Abstract
Hu14.18-IL2 is an immunocytokine (IC) consisting of human IL-2 linked to hu14.18 mAb, which recognizes GD2 disialoganglioside. Phase II clinical trials of intravenous-hu14.18-IL2 (IV-IC) in neuroblastoma and melanoma are underway, and have already demonstrated activity in neuroblastoma. In our Phase II trial, lower neuroblastoma burden at the time of treatment was associated with a greater likelihood of clinical response to IV-IC. We have previously shown that intratumoral-hu14.18-IL2 (IT-IC) compared to IV-IC results in enhanced local and systemic antitumor activity in tumor-bearing mice. We utilized a mouse model to investigate the impact of tumor burden on hu14.18-IL2 treatment efficacy in IV- versus IT-treated animals. Studies presented here describe the analyses of tumor burden at the initiation of treatment and its effects on treatment efficacy, survival, and tumor-infiltrating leukocytes in A/J mice bearing subcutaneous NXS2 neuroblastoma. We show that smaller tumor burden at treatment initiation is associated with increased infiltration of NK and CD8+ T cells and increased overall survival. NXS2 tumor shrinkage shortly after completion of the 3 days of hu14.18-IL2 treatment is necessary for long-term survival. This model demonstrates that tumor size is a strong predictor of hu14.18-IL2-induced lymphocyte infiltration and treatment outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunocytokines (IC) are synthetic fusion proteins that consist of tumor-specific monoclonal antibodies (mAb) linked to an immune-stimulating cytokine. Hu14.18-IL2 is an IC consisting of human interleukin-2 (IL2) linked to each IgG heavy chain of the hu14.18 mAb, which recognizes the GD2 disialoganglioside presence on tumors of neuro-ectodermal origin (i.e., neuroblastoma and melanoma) [1]. ICs are capable of augmenting significant antitumor effects in murine models by targeting the therapy to the tumor and stimulating the immune system to selectively destroy the cancer cells [2–4].
The hu14.18-IL2 IC was tested in adults with melanoma and in children with neuroblastoma [5–8], and clinical activity was seen in children with neuroblastoma [6]. A recent Phase II study through the children’s oncology group (COG) treated recurrent or refractory neuroblastoma patients with hu14.8-IL2 intravenously at 12 mg/m2/day for 3 days every 4 weeks. Seven out of 23 patients with non-bulky disease (evaluable only by sensitive 123I-MIBG scintigraphy or bone marrow histology) showed clinical improvement [6]. In contrast, none of the 13 patients with bulky disease (measurable by standard radiographic criteria) had a measurable response (p = 0.03 for response in the non-bulky vs. bulky disease groups) [6]. Genotyping analyses from these patients showed that responses were also associated with favorable killer immunoglobulin-like receptor (KIR)–KIR ligand relationships, suggesting an in vivo role for NK-mediated antitumor effects [9].
Substantial clinical data indicate that tumor-infiltrating lymphocyte (TIL) number and phenotype have clinical significance and can be predictive of successful immunotherapy. CD3+ and CD8+ TILs were found to correlate with improved survival [10]. A separate study demonstrated that primary melanomas with a brisk TIL infiltrate were less often associated with a positive sentinel lymph node [11]. High levels of intratumoral TILs were associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer patients [12]. Our laboratory also noted in an animal model that intratumoral-IC (IT-IC) compared to IV-IC resulted in increased numbers of activated T- and NK cells within tumors (as measured by augmented expression of the NKG2D activation receptor on these cells), better IC delivery and retention in the tumor, enhanced inhibition of tumor growth, and improved survival [13]. In addition, we previously found that IV treatment with hu14.18-IL2 can effectively eliminate very small subcutaneous tumors and small disseminated metastases in A/J mice with NXS2 neuroblastoma. Greater efficacy was observed if systemic treatment was initiated early after IV seeding of experimental metastases [4]. Our laboratory also demonstrated that intratumoral administration of immunocytokine (IT-IC) induced greater local antitumor effects than IV administration [14].
In our immunotherapy studies of hu14.18-IL2 treatment for NXS2-bearing mice reported here, we compared local versus systemic treatment of the hu14.18-IL2 IC in relation to the size of tumor at the initiation of treatment and the effect on tumor growth, survival, degree of TIL, and lymphocyte subset infiltrate response. Our analyses demonstrate that even within the same treatment group, there is a correlation between low initial tumor burden and increased immune infiltration, between high immune infiltration and improved outcome (namely, tumor growth inhibition and survival), and thus also between low initial tumor burden and improved outcome.
