Abstract
The proinflammatory cytokine interleukin 17 (IL-17) is considered to play a crucial role in diverse human tumors; however, its role in disease progression remains controversial. This study investigated the cellular source and distribution of IL-17 in esophageal squamous cell carcinoma (ESCC) in situ and determined its prognostic value. Immunohistochemistry, immunofluorescence and immunoelectron microscopy were used to identify IL-17-expressing cells in ESCC tissues, paying particular attention to their anatomic localization. Kaplan–Meier analysis and Cox proportional hazards regression models were applied to estimate overall survival in 215 ESCC patients with long-term follow-up (>10 years). The results showed that mast cells, but not T cells or macrophages, were the predominant cell type expressing IL-17 in ESCC tissues. Unexpectedly, these IL-17+ cells were highly enriched in the muscularis propria rather than the corresponding tumor nest (p < 0.0001). The density of IL-17+ cells in muscularis propria was inversely associated with tumor invasion (p = 0.016) and served as an independent predictor of favorable survival (p = 0.007). Moreover, the levels of IL-17+ cells in muscularis propria were positively associated with the density of effector CD8+ T cells and activated macrophages in the same area (both p < 0.0001). This finding suggested that mast cells may play a significant role in tumor immunity by releasing IL-17 at a previously unappreciated location, the muscularis propria, in ESCC tissues, which could serve as a potential prognostic marker and a novel therapeutic target for ESCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal squamous cell carcinoma (ESCC) accounts for more than 90 % of esophageal cancer cases worldwide, ranking eighth in incidence and sixth in mortality [1]. Despite the improved diagnostic and treatment strategies, the overall survival of patients with ESCC remains poor [2, 3]. Tumor invasion into the muscularis propria is a key step in disease progression, is associated with an increase in regional lymph node metastasis and serves as a critical parameter in ESCC staging [4].
Tumor progression is now recognized as the product of evolving crosstalk between different cell types within tumors [5, 6]. Emerging evidence has demonstrated that the infiltrating immune cells and the cytokine milieu at the tumor site can largely determine the biological behavior of human tumors [7]. In ESCC, it has been shown that high densities of intratumoral CD4+ and CD8+ T cells are associated with prolonged patient survival [8], whereas infiltrating dendritic cells can promote ESCC progression by inducing T-cell tolerance [9]. These findings are in accordance with the general view that the inflammatory reaction at a tumor site can either inhibit or promote disease progression, depending on the context [10, 11].
The proinflammatory cytokine interleukin (IL)-17 has recently been identified as a crucial mediator in the pathogenesis of diverse human tumors and is capable of being pro- or antitumorigenic [12–14]. Although the precise underlying mechanisms are not yet clear, this paradox may be accounted for by the IL-17 concentration or the nature and density of IL-17-expressing cells. IL-17 secreted by different cell types may have distinct functional effects on tumor progression due to their varying levels of IL-17 production and other contexts, including localization, the associated cytokine profile and cellular environment [13]. In addition to the well-studied IL-17-producing T cells, IL-17 can be produced in tissues by diverse cell types, such as γδ T cells, NK/NKT cells, macrophages, neutrophils, eosinophils and mast cells [15, 16]. The net IL-17 expression in situ may arise from a broad array of adaptive and innate immune cells. Thus, to understand the complex tissue context, it is important to define the cellular sources of IL-17 in tumors in situ and evaluate their clinical and pathological associations.
Human solid tumors can be anatomically classified into different areas with distinct compositions and functional properties [17–19]. We have recently shown that, in hepatocellular carcinoma, IL-17-expressing cells were predominantly enriched in the area of peritumoral stroma, where they promote disease progression by recruiting neutrophils and stimulating angiogenesis at the edge of adjacent invading tumors [20–22]. In this study, we examine the cellular source and clinical significance of IL-17 in tumors in situ from 215 ESCC patients, paying particular attention to their microlocalization. Unexpectedly, we found that mast cells in the muscularis propria are the predominant sources of IL-17-expressing cells in ESCC tumors in situ, and the density of IL-17+ cells predicts a favorable prognosis and is positively associated with effector CD8+ T cells and activated macrophages in the muscularis propria. These data suggest that the muscularis propria may represent a previously unrecognized region in tumor immunity in esophagus.
