Abstract
B7-H3, a novel B7 family member, positively or negatively regulates T-cell responses. We investigated the clinical relevance and prognostic significance of B7-H3 in hepatocellular carcinoma (HCC). Western blotting showed B7-H3 upregulation in 17 of 24 (70.8 %) HCC tissues compared with nontumor liver tissues (p = 0.028). B7-H3 immunostaining on tissue microarrays containing 240 HCC patient samples indicated that 225 (93.8 %) tumors had aberrant B7-H3 expression, with strong intensity in 79 (32.9 %) cases, whereas B7-H3 expression in peritumor liver cells was weak in most cases (226; 94.2 %). Notably, patients with high/moderate tumor cell B7-H3 expression showed significantly poorer survival (p = 0.009) and increased recurrence (p = 0.002). After multivariable adjustment, high/moderate B7-H3 expression remained significant for an increased risk of recurrence (hazard ratio = 1.79; 95 % confidence interval = 1.19–2.70; p = 0.005). B7-H3 expression correlated with invasive phenotypes like vascular invasion and advanced tumor stage, and the metastatic potential of HCC cell lines. Flow cytometry showed that B7-H3 expression is inversely correlated with proliferation and interferon-γ production by infiltrating T cells. Interferon-γ stimulation significantly upregulated B7-H3 expression in HCC cells in vitro, implicating B7-H3 expression as a feedback mechanism to evade anti-tumor immunity. Importantly, the prognostic value of B7-H3 expression was validated in an independent cohort of 206 HCC patients. Collectively, our data suggest that B7-H3 was abundantly expressed in HCC and was associated with adverse clinicopathologic features and poor outcome. Thus, B7-H3 represents an attractive target for diagnostic and therapeutic manipulation in human HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third most common cause of cancer death worldwide [1]. Most patients present with disease too advanced for curative treatment by resection, transplantation, or local ablation. Although a survival advantage for the multitargeted kinase inhibitor sorafenib in patients with advanced HCC has been suggested, this effect is modest [2]. No other systemic therapy has shown a survival benefit, and recurrence is common, even for curative treatment, highlighting the need for identification of prognostic biomarkers and new therapeutic strategies.
Accumulating evidence suggests that immunotherapy may become a potent therapeutic option for patients with HCC [3]. Stimulating T-cell activation or eliminating inhibitory T-cell signaling to enforce antitumor responses is a prerequisite for immune-based therapies to eradicate human cancers. It is evident that T-cell priming, activation, and tolerance are ultimately governed by costimulatory and coinhibitory signals, in which the B7/CD28 family of coregulatory molecules plays an indispensable role [4]. The prototype ligands of this family, B7-1 and B7-2, have well-defined roles as essential costimulatory molecules involved in T-cell activation. Within the past decade, new members of the B7 family (B7-H1, PD-L2, B7-H3, and B7-H4) have been identified; however, their exact roles in tumor immunity remain unclear. We and others have reported that several of these B7-H molecules (e.g., B7-H1 and B7-H4) are expressed in human cancers and are implicated as coregulatory inhibitors that may induce T-cell anergy or apoptosis upon antigen recognition [5–7].
B7-H3 was discovered in 2001 and was initially identified as a costimulatory molecule on T cells [8]. However, the precise function of B7-H3 remains debatable [9]. Both stimulatory properties, such as promotion of T-cell proliferation and interferon (IFN)-γ production [8, 10], along with inhibitory properties, including impairment of Th1 responses and protection from natural killer cell-mediated lysis [11], have been described. Studies regarding the clinical significance of B7-H3 in human cancers have suggested that higher B7-H3 expression indicates increased risk of recurrence in some cancers [12–14], or paradoxically, correlates with better postoperative survival in others [15, 16], or has no clinical significance [17]. Although expression of B7-H3 in human cancers appears to be a fairly general phenomenon, the literature is currently devoid of clinical observations of B7-H3 in human HCC.
Thus, we investigated the prognostic significance and clinical relevance of B7-H3 expression in a cohort of 240 HCC patients treated with curative resection. We found that tumor cell instead of tumor vasculature expression of B7-H3 was significantly associated with an increased risk of recurrence and aggressive clinicopathologic features. The results were further validated in an independent cohort of 206 HCC patients.