Materials and methods
Mice and declaration of approval
We obtained 7- to 8-week-old female A/J mice from Jackson Laboratories (Bar Harbor, Maine). All mice were housed in university-approved facilities and were handled according to the National Institutes of Health and University of Wisconsin-Madison Research Animal Resource Center (RARC) guidelines.
All experimentation was performed in accordance with the protocols approved by the National Institutes of Health and by the Animal Care and Use Committees of UW-Madison, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Cell lines
NXS2 is a moderately immunogenic, highly metastatic, GD2+ murine neuroblastoma hybrid cell line [2]. The murine NXS2 cell line was grown at 37 °C in a humidified 5 % CO2 atmosphere in Dulbecco’s modified Eagle’s medium (DMEM, Mediatech, Herndon, VA) as previously described [4].
ICs and immunotherapy
The humanized hu14.18-IL2 (APN301, Apeiron Biologics, Vienna, Austria) was supplied by the NCI Biologics Resources Branch (Frederick, MD) via a collaborative relationship with Merck KGaA (Darmstadt, Germany) and Apeiron Biologics.
In vivo tumor growth and treatment
A/J mice were engrafted subcutaneously with 2 × 106 NXS2 cells in 100 μl PBS in the right lower abdomen. Developed tumors were measured with mechanical calipers and allowed to grow until the average volume was 30–150 mm3 (Volume = width × width × length/2). Mice were then randomized into three treatment groups and received 50 μg of IC in 100 μl PBS daily for 3 consecutive days either IV by tail vein injection (IV-IC) or IT by direct injection into the tumor (IT-IC). Control mice were untreated or treated with an equivalent volume of PBS administered by IT injection.
Endpoints for progressive tumor growth
The end point of all tumor growth and survival studies was death of the animal or excessive tumor burden as determined by both tumor size (15 mm in any direction) and the condition and behavior of the animal. These criteria are established by the RARC guidelines. The decision to euthanize an animal was made by an independent observer without regard to treatment group. Time to death/euthanasia was measured from treatment initiation.
Immunohistochemistry and flow cytometry
Mice were killed 48 h after treatment completion and their tumors resected for histology or flow cytometry. Tumors taken for histology were embedded in OCT, flash frozen in liquid nitrogen, and sections stained and quantified using a novel histological method as recently described [13]. Tumors and spleens taken for flow cytometry were resected, disaggregated, and stained, as previously described [13]. Analysis of data was performed using FlowJo software. Fluorochromes Minus Ones (FMOs) were used to distinguish positively stained populations. Results are reported as % positive cells or as mean fluorescence intensity (MFI) units.
Statistical methods
In all figures, p values of <0.05, <0.01, <0.001, and <0.0001 are represented with (*), (**), (***), and (****), respectively. In all scatter plots, asterisks in the legend represent a statistically significant different slope of each treatment groups’ regression line compared to 0. Slope comparisons between regression analyses are tested and similarly indicated with “s” values (s). Regression lines with significantly different slopes cannot be tested for differences in elevation because they will intersect at some point. In cases when slopes are not significantly different, elevation (covariate-adjusted means) comparisons between regression analyses are tested [15] and similarly indicated with “x” values (x). Significance for (s) and (x) values is as follows: <0.05, <0.01, <0.001, and <0.0001 are represented with 1–4 “s” or “x,” respectively. For comparisons between treatment groups, “s” (for slope) or “x” (for elevation) indications are used in place of asterisks, to test whether the slopes or elevations, respectively, of each group’s regression line are statistically significantly different from each other. Contingency tables and graphs were analyzed using a chi-squared statistic test or the Fisher’s exact test (for small samples) to generate p values. Survival curves were generated using the method described by Kaplan and Meier and statistically compared using the log rank (Mantel–Haenszel) test. In figures with histograms, one-tailed or two-tailed (if there was no a priori hypothesis) Student’s t tests were used to determine statistical significance of observed differences between experimental and relevant control values. In graphs with log scales, the data are in some cases transformed by adding “1” [log(x + 1)] in order to avoid negative values from data points close to zero [16]. Data are presented as mean ± standard error of the mean (SEM) and considered statistically significant for p values <0.05. Graphs were generated and significance tests were performed using Prism 5, version 5.04, software (GraphPad). Due to the exploratory nature of the reported statistical inference, no adjustments were made for multiple testing throughout.
