Abstract
Vx-001, an HLA-A*0201 restricted telomerase (TERT)-specific anti-tumor vaccine, is composed of the 9-mer cryptic TERT572 peptide and its optimized variant TERT572Y. We have previously shown that Vx-001 is non-toxic, highly immunogenic and in vaccinated NSCLC patients early specific immune response is associated with prolonged survival. The aim of the present study was to investigate the specific T-cell immune response against Vx-001. Fifty-five patients with chemo-resistant advanced solid tumors were vaccinated with TERT572Y (2 subcutaneous injections) followed by TERT572 peptide (4 subcutaneous injections) every 3 weeks. Specific immune response was evaluated by IFN-γ and perforin ELISpot and intracellular cytokine staining assays. TERT-reactive T cells were detected in 27 (51%) out of 53 evaluable patients after the 2nd vaccination and in 22 (69%) out of 32 evaluable patients after the completion of 6 vaccinations. Immune responses developed irrespective of the stage of disease and disease status before vaccination. Patients with disease progression at study entry who developed a post-vaccination-induced immunological response had a significant overall survival benefit compared to the post-vaccination non-responders. The Vx-001 vaccine is a promising candidate for cancer immunotherapy since it can induce a TERT-specific T-cell immune response that is associated with prolonged survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer immunotherapy is based on the protective role of the immune system against cancer mainly via the capacity of the CD8+ cytotoxic T lymphocytes (CTLs) to recognize and kill cancer cells [1, 2]. CTLs, when recognizing tumoral antigenic peptides on the surface of tumor cells in association with major histocompatibility complex (MHC) class I molecules, may become activated and can lyse the cancer cells expressing these antigens [3].
The human telomerase is a complex of the telomerase RNA subunit (TERC) and the telomerase reverse transcriptase (TERT), which is correlated with telomerase activity. Telomerase adds telomeric repeats to the chromosome ends and therefore prevents the replication-dependent loss of telomere and cellular senescence in highly dividing cells such as tumor cells [4]. TERT is highly expressed in more than 85% of human cancers whereas it is also detected in some rare normal cells and tissues such as hematopoietic precursors, activated B and T cells and germinal B cells [5]. TERT plays a role in the development of human cancer [6], and its inhibition in tumor cells has been shown to lead to growth arrest in vitro [7]. For these reasons, TERT can be considered as an appealing universal tumor antigen.
A major problem of cancer immunotherapy is that almost all human tumor-associated antigens are self-proteins, and therefore, their specific T cells, mainly those with the highest affinity, are often tolerized. Consequently, overcoming tumor-specific self-tolerance is a major goal in tumor immunotherapy. Self-tolerance is commonly directed against “dominant” (high affinity for HLA) but not against “cryptic” (low affinity for HLA) peptides [8, 9]. We have recently demonstrated that a simple way to circumvent this tolerance is to use cryptic peptides, providing their immunogenicity has been previously optimized [10]. Based on this concept, we have developed an optimized “cryptic” peptide, the TERT572Y (YLFFYRKSV), which differs from the native TERT572 at position 1 where a tyrosine has been substituted for an arginine. This substitution enhances its affinity for HLA-A*0201, the most frequently expressed HLA allele found in 45% of Caucasians, Hispanics and Asians [10]. The TERT572Y peptide has been shown to induce tumor immunity but not autoimmunity in HLA-A*0201 transgenic mice [11, 12]. In addition, in vitro studies in healthy volunteer donors and prostate cancer patients have shown that TERT572Y stimulated TERT-specific cytotoxic T lymphocytes (CTLs) with anti-tumor activity [12, 13].
In order to target TERT-expressing tumors in humans, we have developed the Vx-001 vaccine, which is composed of the 9-mer cryptic TERT572 peptide and its optimized variant TERT572Y, administered separately and in sequence. Vx-001 has recently been tested in a phase I/II clinical study in 116 patients with advanced cancer. Analysis of the first 19 patients, enrolled in the phase I part of the trial, showed that the vaccine was non-toxic and highly immunogenic in all tested doses [14]. Moreover, subgroup analysis of 22 vaccinated patients with advanced non-small-cell lung cancer (NSCLC) showed that the vaccine-induced specific early immune response correlated with prolonged survival [15].