Materials and methods
Cell lines
Eight human HCC cell lines were used, including HepG2, Hep3B, PLC/PRE/5, SMCC-7721, MHCC97L, MHCC97H, HCCLM3, and HCCLM6. The latter four HCC cell lines were established at our institute, all from the same parental cell line MHCC97, with stepwise pulmonary metastatic potential (MHCC97L < MHCC97H < HCCLM3 < HCCLM6) as reported elsewhere [18, 19]. Cell lines were all maintained in Dulbecco’s modified Eagle’s Medium supplemented with 10 % fetal bovine serum and 1 % penicillin-streptomycin at 37 °C with 5 % CO2.
Patient cohort and clinical follow-up
Archived tissues were obtained for a cohort of 240 patients who received curative resection of HCC at Liver Cancer Institute, Zhongshan Hospital of Fudan University between 2002 and 2006 [7, 20]. Patients neither showed signs of distant metastasis nor received anticancer therapy before surgery. Follow-up procedures and postoperative treatments according to uniform guidelines were described previously [21]. Conventional clinicopathologic variables are detailed in Supplementary Table S1. The tumor stage was determined according to the 2002 AJCC/UICC tumor-node-metastasis (TNM) classification system [22]. Tumor differentiation was graded using the Edmondson grading system. Liver function was assigned by the Child-Pugh scoring system. Data were censored at the last follow-up for patients without recurrence or death. Time to recurrence or overall survival (OS) was defined as the interval between the time of surgery to that of recurrence or death, respectively.
The median follow-up period was 39.0 months (range, 1.5–95.0 months; standard deviation [SD], 22.7 months). At the last follow-up (March 31st, 2010), 127 (52.9 %) patients were confirmed to have relapsed, including 89 with intrahepatic recurrence, 17 with extrahepatic metastasis, and 21 with both events. A total of 122 (50.8 %) patients had died, including 52 due to liver failure or bleeding from the gastrointestinal tract and the remaining 70 cases due to tumor recurrence.
Fresh tissues from an additional series of 24 HCC patients who underwent resection in 2010 were used for isolation of tumor-infiltrating lymphocytes (TILs) and for flow cytometry and Western blot analyses. Among these 24 patients, 12, 7, and 5 were classified as TNM stage I, II, and IIIA, respectively. Informed consent was obtained, and the study was approved by the Zhongshan Hospital Research Ethics Committee.
Tissue microarray and immunohistochemistry
Tissue microarrays were performed as described previously [7, 20]. Briefly, representative areas, away from necrotic and hemorrhagic materials, were premarked in the paraffin-embedded blocks by hematoxylin and eosin staining. Triplicates of 1-mm-diameter cylinders from the center of the tumor and noncancerous liver tissue, along with samples from different controls (spleen, lymph node, artery, and glioma), were included in each case to ensure reproducibility and homogeneity. Serial sections (4-μm thick) were placed on slides coated with 3-aminopropyltriethoxysilane.
Immunohistochemistry was performed using a two-step protocol (Novocastra) as previously described [7, 20]. Briefly, sections were dewaxed, hydrated, and washed. After neutralization of endogenous peroxidase (0.3 % H2O2 for 20 min) and antigen retrieval (121 °C for 4 min), slides were preincubated with blocking serum and then incubated at 4 °C overnight with each primary antibody. The primary antibodies were as follows: rabbit polyclonal anti-human 4Ig-B7-H3 (Sigma), mouse monoclonal anti-human CD4 (Novocastra), CD8 (Novocastra), and FOXP3 (Abcam). Subsequently, the sections were serially rinsed, incubated with secondary antibodies, and treated with horseradish peroxidase-conjugated streptavidin. Reaction products were visualized using 3,3′-diaminobenzidine tetrahydrochloride and counterstained with hematoxylin. All tissue microarray stainings, including negative-staining controls using phosphate-buffered saline in place of the primary antibodies, were performed in a single experiment.
Evaluation of immunohistochemical staining
Microarrays were evaluated using light microscopy by two investigators blinded to the clinicopathologic data of the patients. Sections were considered to be positive when tumor cells showed cytoplasmic or membranous B7-H3 immunostaining. The B7-H3 immunostaining intensities were scored as follows: 0, negative; 1, weak; 2, moderate; 3, strong [14]. A negative grade represented no tumor cells showing positive immunostaining. For analysis, the B7-H3 immunostaining intensities were classified as follows: the sections scored as 0 and 1 were defined as the low expression group, and sections scored as 2 and 3 were defined as the high expression group. In addition, B7-H3 expression in the tumor vasculature and the vasculature of adjacent nontumor liver tissue was evaluated and recorded as being absent, focal, moderate, or diffuse in nature [12].
Regarding the TIL densities, the entire 1-mm-diameter core was counted manually under ×200 magnification. The results were expressed as the mean ± standard error (SE) of the number of cells/1-mm core for each triplicate.