Results
Initial tumor volume inversely correlates with survival in hu14.18-IL2-treated mice
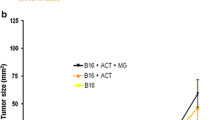
We first sought to determine whether tumor volume at treatment initiation (initial tumor volume) is prognostic or predictive for survival in mice receiving 3 days of IT-IC, IV-IC, or control (IT-PBS). Data were analyzed for survival outcome stratified by initial tumor volume (greater or less than the median tumor volume of 34 mm3). The initial tumor volume was inversely associated with survival (Fig. 1). Hu14.18-IL2-treated mice (both IT-IC and IV-IC) initially bearing tumors smaller than 34 mm3 showed an increased long-term survival percentage (30.8 % survival) compared to IC-treated mice or control-treated mice bearing tumors larger than 34 mm3 (0 % survival). Hu14.18-IL2-treated mice bearing tumors larger than 34 mm3 did not survive significantly longer than control-treated mice initially bearing tumors larger than 34 mm3. These results indicate that smaller initial tumor volume is predictive for improved overall survival induced by hu14.18-IL2 treatment and IC treatment did not provide any survival advantage for the mice with larger initial tumor volume.
Initial tumor volume inversely correlates with survival in hu14.18-IL2-treated mice. A/J mice bearing subcutaneous NXS2 tumors were treated with 3 daily doses of hu14.18-IL2 (either IV or IT) or received control treatment (IT-PBS or no treatment). a Contingency table showing the long-term outcomes of mice treated with IC (hu14.18-IL2 IV or IT) stratified by median initial tumor volume of 34 mm3 or control mice (IT-PBS or untreated). b Kaplan–Meier curves showing number of survival days post-treatment initiation in IC-treated or control groups stratified by median initial tumor volume of 34 mm3. Experiments represent data from 3 independent experiments with a total of 37 mice
Hu14.18-IL2 treatment for mice with smaller initial tumor volume leads to increased tumor NK infiltration and NKG2D expression on tumor NK cells and CD8+ T cells
To examine the effect of initial tumor load on tumor-infiltrating leukocytes (TILs) found 4 days later (2 days after the last treatment) in IT-IC, IV-IC, and control-treated animals, tumors were harvested and analyzed by flow cytometry. In order to make certain that adequate tumor mass was available to enable sufficient cells for flow cytometry analyses, treatment was begun at a later time than that for the experiment shown in Fig. 1. Figure 2 compares TIL populations between mice whose initial tumors were greater than or less than the median tumor volume of 101.6 mm3. The number of leukocytes (CD45+ cells) in IT-IC-treated tumors, as a percentage of total nucleated cells (TNCs), is significantly higher when the initial volume is <101.6 mm3 compared to tumors initially larger than 101.6 mm3 (Fig. 2a). This trend, although not statistically significant, was also seen in control- and IV-IC-treated groups (Fig. 2a). We observed significant increases in leukocyte density after treatment with IT-IC in both the larger and smaller tumors compared to control treatment, while the IV-IC treatment induced a significant increase only in larger tumors compared to control-treated, large tumors. Both NK cells (Fig. 2c) and CD8 T cells (Fig. 2d) contribute to the increased TIL. This was noted in animals with small and large initial tumors. In addition, the percentage of NK cells in IT-IC-treated tumors is significantly higher in smaller initial tumors compared to larger initial tumors (Fig. 2c). This trend, although not significant, is also seen in the IV-IC-treated tumors (Fig. 2c). The CD8+ T cell infiltration is increased in IT-IC- and IV-IC-treated tumors, and is similar regardless of initial tumor burden (Fig. 2d).
Hu14.18-IL2 treatment for mice with smaller initial tumor volume leads to increased tumor NK infiltration and NKG2D expression on tumor NK and CD8+ T cells. A/J mice bearing subcutaneous NXS2 tumors were treated with IT-IC, IV-IC, or untreated. Mice were killed 4 days post-treatment initiation and tumors underwent flow cytometric analysis. Initial tumor volumes of all mice are stratified into groups greater or less than the median value of 101.6 mm3. a post-treatment tumor leukocytes (CD45+ cells) are presented as a percentage of total live nucleated cells (TNCs) within the tumor; b macrophages (F4/80+) are presented as a percentage of leukocytes (CD45+) within the tumor; c natural killer cells (NKp46+) are presented as a percent of mouse leukocytes (CD45+); d cytotoxic T cells (CD8+) are presented as a percent of mouse leukocytes (CD45+); e NKG2D expression levels are presented as MFI on NKp46+- and NKG2A+- activated natural killer cells; and f NKG2D expression levels are presented as MFI on CD8+- and NKG2A+- activated cells. Results represent data from 6 independent experiments with an average of 19 mice within each treatment group
The activation status of infiltrating NKp46+ NK and CD8+ T cell populations is potentially important for antitumor activity. We tested for the expression of the activating NKG2D receptor on NKG2A/C/E+ NKp46+ NK cells (considered “licensed” NK cells) and NKG2A/C/E+ CD8+ cells (considered activated CTL) [17, 18]. NKG2A/C/E+ NKp46+ NK cells in the initially small, IT-IC-treated tumors had significantly increased expression levels (augmented MFI) of the NKG2D effector receptor compared to expression levels seen in initially large, IT-IC-treated tumors (Fig. 2e). This same pattern is seen in CD8+ NKG2A/C/E+ cells (Fig. 2f). Both IV and IT-IC treatments augment the activation of the CD8 cells. This is seen in both the smaller and the larger tumors; however, the activation was significantly greater in the IT-IC-treated smaller tumors compared to IT-IC-treated larger tumors.