Materials and methods
Patients and tissue specimens
Tissue specimens were obtained between January 1996 and December 2002 from 215 patients with pathologically confirmed ESCC at the Cancer Center of Sun Yat-sen University. All patients underwent curative resection for ESCC, which was defined as complete removal of the tumor with no residual cancer [2, 23]. None of the patients had distant metastasis or any neoadjuvant therapies, such as radiotherapy or chemotherapy, before surgery. Individuals with concurrent autoimmune diseases were also excluded. Tumor stages were classified according to the TNM classification (6th edition, 2002). The clinicopathological characteristics of the patients are summarized in Supplementary material Table 1. In addition, fresh muscularis propria associated with tumor tissue (1 mm3) was excised from three patients and used for immunoelectron microscopy. All specimens were anonymously coded in accordance with local ethical guidelines (as stipulated by the Declaration of Helsinki), and written informed consent was obtained. The protocol was approved by the Review Board of the Cancer Center.
Data on patient follow-up were obtained by the Cancer Center Tumor Registry as described previously [17, 20, 23, 24]. Of the 215 patients examined, 149 (69.3 %) died as a direct result of their disease before the end of the observation period. Overall survival was defined as the interval between surgery and death or last observation. The range of overall survival was 2.2–156.7 months. The overall survival rate was 80 % at 1 year, 43.3 % at 3 years, 34.9 % at 5 years and 30.6 % at 10 years.
Immunohistochemistry and immunofluorescence
Specimens were obtained immediately after surgical resection and fixed in 10 % neutral formalin, paraffin-embedded and used for histological assays as previously described [17, 20, 25]. Immunohistochemistry of paraffin sections was carried out using a two-step protocol (Dako A/S, Glostrup, Copenhagen, Denmark). Briefly, 5-μm paraffin sections were first deparaffinized and hydrated, and endogenous peroxidase activity was blocked by incubating the slides in 0.3 % H2O2. Antigen retrieval was performed by microwave treatment in citrate buffer pH 6.0. Sections were blocked with normal sera from the same species from which the secondary antibodies were derived. After overnight incubation at 4 °C with antibodies against human IL-17 (1:300 dilution, R&D Systems, Minneapolis, MN, USA), tryptase (1:500 dilution, Thermo Fisher Scientific, Waltham, MA, USA), CD8 (1:300 dilution, Thermo Fisher Scientific, Waltham, MA, USA), CD68 (1:500 dilution, Dako A/S, Glostrup, Copenhagen, Denmark), CD169 (1:200 dilution, R&D Systems, Minneapolis, MN, USA), or control antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA), the sections were incubated with the secondary antibodies conjugated with horseradish peroxidase (Envision + Dual Link Kit, DAKO, for mouse/rabbit antibodies; donkey anti-goat secondary antibody from Santa Cruz Biotechnology; or R&D Systems for donkey anti-sheep secondary antibody) for 30 min. The enzymatic reaction was developed with peroxidase-labeled secondary antibody and stained with 3,3′-diaminobenzidine tetrahydrochloride using the Envision System (Dako A/S, Glostrup, Copenhagen, Denmark). Sections were then counterstained with hematoxylin (Zymed Laboratories Inc., San Francisco, CA, USA) and mounted in non-aqueous mounting medium.
For double-color immunofluorescence, paraffin-embedded tissue sections were first incubated with goat anti-human IL-17 and rabbit anti-human CD3, mouse anti-human CD68, tryptase or chymase, or sheep anti-human CD169 and mouse anti-human CD68, followed by specimen-paired immunofluorescence secondary antibodies (Life Technologies Inc., CA, USA). For triple-color immunofluorescence, sections were first incubated with goat anti-human IL-17, mouse anti-human chymase and rabbit anti-human tryptase; or with goat anti-human IL-17, mouse anti-human tryptase and rabbit anti-human IL-17, followed by specimen-paired immunofluorescence secondary antibodies (Life Technologies Inc.). Slides were mounted with Vectashield containing DAPI (Vector Laboratories) and analyzed on a fluorescent imaging microscope (BX50; Olympus, Essex, UK). Isotype-matched primary antibodies were used as negative controls (Santa Cruz Biotechnology, Inc.). Images were captured and analyzed using FV10-ASW 1.7 Viewer (Olympus, Essex, UK). Full details of the primary antibodies are listed in Supplementary material Table 2.
Immunoelectron microscopy
Tissues were processed for immunoelectron microscopy as previously described [26]. Ultrathin sections of gold interference color were cut using an ultramicrotome (EMUC6; Leica, Wetzlar, Germany) and collected on formvar-coated nickel slot grids. All immunolabeling steps were performed in a humid chamber at room temperature. Grids were floated on blocking solution, followed by incubation with goat anti-human IL-17 (R&D systems) or control antibody (Santa Cruz Biotechnology, Inc.), and then subjected to secondary labeling with donkey anti-goat IgG coupled with 10-nm gold nanoparticles (Sigma-Aldrich, Missouri, USA). Sections were post-stained in uranyl acetate and with lead stain. Grids were viewed using a JEM 1400 (JEOL, Mitaka, Tokyo) operating at 80 kV.