Western blot analysis
Twenty-four cases with paired tumor and peritumoral liver tissues (five cases with venous tumor thrombi), as well as eight human HCC cell lines were analyzed, including six normal liver tissues from patients with benign hepatic tumors as controls. Western blot analysis was performed as described previously [7, 20] with rabbit polyclonal anti-human 4Ig-B7-H3 (Sigma) and β-actin (Cell Signaling Technology) as an internal loading control.
TIL isolation and flow cytometry
TILs were isolated as described previously [23]. The following antibodies were used: fluorescein isothiocyanate-conjugated anti-CD4, allophycocyanin-conjugated anti-CD8, and R-phycoerythrin-conjugated anti-Ki-67, anti-IFN-γ, and anti-interleukin (IL)-4 (BD Pharmingen). Cells were stimulated for 5 h with Leukocyte Activation Cocktail (BD Pharmingen). Cells were stained with surface markers, fixed and permeabilized with IntraPre Reagent (Beckman Coulter), and finally stained. A portion of each specimen was stained with isotype-matched fluorescent control monoclonal antibodies. Data were acquired on a FACSVantage SE instrument and analyzed with CellQuest software (BD Biosciences).
Induction of B7-H3 in cultured HCC cells and flow cytometry analysis
To determine the effects of IFN-γ, IL-4, and transforming growth factor (TGF)-β1 on expression of B7-H3 in cultured HCC cells, HCCLM3 and HepG2 cells were seeded in 6-well plates (100,000 cells per well) and incubated with 40 ng/ml of recombinant IFN-γ, IL-4, and TGF-β1 (all from PeproTech). After 24 and 48 h of stimulation, cells were harvested and stained with mouse monoclonal anti-human B7-H3-PE (Biolegend) or control mouse monoclonal anti-human IgG-PE (Biolegend). Single-color flow cytometry was analyzed with FlowJo Software (TreeStar).
Independent validation
For further validation, we evaluated B7-H3 expression in another set of tissue microarrays containing a consecutive cohort of 206 HCC patients who underwent liver transplantation between 2001 and 2006 at Liver Cancer Institute, Zhongshan Hospital of Fudan University. Immunohistochemistry, quantification of markers, and statistics were conducted in the same manner. Clinicopathologic characteristics are described in Supplementary Table S1. The median duration of follow-up for this cohort was 48.0 months (range, 3.0–113.5 months; SD, 27.8 months). Of the 206 patients examined, 96 (46.6 %) died before the end of the observation period, and 93 (45.1 %) were diagnosed with tumor recurrence.
Statistics
All statistical analyses were performed using SPSS 15.0 for Windows (SPSS, Inc.). Pearson’s χ2 test and Fisher’s exact test were applied to compare qualitative variables, and quantitative variables were analyzed using Student’s t test or Spearman’s ρ rank correlation coefficient determination. Univariate analysis was calculated by the Kaplan–Meier method (the log-rank test). Multivariate analysis was done using the Cox multivariate proportional hazards regression model in a stepwise manner (backward, conditional). The model included all clinicopathologic variables found to have significant prognostic value in univariate analysis. A two-tailed p < 0.05 was deemed to indicate statistical significance.
Results
B7-H3 is elevated in HCC tissue
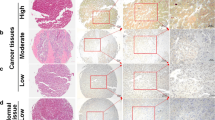
Representative immunostaining images of B7-H3 are presented in Fig. 1. We found that 225 (93.8 %) HCC cases had positive tumor cell B7-H3 expression, with various intensities. A total of 57 (23.8 %) cases showed weak tumor cell B7-H3 intensity, 89 (37.1 %) showed moderate intensity, and 79 (32.9 %) showed marked intensity. In addition, tumor vasculature expression of B7-H3 was apparent in 86.2 % of the tumors. There were 43 (17.9 %), 80 (33.3 %), and 84 (35.0 %) specimens with focal, moderate, and diffuse tumor vasculature B7-H3 expression, respectively. By contrast, peritumor liver cell B7-H3 expression was weak in most cases (226; 94.2 %), with 14 (5.8 %) cases showing moderate intensity and no cases showing marked B7-H3 intensity. However, peritumor liver sinusoid endothelial cells and resident Kupffer cells showed constitutively strong B7-H3 expression. These results were different from the findings in renal cell carcinoma where 360 out of 378 nontumor renal tissues (95.2 %) showed no vasculature B7-H3 expression [12].