The number of F4/80+ macrophages in the tumor as a percentage of CD45+ leukocytes is significantly higher in larger tumors compared to smaller tumors regardless of treatment (Fig. 2b). For all panels in Fig. 2, we have included more detailed correlation comparisons in the online supplementary data (Supplementary Figure 1A-F). We further evaluated which kinds of infiltrating leukocytes (CD45+ cells) are responsible in accounting for overall increases in tumor leukocyte density in the supplementary online text and in Supplementary Figure 2.
Tumor growth inversely correlates with tumor NK cell and CD8+ T cell infiltration following IC treatment
Using immunohistochemistry (IHC), we observed a negative correlation between NKG2A/C/E+ cells and percent tumor growth in IV-IC- and IT-IC-treated tumors but not in PBS-treated tumors (Fig. 3a). These negative slopes indicate that more NK cells correlate with less tumor growth. These same negative correlations were also seen with CD8+ T cells in hu14.18-IL2-treated mice (Fig. 3b). Furthermore, the elevations of the IV-IC lines are significantly higher than those of the IT-IC lines for NK cells (Fig. 3a) and CD8+ cells (Fig. 3b) The different elevations of these lines for IT-IC and IV-IC indicate that, even at similar percentages of NK and T cell infiltration, tumor growth was less in IT-IC-treated animals compared to IV-IC-treated animals. Control-treated mice did not show a significant negative correlation (blue lines in Fig. 3a, b do not have a slope that is significantly different than 0), indicating no significant relationship between these tumor-infiltrating lymphocytes and tumor growth in the absence of IC treatment. Overall, tumors that shrank in size (percent tumor growth <0) tended to have more tumor lymphocyte infiltrate than tumors that grew (Fig. 3a, b). These histological results were confirmed in separate experiments, using flow cytometry (Fig. 3C). We then evaluated the immune profile of treated and untreated tumors, stratified by whether the tumors grew or shrank (Fig. 3D).
Tumor NK cell and CD8+ T cell infiltration inversely correlates with tumor growth following IC treatment. A/J mice bearing subcutaneous NXS2 tumors were treated with IT-IC, IV-IC, or control treatment. Mice were killed 4 days post-treatment initiation and tumors underwent quantitative histological (a, b) or flow cytometric analyses (c, d). a, b After immunohistochemistry, 5 distinct microscopic fields were counted in a blinded fashion within each mouse’s tumor. NKG2A/C/E+ cells (a) and CD8a+ cytotoxic T cells (b) as a percentage of total nucleated cells (TNCs) within the each mouse’s tumor are plotted against each mouse’s percent tumor growth. a, b represent data from 3 independent experiments, with an average of 18 mice total within each treatment group. Horizontal error bars represent standard error of the mean for subsamples within an individual mouse. c Post-treatment tumor leukocytes (CD45+ cells) as a percentage of total live nucleated cells (TNCs) are plotted against each mouse’s percent tumor growth. d Mice are stratified by treatment group and by tumor growth or shrinkage. Within each group, tumor immune cells (CD8+, NKp46+, F4/80+, CD4+) are shown as a percentage of tumor leukocytes (CD45+ cells). Asterisks in d compare tumor immune cell populations between IT-IC-treated mice whose tumor grew versus IT-IC-treated mice whose tumor shrank. c, d represent data from 6 independent experiments with an average of 19 mice total within each treatment group. Note, the IT-IC treatment group consists of 6 mice that had tumors shrink and 15 mice that had tumors grow
Responding IT-IC-treated tumors have higher numbers of CD8+ T cells and NKp46+ NK cells, but fewer of F4/80+ macrophages
Only the IT-IC treatment group had a number of animals with shrinking tumors (as seen in Fig. 3c). The tumor infiltrate in these responding tumors was compared to the infiltrate seen in growing tumors. IT-IC- and IV-IC-treated tumors that grew have similar infiltration profiles (Fig. 3d), while IT-IC-treated tumors that shrank have a different TIL pattern, with significantly higher amounts of CD8+ T cells and NKp46+ NK cells, and significantly lower amounts of F4/80+ macrophages as percentages of CD45+ leukocytes (Fig. 3d). These data suggest that CD8+ T cells and NKp46+ NK cells play important roles in the IC-induced antitumor effect. In contrast, these data suggest that F4/80+ macrophage infiltration may be associated with tumor growth and potentially antagonistic to the antitumor activity of IT-IC (at least in the IT-IC-treated tumors that grew). The amount of CD4+ T cells is not significantly different between IT-IC-treated tumors that grew and IT-IC-treated tumors that shrank (Fig. 3d). Supplementary Fig. 4 shows the raw data for the comparisons in Figure 3D.