Quantification of immunofluorescence
Quantification methods were as previously described [27]. Numbers of single-positive or double-positive cells of interest in each of two representative fields at × 100 magnification (0.5 mm2 per field) were counted manually by two independent, blinded observers. From these numbers, the proportions of cells in different populations were calculated [e.g., the proportion of all tryptase+ (TRY+) mast cells which were IL-17+ was calculated as: (number of IL-17+ TRY+ cells)/(number of IL-17+ TRY+ mast cells + IL-17− TRY+ mast cells)].
Evaluation of immunohistology
Analysis was performed by two independent observers who were blinded to the clinical outcome [17, 21]. To evaluate the density of IL-17+ cells, TRY+ mast cells, CD68+ macrophages, CD169+ macrophages and CD8+ T cells, areas of normal mucosa, tumor nest and muscularis propria were screened at low power (100× magnification) and the five most representative high-power fields were then selected at 400× magnification (0.0768 mm2 per field) using a Hitachi HV-C20A CCD camera (Hitachi, Tokyo, Japan) installed on a Leica AS LMD light microscope (Leica Microsystems) for each area of every specimen. The number of IL-17+ cells or TRY+ mast cells in each area were counted manually and expressed as the mean ± SEM cells per field. There was a significant linear correlation between the counting data of two independent observers, and the average counting by two investigators was applied in the following analysis to minimize interobserver variability.
Statistical analysis
Statistical analyses were performed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). The statistical significance of differences between groups was determined by the Wilcoxon signed-rank test. Cumulative survival time was calculated by the Kaplan–Meier method and analyzed by the log-rank test. A multivariate Cox proportional hazards model was used to estimate adjusted hazard ratios, 95 % confidence intervals (CI) and to identify independent prognostic factors. For categorical analysis, the median value was used as a cutoff to dichotomize the continuous variables (for clinical applications). Analysis of the association between variables was conducted using Spearman’s rank correlation coefficient for continuous variables and χ 2 tests for categorical variables. For the above comparisons, two-tailed p < 0.05 was considered statistically significant.
Results
IL-17+ cells are enriched in the muscularis propria of ESCC tissues
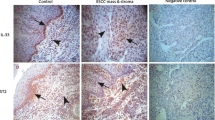
Previous studies of IL-17 expression in tumor tissues focused primarily on T cells stimulated ex vivo with PMA and ionomycin for subsequent flow cytometric analysis [13, 28]. However, such an approach does not define the identity and anatomic localization of the cells expressing intracellular IL-17 in vivo. We therefore examined the tissue location of IL-17-expressing cells in paired tumor and non-tumor tissues from 60 untreated ESCC patients. We divided the tissues into four anatomical regions: normal mucosa (MUN) and muscularis propria (MPN) in non-tumor tissues, and tumor nest (TNT) and muscularis propria (MPT) in tumor tissues (Fig. 1a, c). As shown in Fig. 1, IL-17+ cells were present throughout the tissue, but predominantly located in MPN and MPT (8.5 ± 0.7 and 11.7 ± 0.9 cells/field, respectively) rather than MUN or TNT (0.3 ± 0.1 and 0.4 ± 0.1/field, respectively). Moreover, the number of IL-17+ cells was significantly higher in MPT than in the corresponding MPN (p = 0.003; Fig. 1d).
IL-17+ cells were enriched in the muscularis propria of ESCC tissues. Paraffin-embedded ESCC sections were stained with a goat anti-human IL-17 antibody in paired a non-tumor tissues and b tumor tissues. Scale bar 100 μm. c Higher magnification micrographs of: 1, normal mucosa (MUN); 2, muscularis propria in non-tumor tissues (MPN); 3, tumor nest (TNT); 4, muscularis propria in tumor tissues (MPT). Scale bar 100 μm. d The density of IL-17+ cells was higher in MPN and MPT than in MUN or TNT from paired non-tumor and tumor tissues (n = 60). Results are expressed as mean ± SEM (bars). p < 0.05 was considered significant difference (Wilcoxon signed-rank test)
Mast cells, but not T cells or macrophages, constitute the majority of IL-17-expressing cells in ESCC tissues
To identify the cellular source of IL-17 in ESCC tumor tissues, we performed colocalization studies and calculated the proportion of IL-17+ cells within each cellular subset. We first investigated the colocalization of intracellular IL-17 with the T-cell marker CD3 (Fig. 2a). Unexpectedly, T cells represented only a minority of all IL-17+ cells in both the TNT and MPT of ESCC tissues (2.1 ± 0.6 and 1.3 ± 0.5 %, respectively; n = 9; Fig. 2d).