Representative immunostaining for B7-H3 expression in HCC and peritumor liver tissue. Peritumor liver cells were unstained (a 1 ) or weakly stained (a 2 ) with anti-4Ig-B7–H3 Ab, with limited cases of moderate (b 2 ) staining, whereas liver sinusoid endothelial cells and Kupffer cells (red arrow) show constitutive positive staining. HCC cells show weak (b 1 ), moderate (b 2 ), or strong (b 3 ) staining. Focal (c 1 ), moderate (c 2 ), or diffuse (c 3 ) tumor vasculature staining is observed. Specific cases show strong staining in both tumor cells and tumor vessels (d 1 ), in venous tumor thrombi (d 2 ; green arrow), or in intrahepatic spreading nodules (d 2 ). Magnification, 400×
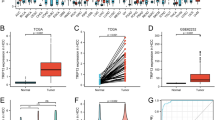
To extend our observations, we tested 24 fresh HCC tissues and 8 human HCC cell lines for B7-H3 expression using Western blot analysis (Fig. 2a). Consistent with our observations in paraffin-embedded HCC tissues, all these HCC tissues and cell lines exhibited expression of B7-H3. In cell lines with a similar genetic background (MHCC97L, MHCC97H, HCCLM3, and HCCLM6), the least aggressive, MHCC97L, displayed the lowest level of B7-H3, whereas the most aggressive, HCCLM3 and HCCLM6, showed the highest levels of B7-H3. In 24 paired samples, B7-H3 was obviously upregulated in 17 of 24 (70.8 %) HCC tissues (Fig. 2b) compared with peritumoral tissues (p = 0.028; Fig. 2c), although peritumor liver sinusoid endothelial cells and Kupffer cells constitutively expressed B7-H3. Specifically, in five cases with macroscopic tumor thrombi, the levels of B7-H3 showed a successive increase from peritumor to tumor tissue, and to the corresponding tumor thrombi (Fig. 2d).
Western blot analysis of B7-H3 expression in HCC tissues and cell lines. a Western blot detection of 4Ig-B7-H3 protein in 24 paired tumors (T) and peritumoral tissues (pT) (including five cases with tumor thrombi [TT]), eight HCC cells lines, and six normal liver tissues (N). β-Actin served as a loading control. b The bar graph illustrates the ratio of the B7-H3 protein level (T/pT). Seventeen of 24 tumors (70.8 %) display upregulated B7-H3 protein levels, compared with the corresponding peritumoral liver tissues. c The B7-H3 protein level obtained by densitometric scanning is significantly upregulated in tumors compared with peritumoral tissues. d B7-H3 expression shows a sequential increase from peritumor tissues to tumor tissues, and to the corresponding tumor thrombi
B7-H3 expression inversely correlated with T-cell proliferation, but showed no association with infiltrating T-cell number
We examined the relationship between tumor B7-H3 expression levels and the functional status of tumor-infiltrating T cells. According to Western blot results, the 24 HCC cases were divided into two groups: B7-H3 high (high B7-H3 expression) and B7-H3 low (low B7-H3 expression) groups. Flow cytometry indicated that the B7-H3 high group was associated with a significantly low proliferation rate and IFN-γ production by CD4+ and CD8+ TILs, whereas IL-4 production of CD4+ TILs did not differ significantly between the two groups (Fig. 3).
Flow cytometry analysis of proliferation and cytokine production by tumor-infiltrating lymphocytes (TILs). TILs were isolated from 24 fresh HCC tissues. The 24 HCC cases were dichotomized into B7-H3 high and B7-H3 low groups, according to the median level of B7-H3 expression as determined by western blot assays. The percentage of Ki-67+ (a) and IFN-γ+ (b) cells in CD4+ and CD8+ T cells, as well as the frequency of IL-4+CD4+ T cells (c) are shown. The gates were based on isotype-matched negative controls. The data shown are representative dot plots from more than five independent experiments. d shows the statistical analysis of these samples
We further investigated whether tumor B7-H3 expression correlated with the density of tumor-infiltrating T cells. Tissue microarrays containing 240 HCC cases were immunostained for CD4+, CD8+, and FOXP3+ T cells (Supplementary Fig. S1A). No significant difference was found in the density of CD8+ T cells (p = 0.95) between B7-H3 high and B7-H3 low tumors (Supplementary Fig. S1B). Although B7-H3 high HCC contained relatively high numbers of CD4+ T cells (p = 0.065) and FOXP3+ regulatory T cells (p = 0.082) compared with B7-H3 low HCC, the differences did not reach statistical significance (Supplementary Fig. S1B).