Increased activated NK cells and CD8+ T cells correlate with tumor volume reduction following Hu14.18-IL2 treatment
NK and CD8+ T cell infiltrations were noted in both the IT-IC- and IV-IC-treated tumors (Fig. 2 and Supplementary Figure 2), but the IT-IC group showed much better tumor shrinkage than the IV-IC group (Fig. 3). We were interested in determining whether the activation status of infiltrating lymphocytes may be important for the observed antitumor effect. We tested for the expression of the effector receptor NKG2D (a known activation marker) on NKG2A/C/E+ NKp46+ NK cells and NKG2A/C/E+ CD8+ TIL to evaluate whether NKG2D expression correlates with tumor growth or shrinkage within the individual treatment groups. NKG2D expression on NK cells (Fig. 4a) and CD8+ T cells (Fig. 4b) in IT-IC-treated tumors correlates with reduction in tumor volume within each treatment group (the higher the NKG2D expression, the smaller the tumor). This correlation is not statistically significant in the IV-IC treatment group (red lines in Fig. 4a, b), suggesting that NKG2D expression may contribute to the enhanced tumor shrinkage in the IT-IC treatment group compared to IV-IC. Untreated mice show a similar relationship between NKG2D on NK cells and CD8+ cells with percent tumor growth (Fig. 4a, b). Thus, even in the absence of treatment, tumors with the greatest spontaneous growth are the ones with the lowest density of NKG2D on their infiltrating lymphocytes. The elevation of the IV-IC line is significantly higher than that of the IT-IC line, suggesting that IV-IC-treated tumors grew more than IT-IC-treated tumors, even when comparing similar levels of NKG2D expression. Other factors in conjunction with NKG2D expression levels are likely contributing to the observed antitumor effect. The data presented in the bar graph show that the expression of the NKG2D activation marker is increased significantly in all treatment groups compared to control animals, and the marker is significantly higher in the IT-IC responding tumors compared to the IT-IC-treated non-responding tumors (Fig. 4c).
NKG2D expression on NK and CD8+ T cells correlates with tumor volume reduction following Hu14.18-IL2 treatment. A/J mice bearing subcutaneous NXS2 tumors were treated with IT-IC or IV-IC or received no treatment. Mice were killed 4 days post-treatment initiation and tumors underwent flow cytometric analysis. Using flow cytometry, post-treatment tumor NKG2D expression (MFI) on activated natural killer cells (a) and cytotoxic T cells (b) is plotted against each mouse’s percent tumor growth. c shows bar graphs of the same data in (a, b). d shows, within each mouse’s tumor, the NKG2D expression (MFI) on double-positive NKp46+, NKG2A/C/E+ NK cells versus that same animal’s NKG2D MFI on double-positive CD8+, NKG2A/C/E+ T cells (x-axis). All panels represent data from 6 independent experiments with an average of 19 mice total within each treatment group
We also tested to see whether augmentation of NKG2D on NK cells and CD8+ cells occurs in parallel in individual mice. We found that, in all treatment groups, augmented NKG2D expression on NK cells positively correlated with augmented NKG2D expression on activated CD8+ cells (Fig. 4d). This suggests that the stimulation and activation of these NK and T cells are not independent, and that the hu14.18-IL2 IC treatment has the ability to activate both NK and T cells when given IT or IV. Additional data are shown in Supplemental Figure 3.