The aim of this study was to investigate the ex vivo reactivity and function of vaccine-induced CTLs in the cohort of patients enrolled in the fixed dose phase II part of the trial. The results reported herein indicate that the vaccination with Vx-001 stimulates TERT572-specific reactive T cells in the peripheral blood in the majority of patients irrespective of the disease stage or clinical status before vaccination. More importantly, the vaccine-induced late immune response correlated with longer overall survival of vaccinated patients.
Materials and methods
Patients
Fifty-five HLA-A*0201-expressing patients with various types of chemo-resistant advanced solid tumors (stages III and IV) were enrolled in this Vx-001 vaccination study. Additional inclusion criteria were as follows: prior administration of all available treatments, which could be considered as “standard of care” including at least one prior chemotherapy regimen; age >18 years; performance status (WHO) of 0–2; adequate hematologic parameters (absolute neutrophil count ≥ 1.500/mm3, absolute lymphocyte count ≥ 1.300/mm3, platelets > 100.000/mm3, hemoglobin > 10 g/dl), and renal (creatinine < 1.5 mg/dl) and hepatic (bilirubin < 1.5 times the upper normal value) function tests. No other concomitant treatment with possible anti-tumor activity (i.e., chemotherapy, radiotherapy and glucocorticosteroids) or administration of immunosuppressive drugs was allowed 4 weeks before or during the course of vaccination. The clinical study was approved by the Ethics and Scientific Committees of the University Hospital of Heraklion and the National Drug Administration (EOF) of Greece and according to the Declaration of Helsinki. All patients signed a written informed consent in order to participate in the clinical study.
Peptides
The Vx-001 vaccine consisted of the 9-mer cryptic native TERT572 (RLFFYRKSV) peptide and its optimized variant TERT572Y (YLFFYRKSV). Both peptides were synthesized at the Faculty of Pharmacy, University of Patras (Greece) by means of solid-phase Fmoc/Bu chemistry. Quality assurance studies included confirmation of identity, sterility and purity (>95% for both peptides) as indicated by analytical high-performance liquid chromatography and were validated for identity by mass spectroscopy, as previously described [15]. No decrease in purity or concentration was observed after more than 2 years of storage at −80°C. Each peptide was prepared as a lyophilized powder for reconstitution and dilution in sterile water.
Vaccination protocol
All patients were vaccinated as previously described [15]. In brief, patients received two subcutaneous (s.c) injections with 2 mg of the optimized TERT572Y peptide followed by four s.c injections with 2 mg of the native TERT572 peptide, administered every 3 weeks. Both peptides were emulsified with Montanide ISA51 (Seppic Inc, Paris, France) immediately prior to injection. Patients received the 6-vaccination schedule unless disease progression occurred, and treatment was discontinued. Patients who completed the 6-vaccination schedule and experienced disease stabilization or objective clinical response received boost vaccinations with the native TERT572 peptide every 3 months until disease progression.
Patient samples for immunomonitoring
Patients’ peripheral blood in EDTA (100 ml) was collected before the first vaccination, after the 2nd and 6th vaccinations and before each boost dose for continuing patients. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Sigma, UK) density centrifugation and cryopreserved in recovery cell culture freezing medium (Gibco/Invitrogen, Paisley, Scotland, UK) at −80°C until used for the immune-assessment assays.