IFN-γ markedly increased B7-H3 expression in HCC cells
Treatment with 40 ng/ml recombinant IFN-γ (Supplementary Fig. S2A) markedly increased the expression of B7-H3 in HCCLM3 (1.97-fold) and HepG2 cells (2.17-fold) after 24 h. Additionally, in HCCLM3 cells, the effect induced by IFN-γ treatment almost disappeared after 48 h (1.12-fold), whereas in HepG2 cells, IFN-γ treatment resulted in a further increase in B7-H3 expression at 48 h (2.84-fold) compared with that at 24 h. By contrast, IL-4 or TGF-β1 treatment induced no significant change in B7-H3 expression in HCCLM3 and HepG2 cells after 24 or 48 h (Supplementary Fig. S2B and C).
Tumor B7-H3 expression and prognosis
Comparisons of clinicopathologic features by B7-H3 expression in tumor cells are summarized in Table 1. B7-H3 expression in tumor cells was significantly associated with the presence of aggressive features, including a higher α-fetoprotein level, advanced TNM stage, poor differentiation, the presence of liver cirrhosis, and vascular invasion. Univariate analysis revealed that patients with tumors with high B7-H3 expression were more likely to experience cancer recurrence (p = 0.002) and death (p = 0.009) compared with patients with tumors with low B7-H3 expression (Table 2). The rates of recurrence-free survival and OS (in parentheses) at 1, 3, and 5 years following resection were 79 % (85 %), 61 % (68 %), and 53 % (58 %) for patients with tumors with low B7-H3 expression compared with 60 % (79 %), 40 % (49 %), and 35 % (41 %) for those in the B7-H3 high group, respectively (Fig. 4a, b).
Kaplan–Meier curves of survival differences among HCC patients. Recurrence-free and overall survival for B7-H3 expression in tumor cells (a, b), instead of B7-H3 expression in tumor vessels (c, d), are statistically significant (log-rank test). Significant differences in recurrence-free and overall survival are validated in an independent cohort according to tumor cell B7-H3 expression (e, f). Cores of one case were detached from the tissue microarray section during immunostaining in the validation cohort
The associations of tumor B7-H3 intensity with patient outcome after adjusting for clinicopathologic features are shown in Table 2. High tumor B7-H3 intensity was significantly associated with an adverse outcome even after multivariate adjustment: patients in the B7-H3 high group were about twice as likely to suffer from tumor recurrence (hazard ratio [HR], 1.79; 95 % confidence interval [CI], 1.19–2.70; p = 0.005) compared with those in the B7-H3 low group.
B7-H3 expression in the tumor vasculature has been shown to be associated with adverse clinical outcome in patients with renal cancer [12] and ovarian carcinoma [24]. However, in the present study, moderate or diffuse B7-H3 expression in the tumor vasculature was not significantly associated with an increased risk of recurrence or death from HCC compared with patients with no B7-H3 expression or focal B7-H3 expression in the tumor vasculature (Table 2; Fig. 4c, d).
Independent validation
The prognostic ability of the B7-H3 expression profile in tumor cells was validated in an independent dataset with multivariate analysis (Supplementary Table S2). The 1-, 3-, and 5-year recurrence-free survival and OS (in parentheses) rates were 79 % (87 %), 73 % (71 %), and 67 % (66 %) for patients with lower B7-H3 expression, compared with 68 % (75 %), 50 % (51 %), and 47 % (47 %) for patients with higher B7-H3 expression, respectively (Fig. 4e, f). Notably, tumor B7-H3 expression allowed us to subclassify TNM stage II patients, with the median recurrence-free survival and OS (in parentheses) times of 96.0 (84.0) versus 84.0 (54.0) months for patients with low versus high expression levels, respectively (p = 0.018, p = 0.026, respectively; log-rank test).
Discussion
We showed that expression of the T-cell coregulatory ligand B7-H3 was low in normal liver parenchyma cells and increased significantly in HCC cells. High B7-H3 staining was observed in a subset of 70 % of tumor specimens and was significantly associated with multiple adverse prognostic features of HCC, including a higher α-fetoprotein level, advanced TNM stage, poor differentiation, liver cirrhosis, and vascular invasion. More importantly, after multivariate adjustment for conventional prognostic factors, high B7-H3 expression persisted as a significant predictor of tumor recurrence following curative resection. The results were further validated in an independent cohort of HCC patients who underwent liver transplantation. Thus, B7-H3 may prove useful for clinical evaluation of HCC patients who undergo curative surgical treatment for their cancer, particularly to identify high-risk patients who are at increased risk of cancer progression.