Large tumor burden at initiation of hu14.18-IL2 therapy negates the antitumor effect
Figure 5a shows a significant direct incremental relationship between initial tumor volume and tumor volume on Day 4 after starting treatment. IT-IC-treated mice (black line) had a significantly steeper slope compared to control mice (blue line) (p = 0.013), indicating an interaction effect of initial tumor volume on IT-IC treatment relative to control mice. Within the IT-IC treatment group, as initial tumor volume increases, the IT-IC antitumor treatment effect gradually disappears. This is demonstrated by all three lines “meeting” at the far right of the graph, as there is no difference in Day 4 tumor volumes between treatment groups at these large initial tumor volumes.
Initial tumor load shows an inhibitory interaction effect on Hu14.18-IL2 treatment and correlates with tumor growth. Four days after randomization and treatment initiation, A/J mice bearing subcutaneous NXS2 were killed for flow cytometric analysis of their tumors. a Shows individual mouse tumor volumes at the day of randomization (initial tumor volume (mm3)) plotted against Day 4 tumor volume. Linear regression models for each treatment group are shown. b shows Day 4 tumor volume data stratified into four quartiles based on initial tumor volume (separated by 33.9, 76.3, 130.9) as well as three treatment groups. All panels represent data from 11 independent experiments with an average of 48 mice total within each treatment group
The data presented as a bar graph in Fig. 5b illustrate decreasing treatment efficacy seen in tumors with increasing initial volumes. Mice were separated into quartiles based on their initial tumor volumes (for this experiment, cutoffs are at 33.86, 76.28, and 130.93 mm3). In the three lowest quartiles (0–130.9 mm3), IT-IC-treated mice had significantly less tumor at Day 4 compared to control mice, whereas there is no difference in the highest quartile (>130.93 mm3). These data indicate that the hu14.18 treatment had no impact on tumor growth when the initial tumors were large.
Early tumor response is predictive of final outcome
We wanted to determine whether the initial response to treatment observed 4 days post-treatment could identify the subset of mice that would be long-term, tumor-free survivors. For ease of biologic interpretation, a cutoff at 0 % tumor growth was used to distinguish growing tumors from shrinking tumors. We observed that all control mice showed tumor growth by 4 days post-treatment initiation and all of these mice eventually died of tumor-related death (Supplementary Figures 5A-B). We also observed that all hu14.18-IL2-treated mice that did not show any tumor shrinkage by 4 days post-treatment initiation eventually died of tumor-related death (Supplementary Figures 5A-B). IC-treated mice that did show tumor shrinkage by 4 days post-treatment initiation had an increased chance (44 %) of long-term, tumor-free survival (determined 70 days following treatment initiation) compared to IT-IC-treated mice that did not show tumor shrinkage by Day 4 (0 % survival) (Supplementary Figure 5A). These data demonstrate that the early antitumor effect of hu14.18-IL2 predicts greater likelihood for long-term overall survival.
Discussion
Initial small tumor load and enhanced TIL infiltration [10–12] have been noted as two independent prognostic factors for therapeutic benefit of immunotherapy and cancer survival. We showed previously that IV-IC was more effective against less-established tumors, possibly reflecting greater IC penetration and less local immune suppression [4]. This report shows that, within a group of genetically identical mice, all receiving identical tumor doses and IC treatment, mice that start treatment with lower initial tumor volume show increased tumor leukocyte infiltration, as well as improved outcome over the mice in that same treatment group that start treatment with larger tumors. When comparing IT-IC-treated mice whose tumors shrank against those whose tumors grew, our histology and flow cytometric data show a statistically significant increase in CD8+ cells and NK cells. Larger tumors had a significant increase in F4/80 macrophages. These cells could possibly be M2-type macrophages that are facilitating tumor growth and inhibiting the adaptive response. These data also suggest that CD8+ cells and NK cells were involved in the process of tumor shrinkage. These results are consistent with our prior results that show IT-IC treatment can be dramatically inhibited in this model by either NK or T cell depletion [13]. Results presented here show, for the first time to our knowledge, that initial small tumor volume correlates with increased rapid TIL infiltration in response to monoclonal antibody-based immunotherapy (in this case, hu14.18-IL2) within identically treated animals. This indicates that, in this model system, small tumor burden and increased TIL infiltration are two linked, rather than independent, factors that are predicative of a favorable in vivo antitumor response to immunotherapy.
