovich D (2004) Mechanisms and functional significance of tumor-induced dendritic-cell defects. Nat Rev Immunol 4:941–952
Chouaib S, Asselin-Paturel C, Mami-Chouaib F, Caignard A, Blay JY (1997) The host-tumor immune conflict: from immunosuppression to resistance and destruction. Immunol Today 18:493–497
Vicari AP, Caux C, Trinchieri G (2002) Tumor escape from immune surveillance through dendritic cell inactivation. Semin Cancer Biol 12:33–42
e Sousa CR (2001) Dendritic cells as sensors of infection. Immunity 14:495–498
Ebata K, Shimizu Y, Nakayama Y, Minemura M, Murakami J, Kato T, Yasumura S, Takahara T, Sugiyama T, Saito S (2006) Immature NK cells suppress dendritic cell functions during the development of leukemia in a mouse model. J Immunol 176:4113–4124
Zhao F, Falk C, Osen W, Kato M, Schadendorf D, Umansky V (2009) Activation of p38 mitogen-activated protein kinase drives dendritic cells to become tolerogenic in ret transgenic mice spontaneously developing melanoma. Clin Cancer Res 15:4382–4390
Vicari AP, Chiodoni C, Vaure C, Ait-Yahia S, Dercamp C, Matsos F, Reynard O, Taverne C, Merle P, Colombo MP, O′Garra A, Trinchieri G, Caux C (2002) Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J Exp Med 196:541–549
Liu Q, Zhang C, Sun A, Zheng Y, Wang L, Cao X (2009) Tumor-educated CD11bhigh Ialow regulatory dendritic cells suppress T cell response through arginase I. J Immunol 182:6207–6216
Perrot I, Blanchard D, Freymond N, Isaac S, Guibert B, Pacheco Y, Lebecque S (2007) Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol 178:2763–2769
Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J (1999) In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med 190:1417–1426
Thomachot MC, Bendriss-Vermare N, Massacrier C, Biota C, Treilleux I, Goddard S, Caux C, Bachelot T, Blay JY, Menetrier-Caux C (2004) Breast carcinoma cells promote the differentiation of CD34+ progenitors towards 2 different subpopulations of dendritic cells with CD1a(high)CD86(−)Langerin- and CD1a(+)CD86(+)Langerin+ phenotypes. Int J Cancer 110:710–720
Almand B, Resser JR, Lindman B, Nadaf S, Clark I, Kwon ED, Carbone DP, Gabrilovich DI (2000) Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res 6:1755–1766
Della Bella S, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A, Clerici M, Greco M, Villa ML (2003) Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer 89:1463–1472
Diao J, Zhao J, Winter E, Cattral MS (2010) Recruitment and differentiation of conventional dendritic cell precursors in tumors. J Immunol 184:1261–1267
Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin A, Corzo A, Cho HI, Celis E, Lennox B, Knight SC, Padhya T, McCaffrey TV, McCaffrey JC, Antonia S, Fishman M, Ferris RL, Kagan VE, Gabrilovich DI (2010) Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med 16:880–886
Norian LA, Rodriguez PC, O′Mara LA, Zabaleta J, Ochoa AC, Cella M, Allen PM (2009) Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via l-arginine metabolism. Cancer Res 69:3086–3309
Figel AM, Brech D, Prinz PU, Lettenmeyer UK, Eckl J, Turqueti-Neves A, Mysliwietz J, Anz D, Rieth N, Münchmeier N, Buchner A, Porubsky S, Siegert SI, Segerer S, Nelson PJ, Nößner E (2011) Human renal cell carcinoma induces a dendritic cell subset that uses T-cell crosstalk for tumor-permissive milieu alterations. Am J Pathol 179:436–451
Egeter O, Mocikat R, Ghoreschi K, Dieckmann A, Röcken M (2000) Eradication of disseminated lymphomas with CpG-DNA-activated Th1 cells from non-transgenic mice. Cancer Res 60:1515–1520
Ziegler A, Heidenreich R, Braumüller H, Wolburg H, Weidemann S, Mocikat R, Röcken M (2009) EpCAM, a human tumor-associated antigen, promotes Th2 development and tumor immune evasion. Blood 113:3494–3502
Müller-Hermelink N, Braumüller H, Pichler B, Wieder T, Mailhammer R, Schaak K, Ghoreschi K, Yazdi A, Haubner R, Sander CA, Mocikat R, Schwaiger M, Förster I, Huss R, Weber WA, Kneilling M, Röcken M (2008) TNFR1 signaling and IFN-γ signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell 13:507–518