Substantive research has been performed regarding the role of B7-H3 in tumor immunity; however, the pathophysiologic function of B7-H3 has yet to be fully elucidated. Both negative and positive immunologic functions for B7-H3 have been reported. Although, in the present study, we have suggested a detrimental role for B7-H3 in HCC patients, in murine synergistic HCC models, intratumoral administration of a B7-H3-expressing plasmid and arsenic trioxide or a vasostatin-expressing plasmid synergized to completely eradicate established tumors [25, 26]. In the mouse HCC models, B7-H3 therapy activated CD8+ and natural killer cells and increased their infiltration into tumors [25, 26]. Likewise, opposing roles regarding B7-H3 in mice experiments versus human clinical settings have also been recorded in colon cancer [27, 28]. The contrasting roles of B7-H3 can likely be attributed to multiple receptors on different cells. Indeed, a costimulatory receptor for mouse B7-H3 that preferentially enhances mouse CD8+ T-cell activation (TREM-like transcript 2) has been identified recently [10]. However, Leitner and colleagues found no evidence for such an interaction between TREM-like transcript 2 and B7-H3 in both mice and humans [29], and to date, cognate coinhibiting receptors for B7-H3 have not been elucidated.
Additionally, earlier observations of human gastric [16] and pancreatic carcinoma [15] have implicated that B7-H3-positive tumor cells are associated with improved patient survival, whereas subsequent studies in the same cancers yielded opposite results [30, 31]. More recently, B7-H3 has been overwhelmingly implicated as a potent inhibitor of T-cell activity. For example, in neuroblastoma [32], breast [14], lung [33], and prostate [6, 34] cancers, patients with strong B7-H3 expression are more likely to show disease spread. Additionally, the B7-H3-positive vasculature of ovarian carcinoma implies increased recurrence and reduced survival [24]. Furthermore, either B7-H3 expression in tumor cells or diffuse B7-H3 expression in the tumor vasculature significantly predicts an increased risk of death from renal cell carcinoma [12]. Evidence from mechanistic studies has revealed that both mouse and human B7-H3 inhibit T-cell activation and production of effector cytokines such as IFN-γ through regulation of NFTA, NF-κB, and AP-1, three major signaling pathways through which the T-cell receptor regulates gene transcription [8, 10]. Based on the strong correlation between B7-H3 intensity and adverse clinicopathologic features of human HCC, we surmise that B7-H3 functions as an inhibitor of T cell-mediated immunity that promotes aggressive clinical behavior of HCC. Supporting our hypothesis, microRNA miR-29, a newly identified regulator of B7-H3 protein expression that decreases B7-H3 translation [35], is markedly downregulated in HCC and plays an important role in apoptosis and molecular etiology of HCC [36]. Besides HCC, downregulation of miR-29 was reported in neuroblastoma [35], ovarian carcinoma [37], and other malignancies [38, 39], which may lead to feedback upregulation of B7-H3 and consequent immune evasion in these tumors.
To further provide insight into the clinical significance of B7-H3 in HCC, we performed Western blotting on 24 pairs of tumor and peritumor liver tissues, including five cases with tumor thrombi. Protein expression of B7-H3 was significantly higher in tumors than in peritumor liver tissues, with the most prominent expression detected in the corresponding tumor thrombi. This result was consistent with the strong staining of B7-H3 in intrahepatic metastases and venous tumor thrombosis of HCC by immunhistochemistry (Fig. 1d). Furthermore, although the numbers of CD4+, CD8+, and FOXP3+ TILs showed no significant difference between B7-H3 high and B7-H3 low tumors, flow cytometry results indicated that B7-H3 high tumors were associated with reduced proliferation and IFN-γ production by TILs. Therefore, tumor cells spreading regionally in the peritumor liver and systemically in the peripheral circulation that express B7-H3 may have a significant survival advantage. Conversely, the presence of IFN-γ could significantly upregulate B7-H3 expression in HCC cells, suggesting that B7-H3 may act as a feedback mechanism to evade anti-tumor immunity. We envision a mechanistic role for B7-H3 in promoting HCC progression, based on its potential to impair T cell-mediated immunity.
In addition to cancer cells, B7-H3 is highly expressed in endothelial cells of the HCC tumor vasculature and is constitutively expressed in sinusoid endothelial cells of normal liver tissue. However, our present study failed to reveal any significant prognostic value of B7-H3 expression in the tumor vasculature of HCC. This does not exclude the biological significance of B7-H3 expression in tumor vasculature of HCC. Conversely, the question of pre-empting toxicity to the normal liver sinusoid endothelial cells has to be addressed in future B7-H3-targeted therapeutic strategies.