Mast cells, but not T cells or macrophages, constitute the majority of IL-17-expressing cells in ESCC tissues. Paraffin-embedded ESCC sections (n ≥ 9) were subjected to double-color immunofluorescence for IL-17 (green) and a the T-cell marker CD3, b macrophage marker CD68 or c mast cell marker TRY (red), with DAPI counterstain (blue). These high-power fields at × 600 magnification in the muscularis propria illustrate the colocalization of IL-17 with the indicated markers (yellow color; white arrows). Scale bar 50 μm. d–f Numbers and proportions of IL-17+ cells in tumor nest (TNT) and muscularis propria (MPT) of ESCC tumor tissues. Cell numbers were calculated as cells per mm2. d The numbers of CD3+ T cells, IL-17+ T cells and the percentages of all CD3+ T cells or IL-17+ cells which were double-positive for CD3 and IL-17 (from left to right, n = 9). e The numbers of CD68+ macrophages, IL-17+ macrophages and the percentages of all CD68+ macrophages or IL-17+ cells which were double-positive (from left to right, n = 10). (f) The number of TRY+ mast cells, IL-17+ mast cells and the percentages of all TRY+ mast cells or IL-17+ cells which were double-positive (from left to right, n = 19). Results are expressed as mean ± SEM (bars). p < 0.05 was considered significant difference (Wilcoxon signed-rank test)
In addition to T cells, recent studies have shown that IL-17 can be produced by innate immune cells, such as macrophages and mast cells in inflammatory tissues [27, 29–31]. We therefore performed double-color immunofluorescence for IL-17 with the macrophage marker CD68 or with the mast cell-specific enzyme tryptase (TRY). Although macrophages were abundant in ESCC tissues, most did not express IL-17 (Fig. 2b, e). Macrophages represented a minority of all IL-17+ cells in both TNT and MPT of ESCC tissues (3.6 ± 1.2 and 5.0 ± 0.8 %, respectively; n = 10; Fig. 2e). In contrast, most IL-17+ cells costained brightly with TRY (Fig. 2c and Supplementary material Fig. 1). Although the levels of TRY+ mast cells were significantly higher in MPT (292 ± 33/mm2) than in TNT (155 ± 26/mm2; n = 19; p = 0.001), mast cells represented the majority of IL-17+ cells in both the MPT and TNT of ESCC tissues (97 ± 6.2 and 71.9 ± 7.0 %, respectively; Fig. 2f). Moreover, both the number and proportion of IL-17+ TRY+ mast cells were significantly higher in MPT (224 ± 26/mm2 and 77 ± 3.2 %) than the corresponding TNT tissues (28 ± 6/mm2 and 19.1 ± 3.7 %; Fig. 2f), which is consistent with the finding that IL-17+ cells were enriched in the MPT of ESCC tissues (Fig. 1d). The specificity of IL-17 staining was further confirmed by triple-color immunofluorescence with mouse anti-TRY and two antibodies from goat and rabbit that recognize different epitopes of the human IL-17 protein, showing good colocalization of IL-17 in TRY+ mast cells in ESCC tissues (Supplementary material Fig. 2).
The results described above indicate that the majority of IL-17-expressing cells in ESCC tissues in situ are mast cells. We next determined the subcellular localization of IL-17 within mast cells by immunogold staining and transmission electron microscopy. The mast cells were identified on the basis of their polygonal morphology and abundant, densely packed specific granules in the cytoplasm [32]. As shown in Fig. 3a, most of the gold particles were closely associated with secretory granules, demonstrating constitutive expression of IL-17 in mast cells.
IL-17 is expressed in mast cells. Specimens were dissected from ESCC patients immediately after surgery and examined by immunoelectron microscopy. One of three representative micrographs is shown in the left panels. Boxes show area enlarged in the right panels. a Immunogold detection of IL-17 was closely associated with secretory granules. b Isotype control showed no signal. N, nuclear; G, secretory granules. Scale bar 500 nm
Human mast cells can be divided into two subsets depending on the expression of proteases in their granules. Mast cells expressing only tryptase are typically located in the respiratory and intestinal mucosa, whereas mast cells that contain tryptase, chymase and other proteases are found in connective tissues and are defined as connective tissue mast cells (MCTC) [33]. We therefore used triple-color immunofluorescence to identify the mast cell subsets. The results showed that most IL-17-expressing cells costained brightly with tryptase and chymase, suggesting that MCTC represented the majority of IL-17-expressing cells in ESCC tissues (Supplementary material Fig. 3).