We found that NKG2D expression on CD8+ cells and on NK cells inversely correlated with tumor growth, which suggested a role of NKG2D expression on NK cells and on CD8 cells in tumor shrinkage. Interestingly, in individual mice, the level of augmented NKG2D expression on activated NK cells was found to correlate directly with the level of augmented NKG2D on CD8+ cells. This suggests a shared relationship for the level of augmented NKG2D on NK and on CD8+ TILs, which also is involved in helping to inhibit tumor growth. Studies by Verneris et al. [19] show that ligation of NKG2D on NK cells directly induces cytotoxicity. They also showed that NKG2D expression is up-regulated upon IL-2 activation and expansion of CD8+ T cells. NKG2D triggering accounts for the majority of MHC-unrestricted cytotoxicity of activated and expanded CD8+ T cells [20].
Tumor shrinkage detected 4 days after starting treatment was predictive of increased long-term survival, while tumor progression detected 4 days after starting treatment was predictive of continued tumor progression and ultimate death due to progressive disease. As adaptive T cell responses normally take >5 days to develop, it seems unlikely that an adaptive T cell response can account for this shrinkage seen on Day 4 and this helps explain why the number of CD8+ T cells is similar in small and large tumors, and that the increase in T cells seen with treatment is similar in animals with both small and large tumors. It may be that a combination of innate immune responses, antibody-dependent cell-mediated cytotoxicity, and NKG2D-induced antitumor effects (by NK cells and CD8+ T cells) may account for the initial tumor shrinkage, which is essential (in this model) for resultant long-term tumor-free survival. This requirement for early tumor shrinkage in order to develop long-term benefit in this mouse model (where a neutralizing mouse antihuman antibody response limits treatment to a single course of therapy) is different from the slower detection of antitumor responses recently described for clinical immune benefit obtained with multiple courses of ipilimumab. As immune benefit with ipilimumab is mediated by an adaptive T cell response, occasional patients show tumor growth during and shortly after treatment, followed by late detection of antitumor effects (tumor shrinkage) [21, 22].
Data presented here indicate that the final response to tumor immunotherapy, either beneficial or ineffective, is determined (at least in part) by quantitative factors that might be manipulated to enhance antitumor efficacy or measured for prognostic purposes. Some of these quantitative factors are already being used in clinical decision making. Clinical stage of disease is a primary factor used to predict prognosis and help with treatment assignation. Based on these preclinical data, as well as our clinical Phase II data showing greater likelihood of response for patients with less “bulky disease” [6], we hypothesize that patients should be treated with IC (either IT-IC or IV-IC) when tumor volume is at a minimum, as larger tumor size interferes with the ability of IC treatment to mediate beneficial antitumor effects. Tumor growth patterns soon after IC treatment initiation may also be studied as a potential predictor of long-term benefit. If these murine studies translate to the clinical setting, patients with tumors that have a reduction in size within the first few days post-treatment initiation may have a greater possibility for long-term benefit. Soon after IC treatment initiation, tumor infiltration might be used as an indicator of treatment efficacy. Patients with high CD8+ T cell, high NKp46+ NK cell, and low M2 macrophage tumor-infiltrating populations after initial treatment may have a higher likelihood of experiencing a more potent antitumor effect. The NKG2D expression of NK cells and activated CD8+ T cells may also be indicative of the potential for a more potent IC-induced antitumor effect.
References
Gillies SD, Reilly EB, Lo KM, Reisfeld RA (1992) Antibody-targeted interleukin 2 stimulates T-cell killing of autologous tumor cells. Proc Natl Acad Sci USA 89(4):1428–1432
Lode HN, Xiang R, Varki NM, Dolman CS, Gillies SD, Reisfeld RA (1997) Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow. J Natl Cancer Inst 89(21):1586–1594
Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA (1998) Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood 91(5):1706–1715
Neal ZC, Yang JC, Rakhmilevich AL, Buhtoiarov IN, Lum HE, Imboden M, Hank JA, Lode HN, Reisfeld RA, Gillies SD, Sondel PM (2004) Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin Cancer Res 10(14):4839–4847