The main limitation of the present study was its retrospective nature. To compensate for this limitation, we validated the prognostic value of B7-H3 in an independent cohort of 206 HCC patients who underwent liver transplantation. Similar to hepatectomy, liver transplantation is a curative option that simultaneously removes both HCC and the diseased liver. However, as with all initial series that propose the prognostic significance of molecular biomarkers, our current findings still require independent external validation.
In conclusion, the current study indicates that B7-H3 is highly expressed in human HCC and is associated with multiple aggressive tumor features. Particularly, B7-H3 serves as an independent prognostic factor for recurrence in HCC patients who receive curative treatments like tumor resection or liver transplantation. Given the proposed immune inhibitory mechanisms of B7-H3, B7-H3 may not only help to predict which patients are at a higher risk of recurrence, thus facilitating patient selection for more aggressive treatment, but also may represent an attractive target for therapeutic manipulation in the future for multimodal management of HCC.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- TNM:
-
Tumor-node-metastasis
- SD:
-
Standard deviation
- OS:
-
Overall survival
- TIL:
-
Tumor-infiltrating lymphocytes
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Jemal A, Bray F, Center M, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, de Oliveira AC, Santoro A, Raoul J-L, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz J-F, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, The SISG (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390
Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH (2009) A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 49(1):124–132
Zou W, Chen L (2008) Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 8(6):467–477
Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W (2006) B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med 203(4):871–881
Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, Scardino PT, Sharma P, Allison JP (2007) B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A 104(49):19458–19463
Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J (2009) Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 15(3):971–979
Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L (2001) B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol 2(3):269–274
Hofmeyer KA, Ray A, Zang X (2008) The contrasting role of B7-H3. Proc Natl Acad Sci U S A 105(30):10277–10278
Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M (2008) Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci U S A 105(30):10495–10500
Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, Wakeham A, Itie A, Chung S, Da Costa J, Arya S, Horan T, Campbell P, Gaida K, Ohashi PS, Watts TH, Yoshinaga SK, Bray MR, Jordana M, Mak TW (2003) The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol 4(9):899–906
Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, Thompson RH, Boorjian SA, Dong H, Leibovich BC, Blute ML, Kwon ED (2008) Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res 14(16):5150–5157
Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML, Cheville JC, Sebo TJ, Kwon ED (2007) B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res 67(16):7893–7900
Arigami T, Narita N, Mizuno R, Nguyen L, Ye X, Chung A, Giuliano AE, Hoon DS (2010) B7-h3 ligand expression by primary breast cancer and associated with regional nodal metastasis. Ann Surg 252(6):1044–1051
Loos M, Hedderich DM, Ottenhausen M, Giese NA, Laschinger M, Esposito I, Kleeff J, Friess H (2009) Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer 9:463
Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, Zhao JM, Zhang GB, Zhang XG (2006) Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol 12(3):457–459
Quandt D, Fiedler E, Boettcher D, Marsch W, Seliger B (2011) B7-H4 expression in human melanoma: its association with patients’ survival and anti tumor immune response. Clin Cancer Res 17(10):3100–3111
Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue Q, Gao DM, Bao WH (2001) Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J Gastroenterol 7(5):630–636
Li Y, Tang Y, Ye L, Liu B, Liu K, Chen J, Xue Q (2003) Establishment of a hepatocellular carcinoma cell line with unique metastatic characteristics through in vivo selection and screening for metastasis-related genes through cDNA microarray. J Cancer Res Clin Oncol 129(1):43–51
Xu YF, Yi Y, Qiu SJ, Gao Q, Li YW, Dai CX, Cai MY, Ju MJ, Zhou J, Zhang BH, Fan J (2010) PEBP1 downregulation is associated to poor prognosis in HCC related to hepatitis B infection. J Hepatol 53(5):872–879