A high density of IL-17+ cells in MPT is inversely associated with tumor invasion and predicts a favorable prognosis in ESCC patients
Based on our observations that most IL-17-expressing cells were mast cells and that they were highly enriched in MPT, but not in the corresponding TNT in ESCC tumor tissues, we predicted that these cells might be associated with disease progression. To test this assumption, 215 ESCC patients who had received curative resection with long-term follow-up data (>10 years) were divided into two groups according to the median value of IL-17+ cells in MPT (IL-17 +MP cells), TRY+ cells in MPT (TRY +MP cells) or TRY+ cells in TNT (TRY +TN cells). Kaplan–Meier analysis revealed a positive association between the density of IL-17 +MP cells and overall survival (n = 215; p = 0.005; Fig. 4a). Patients with higher IL-17 +MP cell density had significantly longer overall survival (median 41.95 months) than those with lower IL-17 +MP cell density (median 19.73 months). In addition, the density of IL-17 +MP cells was inversely associated with tumor invasion (p = 0.016; Supplementary material Table 3). However, mast cell numbers in MPT or TNT did not correlate with the survival of ESCC patients (Fig. 4b, c). When the clinicopathological variables that were significant in univariate analysis were adopted as covariates, multivariate analysis revealed that the density of IL-17 +MP cells was an independent prognostic factor for overall survival (p = 0.007; Table 1). These results suggest that IL-17+ cell density is inversely associated with progression of ESCC and might serve as an independent predictor of good survival.
Accumulation of IL-17+ cells in the muscularis propria predicts favorable prognosis in ESCC patients. The patients (n = 215) were divided into two groups (high or low) according to the median values for: a IL-17+ cell density in muscularis propria (IL-17 +MP cells, median = 8.6); b TRY+ cell density in the muscularis propria (TRY +MP cells, median = 13.6); or c TRY+ cell density in the tumor nest (TRY +TN cells, median = 3). Cell densities were obtained in ×400 fields (0.0768 mm2 per field) from different anatomic locations in tumor tissues. Dashed lines low-density groups; solid lines high-density groups. Overall survival was calculated by the Kaplan–Meier method and analyzed by the log-rank test
IL-17 +MP cells are positively associated with effector immune cells in the same microenvironment
It is generally agreed that IL-17 may not directly mediate antitumor activity, but can indirectly promote antitumor immunity by facilitating the recruitment of other effector immune cells, such as CD8+ T cells [12, 13]. A recent study in mouse showed that the CD169+ macrophage subset dominates antitumor immunity by cross-presenting tumor antigens to CD8+ T cells [34]. To investigate whether such a mechanism is also involved in tumor immunity in the muscularis propria, we examined the distribution of these effector cells and their association with IL-17+ cells in ESCC tissues. The number of CD8+ T cells was indeed significantly higher in the MPT than the TNT of the same ESCC patients (Fig. 5a). Although there was no obvious difference in macrophage density (using CD68 as a pan-macrophage marker) between MPT and TNT, CD169+ macrophages were more prominent in MPT (Fig. 5b, c, Supplementary material Fig. 4).
IL-17+ cells are positively associated with effector immune cells in the muscularis propria of ESCC tumor tissues. a–c Analysis of the density of CD8+ T cells, CD169+ macrophages or CD68+ macrophages in paired tumor nest (TNT) and muscularis propria (MPT) of ESCC tumor tissues (n = 60). Bars show the mean values from five most representative ×400 fields (0.0768 mm2 per field). d–i The association between IL-17+ cells and other effector immune cells in ESCC tumor tissues (n = 60). d–f Positive associations between IL-17 +MP cells and CD8 +MP T cells, CD169 +MP macrophages or CD68 +MP macrophages in the same anatomic location (MPT). g–i No associations between IL-17 +MP cells and CD8 +TN T cells, CD169 +TN macrophages or CD68 +TN macrophages in different anatomic locations (MPT vs. TNT). p < 0.05 was considered significant difference (Spearman’s rank correlation coefficient test)
These data prompted us to further examine the correlations between the densities of IL-17 +MP cells and CD8+ T cells or macrophages in serial sections of ESCC tissues. Spearman’s rank correlation coefficient tests revealed that the level of IL-17+ cells in muscularis propria was positively correlated with the density of CD8+ T cells, CD169+ macrophages and CD68+ macrophages in the same anatomic location (all p < 0.001; Fig. 5d, f). However, the density of IL-17 +MP cells showed no correlation with the levels of CD8+ T cells, CD169+ macrophages or CD68+ macrophages in the tumor nest (Fig. 5g, i). These results suggest that the antitumor activity of IL-17+ cells in the muscularis propria of ESCC tissues may be mediated by recruiting CD8+ T cells and the CD169+ macrophage subset into the same microenvironment.