Enzyme-linked immunosorbent spot (ELISpot) assays
The IFN-γ and Perforin ELISpot assays were used to assess the reactivity and cytotoxic activity of specific T cells, respectively, in response to TERT572 and TERT572Y peptides. Both assays were performed according to the manufacturer’s protocol (IFN-γ ELISPOT, Diaclone, Besancon, France; Perforin ELISPOT, Mabtech, Sweden). The kits provided all the reagents used, unless otherwise stated. Briefly, nitrocellulose bottomed 96-well plates (MultiScreen MAIP N45; Millipore) were pre-wetted with 50 μl of 70% EtOH in distilled water for 2 min at room temperature (RT). The plates were washed five times with 200 μl sterile water and coated with capture anti-human IFN-γ or 30 μg/ml coating anti-human perforin (Pf-80/164) in PBS overnight at 4°C. The wells were washed with PBS and blocked for 1 h at room temperature (RT) with 200 μl RPMI-1640 (Gibco/Invitrogen, Paisley, Scotland, UK) supplemented with 10% human (AB) serum (PAA, Linz, Austria). Then, 50 μl of thawed 2x105 PBMCs in RPMI-1640 supplemented with 10% AB serum was added in the presence or absence of 10 μM of peptide (TERT572 or TERT572Y), and the cells were incubated overnight at 37°C in 5%CO2 in air. After incubation, the cell suspension was discarded and the plates were washed with 200 μl PBS. Then, 100 μl of detection anti-human biotinylated IFN-γ 1% (v/v) BSA/PBS or 1 μg/ml in PBS/1% BSA anti-human biotinylated perforin (Pf-344-biotin) was added and incubated for 2 h at RT. After 6 washes, 100 μl of alkaline phosphatase-conjugated streptavidin diluted at a ratio of 1:1,000 (v/v) in PBS was added and incubated for 1 h at RT. After washing, peroxidase substrate NBT/BCIP was added and incubated for 40 min. Upon appearance of dark spots in the negative control wells, the reaction was terminated by washing with running tap water. The spots were counted using Axio Imager.M1 analyzer and KS Elispot software (Zeiss, Germany).
In all ELISpot tests, six wells were tested for each group in three independent experiments. Negative controls were the cells alone (spontaneous IFN-γ and perforin release), whereas positive controls were cells treated with 5 μg/ml concanavalin A (ConA; Sigma, UK) or 5 μg/ml staphylococcus enterotoxin B (SEB; Sigma, UK) for IFN-γ and perforin ELISpot assays, respectively. The definition of a positive response at the IFN-γ ELISpot assay included a difference of more than 10 spot-forming cells and a statistically significant difference (P ≤ 0.05) between peptide-stimulated and negative control wells using the Student’s t test. The number of the vaccine-reactive T cells above background was calculated as the difference between the numbers of the counted cells in peptide-stimulated and non-stimulated wells. Responses after the 2nd and 6th vaccination were normalized by subtracting the pre-vaccination responses. Results are presented as the number of peptide-reactive cells per 2 × 105 PBMCs. The definition of a positive response at the perforin ELISpot assay included a statistically significant difference (P ≤ 0.05) between peptide-stimulated and negative control wells using the Student’s t test. Results are presented as the number of peptide-reactive cells per 5 × 105 PBMCs.
Intracellular cytokine staining
Peptide-specific CD8+ T cells were identified by IFN-γ intracellular staining using flow cytometry. Thawed 106 PBMCs were incubated in RPMI-1640 supplemented with 10% (v/v) AB serum in the presence or absence of 10 μg/ml peptides or 5 μg/ml SEB, overnight at 37°C in 5%CO2 in air. Brefeldin A (10 μg/ml; Sigma, UK) was added 1 h after the initiation of the stimulation in order to inhibit the secretion of newly synthesized cytokines. The cells were then washed with FACS buffer (1% (v/v) FCS/0.1% (v/v) sodium azide in PBS) and stained with fluorochrome-conjugated monoclonal antibodies against cell surface molecules (anti-CD8-PerCP and anti-CD3-APC; BD Biosciences, UK) for 30 min at 4°C. After washing, the cells were fixed and permeabilized by using the Cytofix/Cytoperm kit (BD Biosciences, UK) for 20 min at 4°C. After washing twice with Perm/wash solution, cells were stained with FITC-conjugated anti-IFN-γ (BD Biosciences, UK) for 40 min at 4°C. Cells were washed with Perm/wash solution and resuspended in BD Cell Fix buffer (BD Biosciences, UK) and analyzed by flow cytometry using FACSCalibur (BD Biosciences, UK); the acquired cytofluorographic data were analyzed using Cell Quest Pro software (BD Biosciences, UK). Results are expressed as the percentages of CD8+ IFN-γ+ T cells of the gated CD3+ T cells in the dot plots. The number of T cells in the graphs was calculated as: (2x105/100) × [(experimental − spontaneous CD8+ IFN-γ+-releasing cells)].