Sun HC, Zhang W, Qin LX, Zhang BH, Ye QH, Wang L, Ren N, Zhuang PY, Zhu XD, Fan J, Tang ZY (2007) Positive serum hepatitis B e antigen is associated with higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B-related hepatocellular carcinoma. J Hepatol 47(5):684–690
Greene FL, Page DL, Fleming ID et al (2002) AJCC cancer staging manual, 6th edn. Springer, Chicago, pp 131–144
Unitt E, Rushbrook SM, Marshall A, Davies S, Gibbs P, Morris LS, Coleman N, Alexander GJ (2005) Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology 41(4):722–730
Zang X, Sullivan P, Soslow R, Waitz R, Reuter V, Wilton A, Thaler H, Arul M, Slovin S, Wei J (2010) Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol 23(8):1104–1112
Luo L, Qiao H, Meng F, Dong X, Zhou B, Jiang H, Kanwar J, Krissansen G, Sun X (2006) Arsenic trioxide synergizes with B7H3-mediated immunotherapy to eradicate hepatocellular carcinomas. Int J Cancer 118(7):1823–1830
Ma L, Luo L, Qiao H, Dong X, Pan S, Jiang H, Krissansen GW, Sun X (2007) Complete eradication of hepatocellular carcinomas by combined vasostatin gene therapy and B7H3-mediated immunotherapy. J Hepatol 46(1):98–106
Lupu CM, Eisenbach C, Lupu AD, Kuefner MA, Hoyler B, Stremmel W, Encke J (2007) Adenoviral B7-H3 therapy induces tumor specific immune responses and reduces secondary metastasis in a murine model of colon cancer. Oncol Rep 18(3):745–748
Sun J, Chen L, Zhang G, Jiang J, Zhu M, Tan Y, Wang H, Lu B, Zhang X (2010) Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer Immunol Immunother 59(8):1163–1171
Leitner J, Klauser C, Pickl WF, Stockl J, Majdic O, Bardet AF, Kreil DP, Dong C, Yamazaki T, Zlabinger G, Pfistershammer K, Steinberger P (2009) B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur J Immunol 39(7):1754–1764
Yamato I, Sho M, Nomi T, Akahori T, Shimada K, Hotta K, Kanehiro H, Konishi N, Yagita H, Nakajima Y (2009) Clinical importance of B7-H3 expression in human pancreatic cancer. Br J Cancer 101(10):1709–1716
Arigami T, Uenosono Y, Hirata M, Yanagita S, Ishigami S, Natsugoe S (2011) B7-H3 expression in gastric cancer: a novel molecular blood marker for detecting circulating tumor cells. Cancer Sci 102(5):1019–1024
Gregorio A, Corrias MV, Castriconi R, Dondero A, Mosconi M, Gambini C, Moretta A, Moretta L, Bottino C (2008) Small round blue cell tumours: diagnostic and prognostic usefulness of the expression of B7-H3 surface molecule. Histopathology 53(1):73–80
Zhang G, Xu Y, Lu X, Huang H, Zhou Y, Lu B, Zhang X (2009) Diagnosis value of serum B7-H3 expression in non-small cell lung cancer. Lung Cancer 66(2):245–249
Chavin G, Sheinin Y, Crispen PL, Boorjian SA, Roth TJ, Rangel L, Blute ML, Sebo TJ, Tindall DJ, Kwon ED, Karnes RJ (2009) Expression of immunosuppresive B7-H3 ligand by hormone-treated prostate cancer tumors and metastases. Clin Cancer Res 15(6):2174–2180
Xu H, Cheung IY, Guo HF, Cheung NK (2009) MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res 69(15):6275–6281
Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y, Jia WH, Zhuang SM (2010) Effects of MicroRNA‐29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 51(3):836–845
Flavin R, Smyth P, Barrett C, Russell S, Wen H, Wei J, Laios A, O’Toole S, Ring M, Denning K, Li J, Aherne S, Sammarae D, Aziz NA, Alhadi A, Finn SP, Loda M, Sheils O, O’Leary JJ (2009) miR-29b expression is associated with disease-free survival in patients with ovarian serous carcinoma. Int J Gynecol Cancer 19(4):641–647
Sengupta S, den Boon JA, Chen I-H, Newton MA, Stanhope SA, Cheng Y-J, Chen C-J, Hildesheim A, Sugden B, Ahlquist P (2008) MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci U S A 105(15):5874–5878
Garzon R, Heaphy CE, Havelange V, Fabbri M, Volinia S, Tsao T, Zanesi N, Kornblau SM, Marcucci G, Calin GA, Andreeff M, Croce CM (2009) MicroRNA 29b functions in acute myeloid leukemia. Blood 114(26):5331–5341
Acknowledgments
This study was supported by the Major Program of NSFC (No.81030038), National Key Sci-Tech Project (2008ZX10002-019), Shanghai Rising-Star Program (No. 10QA1401300), National Natural Science Foundation of China (No. 30872379 & No. 30901432), and “Chen Guang” project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (No. 11CG02).
Conflict of interest
The authors declare no competing interests to this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tai-Wei Sun, Qiang Gao, and Shuang-Jian Qiu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, TW., Gao, Q., Qiu, SJ. et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol Immunother 61, 2171–2182 (2012). https://doi.org/10.1007/s00262-012-1278-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-012-1278-5