Discussion
Although production of the proinflammatory cytokine IL-17 has been investigated in patients with diverse types of cancer, its role in tumor progression is still controversial [13]. The present study showed that mast cells, but not T cells, were the predominant IL-17-expressing cells in ESCC tissues in situ. These cells accumulated in the muscularis propria rather than the corresponding tumor nest, and increased IL-17 +MP cell density was associated with prolonged survival in ESCC patients. Moreover, we have provided evidence that the density of IL-17+ cells in the muscularis propria was positively associated with the numbers of effector immune cells in the same area of ESCC tissues, and inversely associated with tumor invasion. These data suggest that mast cells play a significant role in tumor immunity by releasing IL-17 at a previously unappreciated location, the muscularis propria, in ESCC tissues.
The opposing effects reported for IL-17 on tumor progression may be partly due to its diverse cellular sources in different tumor microenvironments [13]. IL-17-expressing T cells may contribute to protective human tumor immunity in ovarian cancer, whereas IL-17-expressing macrophages could promote the invasiveness of tumor cells in breast cancer [30, 35]. In the present study, we found that although the densities of CD3+ T cells are significantly higher than mast cells in ESCC tissues, T cells represented only a minority of all IL-17-expressing cells in both tumor nest and muscularis propria of ESCC tissues in situ. However, we observed that IL-17 colocalized with tryptase, a highly specific marker for mast cells, in ESCC tissues. Moreover, electron microscopy provided further evidence for subcellular localization of IL-17 within mast cells. The specificity of IL-17 staining was further confirmed by triple-color immunofluorescence with mouse anti-TRY and two Abs from goat and rabbit that recognize different epitopes of the human IL-17 protein.
Mast cells can promote or inhibit the development of tumors, due to their production of a variety of cytokines [36–38]. However, production of IL-17 by mast cells has not previously been investigated in human tumors [36]. Our present study therefore suggests a more important role for mast cells in tumor progression than previously appreciated, mediated by the production of IL-17. In line with our results, IL-17-producing mast cells have recently emerged as crucial mediators in the pathogenesis of diverse inflammatory diseases [16, 39]. In vitro studies have also revealed that mast cells can produce IL-17 in response to various inflammatory stimuli [29].
Anatomic localization may be another crucial context, which determines the effects of IL-17-producing cells on tumor progression [17, 18]. It is generally agreed that the mucosa and the submucosa, but not the muscularis propria, are the predominant sites of chronic inflammation in gastrointestinal tissues [40, 41]. We were therefore surprised to find that a significant number of leukocytes, including IL-17+ cells, CD8+ T cells and CD169+ macrophages, were enriched in the muscularis propria of ESCC tissues. Moreover, we found that the number of IL-17+ cells in the muscularis propria served as an independent predictor for favorable survival in ESCC. These data suggested that the muscularis propria microenvironment might also affect tumor immunity and regulate the progression of ESCC. In accordance with our results, previous studies have shown that mature dendritic cells are predominantly distributed in the muscularis propria, rather than the mucosa or submucosa areas of the mouse intestine [42]. In addition, Lv et al. [43] reported that a high density of IL-17+ cells was associated with improved survival in ESCC, but was not an independent predictor in multivariate analysis. This discrepancy is probably due to their study not evaluating IL-17+ cells in distinct anatomic locations, whereas our studies have shown that the mean density of IL-17+ cells in the muscularis propria is about 20-fold higher than in the tumor nest (data not shown).
Although antitumor effects of IL-17 have been observed in human tumors, the mechanisms are presently unclear [12]. It has been suggested that IL-17+ cells might mediate their antitumor activity indirectly, by facilitating the recruitment of other effector immune cells [13]. In line with this possibility, we observed that IL-17RA, the predominant IL-17 receptor, which can be expressed by both tumor and stromal cells throughout the tissue (data not shown), and the levels of IL-17 +MP cells were inversely associated with tumor invasion, and positively associated with effector CD8 +MP T cells and activated CD169 +MP macrophages in the same microenvironment. These data are consistent with several lines of evidence. In patients with prostate cancer, a high level of involvement of IL-17+ cells in inflammation is associated with a lower pathologic stage [44]. In primary ovarian cancer, IL-17 plays an indirect role in antitumor immunity by promoting effector T cell and NK cell trafficking to the tumor microenvironment [35]. Moreover, IL-17 can induce the production of antitumor cytokines from macrophages [45].