TERT572Y tetramer staining and clones
TERT572Y-specific CD8+ T cells were obtained from the PBMCs of a responding vaccinated patient by flow cytometry sorting of TERT572Y-tetramer+/CD8+ T cells. 1 × 106 thawed unstimulated PBMCs were incubated with phycoerythrin-conjugated (PE)-HLA-A*0201/TERT572Y (Proimmune Ltd, UK) for 30 min at RT, and then anti-CD8-APC and anti-CD3-FITC (BD Biosciences, UK) were added and incubated for an additional 30 min at 4°C. Cells were washed once and sorted by a flow cytometry cell sorting. Sorted cells were used to set up limiting dilution cultures and in vitro expanded in the presence of 1 μg/ml PHA and 150 U/ml rIL-2 for 7 days and used for the chromium-release assay.
Chromium-release assay
Antigen recognition was assessed using target cell lines [TERT+ (N18/TERT and NA8) and TERT− (N418 and Me290) kindly provided by Prof. P. Romero, Ludwig Center for Cancer Research, Lausanne, Switzerland] labeled with 51Cr for 1 h at 37°C and washed three times. Labeled target cells (1,000 cells in 50 μl) were then added to varying numbers of effector cells (100 μl) in V-bottom microwells. Chromium release was measured in the supernatant (100 μl) harvested after 4-h incubation at 37°C. The percentage-specific lysis was calculated as: 100 × [(experimental − spontaneous release)/(total − spontaneous release)].
Statistical analysis
Progression-free (PFS) and overall survival (OS) was estimated from the date of study entry to the date of the first evidence of disease progression and the last contact or death, respectively. PFS and OS of all patients or their subgroup according to progressive disease (PD) or stable disease (SD) at study entry was compared according to the development of early (after the 2nd vaccination) or late (at the completion of 6-vaccination) immune response by the log-rank test. The probability of survival was estimated using the Kaplan–Meier method. The 95% confidence interval (95% CI) was calculated. The frequencies of vaccine responding patients were compared using the paired t test. All tests were considered significant when the resulting P value was < 0.05.
Results
Patients’ demographics and vaccine administration
Fifty-five HLA-A*0201-positive patients with chemo-resistant advanced solid tumors were enrolled in this study. Patients’ demographics are presented in Table 1. The enrolled patients had various tumor types, including breast cancer (20%), melanoma (13%) and prostate cancer (20%) among others. At study entry, 93% of patients had distant metastases (Stage IV) and 71% presented documented disease progression (PD) during the last chemotherapy regimen. Twenty (36%) patients had received ≥2 chemotherapy regimens before enrollment. All patients received at least the first two vaccinations and 34 (62%) completed the 6-vaccination schedule. The vaccination protocol was prematurely terminated in the remaining patients due to disease progression. Moreover, nine (16.4%) patients received at least one boost vaccination.
TERT-specific T-cell responses
Baseline immune reactivity to TERT572 peptide was assessed in 55 patients, and the vaccine-induced immune response after the 2nd vaccination was assessed in all but two patients; in addition, the immune response was assessed in 32 (94%) out of 34 patients after the 6th vaccination (post-vaccination). Immune responses were evaluated by detecting peripheral blood TERT-specific T cells using the IFN-γ ELISpot assay. Moreover, the immune response was also evaluated by the IFN-γ intracellular staining assay in 35 out of 55 (63.6%) patients in whom sufficient PBMCs were available.