Osenga K, Hank J, Albertini M, Gan J, Sternberg A, Seeger R, Matthay K, Reynolds P, Krailo M, Adamson P, Reisfeld R, Gillies S, Sondel P (2006) A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the children’s oncology group. J Clin Cancer Res 12(6):1750–1759
Shusterman S, London WB, Gillies SD, Hank JA, Voss SD, Seeger RC, Reynolds CP, Kimball J, Albertini MR, Wagner B, Gan J, Eickhoff J, DeSantes KB, Cohn SL, Hecht T, Gadbaw B, Reisfeld RA, Maris JM, Sondel PM (2010) Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a children’s oncology group (COG) phase II study. J Clin Oncol 28(33):4969–4975
King DM, Albertini MR, Schalch H, Hank JA, Gan J, Surfus J, Mahvi D, Schiller JH, Warner T, Kim K, Eickhoff J, Kendra K, Reisfeld R, Gillies SD, Sondel P (2004) Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol 22(22):4463–4473
Ribas A, Kirkwood JM, Atkins MB, Whiteside TL, Gooding W, Kovar A, Gillies SD, Kashala O, Morse MA (2009) Phase I/II open-label study of the biologic effects of the interleukin-2 immunocytokine EMD 273063 (hu14.18-IL2) in patients with metastatic malignant melanoma. J Transl Med 7:68–78
Delgado DC, Hank JA, Kolesar J, Lorentzen D, Gan J, Seo S, Kim K, Shusterman S, Gillies SD, Reisfeld RA, Yang R, Gadbaw B, DeSantes KB, London WB, Seeger RC, Maris JM, Sondel PM (2010) Genotypes of NK cell KIR receptors, their ligands, and Fcγ receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res 70(23):9554–9561
Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW (2011) The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 105(1):93–103
Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS (2007) Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol 25(7):869–875
Horne ZD, Jack R, Gray ZT, Siegfried JM, Wilson DO, Yousem SA, Nason KS, Landreneau RJ, Luketich JD, Schuchert MJ (2011) Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res 171(1):1–5
Yang RK, Kalogriopoulos NA, Rakhmilevich AL, Ranheim EA, Seo S, Kim K, Alderson KL, Gan J, Reisfeld RA, Gillies SD, Hank JA, Sondel PM (2012) Intratumoral hu14.18-IL2 (IC) induces local and systemic antitumor effects that involve both activated T- and NK cells as well as enhanced IC retention. J Immunol 189(5):2656–2664
Johnson EE, Lum HD, Rakhmilevich AL, Schmidt BE, Furlong M, Buhtoiarov IN, Hank JA, Raubitschek A, Colcher D, Reisfeld RA, Gillies SD, Sondel PM (2008) Intratumoral immunocytokine treatment results in enhanced antitumor effects. Cancer Immunol Immunother 57(12):1891–1902
Zar JH (1984) Comparing simple linear regression equations. In: Zar JH (ed) Biostatistical analysis, 2nd edn. Prentice-Hall, Englewood Cliffs, pp 363–378
van Belle G, Fisher LD, Heagerty PJ, Lumley T (2004) Analysis of variance, 2nd edition biostatistics: a methodology for the health sciences. Wiley, Hoboken, pp 357–427
Vance RE, Jamieson AM, Cado D, Raulet DH (2002) Implications of CD94 deficiency and monoallelic NKG2A expression for natural killer cell development and repertoire formation. Proc Natl Acad Sci USA 99:868–873
McMahon CW, Zajac AJ, Jamieson AM, Corral L, Hammer GE, Ahmed R, Raulet DH (2002) Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8(+) T cells. J Immunol 169(3):1444–1452
Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS (2004) Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood 103(8):3065–3072
Karimi M, Cao TM, Baker JA, Verneris MR, Soares L, Negrin RS (2005) Silencing human NKG2D, DAP10, and DAP12 reduces cytotoxicity of activated CD8+ T cells and NK cells. J Immunol 175(12):7819–7828
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723
Saenger YM, Wolchok JD (2008) The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun 8:1–7
Acknowledgments
We thank Dr. Bartosz Grzywacz for helpful discussions. The humanized hu14.18-IL2 (APN301, Apeiron Biologics, Vienna, Austria) was supplied by the NCI Biologics Resources Branch (Frederick, MD) via a collaborative relationship with Merck KGaA (Darmstadt, Germany) and Apeiron Biologics. This work was supported by National Institutes of Health Grants CA032685, CA87025, CA166105, CA14520, GM067386, Department of Defense grant W81XWH-08-1-0559 and grants from the Midwest Athletes for Childhood Cancer Fund, the Crawdaddy Foundation, The Evan Dunbar Foundation, and the University of Wisconsin-Madison Institute for Clinical and Translational Research (ICTR) TL1 Training Grant 1TL1RR025013-01.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, R.K., Kalogriopoulos, N.A., Rakhmilevich, A.L. et al. Intratumoral treatment of smaller mouse neuroblastoma tumors with a recombinant protein consisting of IL-2 linked to the Hu14.18 antibody increases intratumoral CD8+ T and NK cells and improves survival. Cancer Immunol Immunother 62, 1303–1313 (2013). https://doi.org/10.1007/s00262-013-1430-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-013-1430-x