Emerging evidence indicates that it is not inflammation per se but inflammatory “context” that determines the ability of proinflammatory factors to facilitate or prevent tumor growth [10, 11, 13]. Our results suggest that mast cells may play a more prominent role than previously appreciated, by releasing IL-17 at the site of the muscularis propria in ESCC tissues. Moreover, increased IL-17 +MP cells are a potential prognostic marker in ESCC and were positively associated with other effector immune cells in the same location. This study confirms that the relationship between IL-17-producing cells and tumor immunopathology is highly dependent on context, including cellular source, anatomic location and the associated cellular microenvironment. A better understanding of these contexts could be used to develop new cancer immunotherapies.
Abbreviations
- IL-17:
-
Interleukin 17
- ESCC:
-
Esophageal squamous cell carcinoma
- TRY:
-
Tryptase
- CHY:
-
Chymase
- MUN :
-
Normal mucosa
- MPN :
-
Muscularis propria in non-tumor tissues
- TNT :
-
Tumor nest
- MPT :
-
Muscularis propria in tumor tissues
- IL-17 +MP cells:
-
IL-17+ cells in MPT
- TRY +MP cells:
-
TRY+ cells in MPT
- TRY +MP cells:
-
TRY+ cells in TNT
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. doi:10.3322/caac.20107
Enzinger PC, Mayer RJ (2003) Esophageal cancer. N Engl J Med 349(23):2241–2252. doi:10.1056/NEJMra035010
Cooper SL, Russo JK, Chin S (2012) Definitive chemoradiotherapy for esophageal carcinoma. Surg Clin North Am 92(5):1213–1248. doi:10.1016/j.suc.2012.07.013
Rice TW, Zuccaro G Jr, Adelstein DJ, Rybicki LA, Blackstone EH, Goldblum JR (1998) Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg 65(3):787–792
Mueller MM, Fusenig NE (2004) Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 4(11):839–849. doi:10.1038/nrc1477
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21(3):309–322. doi:10.1016/j.ccr.2012.02.022
Fridman WH, Pages F, Sautes-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12(4):298–306. doi:10.1038/nrc3245
Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Murakami S, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H (2003) CD4 + and CD8 + T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res 63(7):1555–1559
Liu J, Lu G, Li Z, Tang F, Liu Y, Cui G (2010) Distinct compartmental distribution of mature and immature dendritic cells in esophageal squamous cell carcinoma. Pathol Res Pract 206(9):602–606. doi:10.1016/j.prp.2010.03.011
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444. doi:10.1038/nature07205
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140(6):883–899. doi:10.1016/j.cell.2010.01.025
Murugaiyan G, Saha B (2009) Protumor vs antitumor functions of IL-17. J Immunol 183(7):4169–4175. doi:10.4049/jimmunol.0901017
Zou W, Restifo NP (2010) T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol 10(4):248–256. doi:10.1038/nri2742
Huang G, Wang Y, Chi H (2012) Regulation of TH17 cell differentiation by innate immune signals. Cell Mol Immunol 9(4):287–295. doi:10.1038/cmi.2012.10
Cua DJ, Tato CM (2010) Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol 10(7):479–489. doi:10.1038/nri2800
de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, Idu MM, van Maldegem F, Aten J, van der Wal AC (2010) Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J Pathol 220(4):499–508. doi:10.1002/path.2667
Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L (2009) Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 206(6):1327–1337. doi:10.1084/jem.20082173
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313(5795):1960–1964. doi:10.1126/science.1129139
Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M (2008) Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 14(5):518–527. doi:10.1038/nm1764
Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L (2009) Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol 50(5):980–989. doi:10.1016/j.jhep.2008.12.033
Kuang DM, Peng C, Zhao Q, Wu Y, Chen MS, Zheng L (2010) Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology 51(1):154–164. doi:10.1002/hep.23291
Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L (2011) Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol 54(5):948–955. doi:10.1016/j.jhep.2010.08.041
Lin SH, Chang JY (2010) Esophageal cancer: diagnosis and management. Chin J Cancer 29(10):843–854
Zhu ZH, Sun BY, Ma Y, Shao JY, Long H, Zhang X, Fu JH, Zhang LJ, Su XD, Wu QL, Ling P, Chen M, Xie ZM, Hu Y, Rong TH (2009) Three immunomarker support vector machines-based prognostic classifiers for stage IB non-small-cell lung cancer. J Clin Oncol 27(7):1091–1099. doi:10.1200/JCO.2008.16.6991