Braumüller H, Wieder T, Brenner E, Aßmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, Ranta F, Ullrich S, Mocikat R, Braungart K, Mehra T, Fehrenbacher B, Berdel J, Niessner H, Meier F, van den Broek M, Häring HU, Handgretinger R, Quintanilla-Martinez L, Fend F, Pesic M, Bauer J, Zender L, Schaller M, Schulze-Osthoff K, Röcken M (2013) T-helper-1-cell cytokines drive cancer into senescence. Nature 494:361–365
Mocikat R, Braumüller H, Gumy A, Egeter O, Ziegler H, Reusch U, Bubeck A, Louis J, Mailhammer R, Riethmüller G, Koszinowski U, Röcken M (2003) Natural killer cells activated by MHC class I-low targets prime dendritic cells to induce protective CD8 T cell responses. Immunity 19:561–569
Adam C, King S, Allgeier T, Braumüller H, Lüking C, Mysliwietz J, Kriegeskorte A, Busch DH, Röcken M, Mocikat R (2005) DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood 106:338–344
Brenner C, King S, Przewoznik M, Wolters I, Adam C, Bornkamm G, Busch D, Röcken M, Mocikat R (2010) Requirements for control of B-cell lymphoma by NK cells. Eur J Immunol 40:494–504
Przewoznik M, Hömberg N, Naujoks M, Pötzl J, Münchmeier N, Brenner C, Anz D, Bourquin C, Nelson P, Röcken M, Mocikat R (2012) Recruitment of natural killer cells in advanced stages of endogenously arising B-cell lymphoma: implications for therapeutic cell transfer. J Immunother 35:217–222
Mocikat R, Selmayr M, Thierfelder S, Lindhofer H (1997) Trioma-based vaccination against B cell lymphoma confers long-lasting tumor immunity. Cancer Res 57:2346–2349
Strehl J, Selmayr M, Kremer J-P, Hültner L, Lindhofer H, Mocikat R (1999) Gene therapy of B-cell lymphoma with cytokine gene-modified trioma cells. Int J Cancer 83:113–120
Kronenberger K, Dieckmann A, Selmayr M, Strehl J, Wahl U, Lindhofer H, Kraal G, Mocikat R (2002) Impact of the lymphoma idiotype on in vivo tumor protection in a vaccination model based on targeting antigens to antigen-presenting cells. Blood 99:1327–1331
Kronenberger K, Nößner E, Frankenberger B, Wahl U, Dreyling M, Hallek M, Mocikat R (2008) A polyvalent cellular vaccine induces T-cell responses against specific self antigens overexpressed in chronic-lymphocytic B-cell leukemia. J Immunother 31:723–730
Kovalchuk AL, Qi CF, Torrey TA, Taddesse-Heath L, Feigenbaum L, Park SS, Gerbitz A, Klobeck G, Hoertnagel K, Polack A, Bornkamm GW, Janz S, Morse HC 3rd (2000) Burkitt lymphoma in the mouse. J Exp Med 192:1183–1190
Chomarat P, Banchereau J, Davoust J, Palucka AK (2000) IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol 1:510–514
Gabrilovich DI, Chen HL, Girgis KR, Cunningham KT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP (1996) Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 2:1096–1103
Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG (2005) Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+ CD4+ Tr1 cells. Blood 105:1162–1169
Gagliani N, Magnani CF, Huber S, Gianolini MF, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F, Di Serio C, Bacchetta R, Andreani M, Brockmann L, Gregori S, Flavell RA, Roncarolo M-G (2013) Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 19:739–746
O′Garra A, Vieira P (2007) Th1 cells control themselves by producing IL-10. Nat Rev Immunol 7:425–428
Acknowledgments
The work was supported by grants from Wilhelm-Sander-Stiftung (2010.108.1) and from Deutsche Krebshilfe (109036 and 109037). We thank A. Geishauser and M. Hagemann for expert technical assistance and S. Edelmann for providing OT-I and OT-II mice. This paper includes parts of the doctoral theses of Marcella Naujoks, Jakob Weiß, Tanja Riedel and Margarethe Przewoznik at the Ludwig-Maximilians-Universität München.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Marcella Naujoks and Jakob Weiß have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Naujoks, M., Weiß, J., Riedel, T. et al. Alterations of costimulatory molecules and instructive cytokines expressed by dendritic cells in the microenvironment of an endogenous mouse lymphoma. Cancer Immunol Immunother 63, 491–499 (2014). https://doi.org/10.1007/s00262-014-1538-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-014-1538-7