TERT-specific IFN-γ-producing T cells were detected in 27 (51%) out of 53 patients after the 2nd vaccination and in 22 (69%) out of 32 after the completion of the 6 vaccinations (post-vaccination), using the IFN-γ ELISpot assay. Two representative pictures from the ELISpot assay of a “weak” (Suppl. Figure 1A-available online) and a “strong” (Suppl. Figure 1B-available online) responder have been added as Supplementary Fig. 1 (Suppl. Figure 1A, B-available online). The mean (±standard deviation) frequency of spot-forming cells was 6 ± 11 per 2 × 105 PBMCs before vaccination (background frequency), 19.1 ± 50.7 per 2 × 105 PBMCs after the 2nd vaccination and 29 ± 62 per 2 × 105 PBMCs post-vaccination. The TERT-specific frequencies after the 2nd and the 6th vaccinations were statistically different compared to the baseline (pre-vaccination; P < 0.01, paired t test) (Fig. 1a). Four (12.1%) patients increased by >twofold their immune response, three (9.1%) did not change and nine (27.3%) had a >twofold decrease in peptide-specific immunity observed between the 2nd and 6th vaccinations. Intracellular staining of PBMCs for TERT peptides-induced IFN-γ production confirmed these T-cell-specific immune responses (Fig. 1b, c).
TERT572-specific T-cell responses in patients vaccinated with Vx-001. a Frequencies of specific T cells to TERT peptides in vaccinated patients prior to vaccination and after the 2nd and 6th vaccination (post-vaccination) using IFN-γ ELISpot assay, and b IFN-γ intracellular staining, c TERT572 and TERT572Y reactive CD8+ T cells after the 2nd and 6th vaccination in two representative patients as assessed by IFN-γ ICS. Percentages in the dot plots are for CD3+ CD8+ IFN-γ+ T cells [***P < 0.0001; **P < 0.001 and *P < 0.05; SFC = spot-forming cells]
Functional analysis of TERT-specific T cells
In order to determine the cytotoxic activity of TERT-specific IFN-γ-producing T cells induced by Vx-001, an ex vivo perforin ELISpot assay was performed with PBMCs from 6 selected patients for whom biological samples were available from post-vaccination time point. Patients’ demographics are presented in Supplementary Table 1. For all those 6 patients, an immunological response had previously been demonstrated by the IFN-γ ELISpot assay. Results showed that five (83%) patients presented CTL activity with the ability to specifically produce detectable levels of perforin ex vivo in the presence of TERT572 peptide (Fig. 2a). Three of these six patients were also able to produce perforin in response to the optimized TERT572Y peptide. In one patient, T-cell-specific perforin release could not be detected in response to either peptide.
a Frequencies of cytotoxic-specific T cells to TERT572 peptide as assessed by perforin production in the post-vaccination samples of four representative patients using ELISpot. Dashed line represents the threshold for “positive” response [***P < 0.0001; **P < 0.001 and *P < 0.05; SFC = spot-forming cells] b The lytic activity of the sorted TERT572Y-tetramer+/CD8+ T cells from one strongly responding patient was assessed by a chromium-release assay against TERT+ and TERT− cell lines
In addition, the functional specificity of sorted hTERT572Y-tetramer+ CD8+ T cells from one vaccinated patient was assessed in recognizing and killing of TERT-expressing cells by a chromium-release assay. The TERT+/HLA-A*0201+ (N18/TERT and NA8) but not the TERT−/HLA-A*0201+ (N418 and Me290) cells lines were lysed by hTERT572Y-tetramer sorted CD8+ T cells (Fig. 2b).
Immune responses according to disease stage and clinical status
In patients with locally advanced disease (stage III), TERT-specific IFN-γ-producing T cells could be detected in three out of four patients after the 2nd vaccination and in two out of three patients after the completion of the 6-vaccination protocol. One patient significantly enhanced its immune response, whereas one had a decrease in peptide-specific immunity after more than 3 vaccinations. Moreover, in patients with stage IV disease, TERT-specific IFN-γ-producing T cells were induced in 24 (49%) out of 49 patients after the 2nd vaccination and 20 (69%) out of 29 patients after the completion of the vaccination protocol (Fig. 3a). Three (10%) patients increased further their immune response, three (10%) did not change, and seven (24%) had a twofold decrease in peptide-specific immunity observed between the 3rd and after the 6th vaccination. The TERT-specific frequencies after the 2nd and the 6th vaccination were statistically different compared to the baseline (pre-vaccination; P < 0.001, paired t test).