Ding T, Xu J, Wang F, Shi M, Zhang Y, Li SP, Zheng L (2009) High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol 40(3):381–389. doi:10.1016/j.humpath.2008.08.011
Takahashi N, Vanlaere I, de Rycke R, Cauwels A, Joosten LA, Lubberts E, van den Berg WB, Libert C (2008) IL-17 produced by Paneth cells drives TNF-induced shock. J Exp Med 205(8):1755–1761. doi:10.1084/jem.20080588
Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, Villanueva EC, Shah P, Kaplan MJ, Bruce AT (2011) Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol 187(1):490–500. doi:10.4049/jimmunol.1100123
Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R (2007) Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 8(9):950–957. doi:10.1038/ni1497
Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, Melendez AJ, McInnes IB (2010) Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol 184(7):3336–3340. doi:10.4049/jimmunol.0903566
Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, Yilmazer A, Paish EC, Ellis IO, Patel PM, Jackson AM (2008) IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res 10(6):R95. doi:10.1186/bcr2195
Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y (2003) Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52(1):65–70
Abraham SN, St John AL (2010) Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol 10(6):440–452. doi:10.1038/nri2782
Galli SJ, Grimbaldeston M, Tsai M (2008) Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol 8(6):478–486. doi:10.1038/nri2327
Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, Kanagawa O, Fujii S, Tanaka M (2011) CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity 34(1):85–95. doi:10.1016/j.immuni.2010.12.011
Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W (2009) Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114(6):1141–1149. doi:10.1182/blood-2009-03-208249
Khazaie K, Blatner NR, Khan MW, Gounari F, Gounaris E, Dennis K, Bonertz A, Tsai FN, Strouch MJ, Cheon E, Phillips JD, Beckhove P, Bentrem DJ (2011) The significant role of mast cells in cancer. Cancer Metastasis Rev 30(1):45–60. doi:10.1007/s10555-011-9286-z
Kalesnikoff J, Galli SJ (2008) New developments in mast cell biology. Nat Immunol 9(11):1215–1223. doi:10.1038/ni.f.216
Welsh TJ, Green RH, Richardson D, Waller DA, O’Byrne KJ, Bradding P (2005) Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol 23(35):8959–8967. doi:10.1200/JCO.2005.01.4910
Noordenbos T, Yeremenko N, Gofita I, van de Sande M, Tak PP, Canete JD, Baeten D (2012) Interleukin-17-positive mast cells contribute to synovial inflammation in spondyloarthritis. Arthr Rheum 64(1):99–109. doi:10.1002/art.33396
Connor SJ, Paraskevopoulos N, Newman R, Cuan N, Hampartzoumian T, Lloyd AR, Grimm MC (2004) CCR2 expressing CD4 + T lymphocytes are preferentially recruited to the ileum in Crohn’s disease. Gut 53(9):1287–1294. doi:10.1136/gut.2003.028225
Villablanca EJ, Cassani B, von Andrian UH, Mora JR (2011) Blocking lymphocyte localization to the gastrointestinal mucosa as a therapeutic strategy for inflammatory bowel diseases. Gastroenterology 140(6):1776–1784. doi:10.1053/j.gastro.2011.02.015
Flores-Langarica A, Meza-Perez S, Calderon-Amador J, Estrada-Garcia T, Macpherson G, Lebecque S, Saeland S, Steinman RM, Flores-Romo L (2005) Network of dendritic cells within the muscular layer of the mouse intestine. Proc Natl Acad Sci U S A 102(52):19039–19044. doi:10.1073/pnas.0504253102
Lv L, Pan K, Li XD, She KL, Zhao JJ, Wang W, Chen JG, Chen YB, Yun JP, Xia JC (2011) The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS ONE 6(3):e18219. doi:10.1371/journal.pone.0018219
Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG (2008) Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res: Off J Am Assoc Cancer Res 14(11):3254–3261. doi:10.1158/1078-0432.CCR-07-5164
Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP (1998) IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol 160(7):3513–3521
Acknowledgments
This work was supported by Project Grants from the National Basic Research Program of China (2010CB529904 and 2011CB811305) and the National Natural Science Foundation of China (81230073 and 91029737).
Conflict of interest
The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Bo Wang and Lian Li have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, B., Li, L., Liao, Y. et al. Mast cells expressing interleukin 17 in the muscularis propria predict a favorable prognosis in esophageal squamous cell carcinoma. Cancer Immunol Immunother 62, 1575–1585 (2013). https://doi.org/10.1007/s00262-013-1460-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-013-1460-4