TERT572-specific T-cell responses according to disease stage and disease status before study entry and disease evolution in vaccinated patients. Magnitude of T-cell response to TERT572 peptide in vaccinated patients prior to vaccination and after the 2nd and 6th vaccinations (post-vaccination) according to the a disease stage and b disease status using ELISpot. [SFC = spot-forming cells; [***P < 0.0001; **P < 0.002, paired t test]
Vx-001 was similarly immunogenic in patients who entered the vaccination protocol with either stable (SD) or progressive disease (PD), as almost 70% of the patients had developed TERT-specific IFN-γ-producing T cells after the completion of the 6-vaccination protocol (Fig. 3b).
Kinetics of TERT-specific T-cell response
PBMCs from 12 random patients were also collected before each vaccination dose in order to assess the kinetics of induction of peptide-specific T-cell responses. As shown in Fig. 4 A, the induction of peptide-specific immune response varied from patient to patient; however, the majority of patients mounted an immune response after the 2nd administration of the TERT572Y peptide. Patients were also able to generate an immune response at different time points during the course of the 6-vaccination protocol, but no patient developed an immune response after the administration of only the first vaccination dose. The magnitude of vaccine-induced T-cell response after the completion of the 6 vaccinations was similar in all patients independently of the time of the induction of the TERT-specific immune response.
Kinetics of TERT-specific T-cell response development after immunization. TERT-specific immune response during the course of six cycles of vaccination a and in boosted patients b as assessed by IFN-γ ELISpot assay c Immune response in two representative boosted patients after the 8th (#45) and 10th (#13) boosts as assessed by IFN-γ ICS. The data in the graphs are presented as the mean value of 3 independent experiments. Background frequencies have been subtracted. [SFC = spot-forming cells]
Moreover, prolonged vaccination maintained the number of peptide-specific CD8+ T cells in nine (82%) out of 11 patients who received boost vaccinations with the native TERT572 peptide as assessed by IFN-γ ELISpot assay (Fig. 4b) and IFN-γ intracellular staining (Fig. 4c).
Immune response and clinical outcome
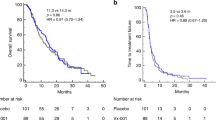
In order to determine whether there is an association between the development of TERT-specific IFN-γ immune reactivity and PFS as well as OS, the outcome of patients enrolled onto the vaccination protocol was analyzed. Overall, there was no significant difference in either PFS or OS between patients who developed an early (after the 2nd vaccination) or late (post-vaccination) immunological response during vaccination versus the ones who did not (Fig. 5a, b).
Progression-free and overall survival of all vaccinated patients. The progression-free and overall survival of all patients was assessed according to the presence (green line) of absence (blue line) of TERT-specific immune response after the 2nd vaccination (a) and at the completion of the 6-vaccination protocol (b) Overall survival of vaccinated patients with PD at study entry according to post-vaccination immune response (c)
However, in the subgroup analysis of patients who entered the study with progressive disease, those who developed a late immune response had a significantly longer OS compared with that of patients without a post-vaccination immune response (28.6 months vs. 13.1 months; log-rank test P = 0.01; Fig. 5c). The enrollment of patients entering the study with progressive disease was not biased with regard to the cancer type. The patients had various cancer types such as renal 13%, breast 13%, cholangio/pancreas 18%, prostate 26%, ovarian 3%, melanoma 13%, hepatocellular 5%, colorectal 3%, head and neck 3%, other 3%. Their life expectancy was also variable according to the disease type and previous treatment but in most cases estimated to be 6 months or less. In addition, the induction of either early or late immune response versus no-response (χ2 = 8.3, P = 0.5 and χ2 = 8.9, P = 0.3, respectively) in the progressive disease patients was irrespective of their cancer type.
Discussion
In a previous study, we showed that CD8+ T-cell immune responses could be detected in 22 HLA-A*0201 patients with advanced NSCLC vaccinated with Vx-001 [15]. In the present study, we assessed a larger cohort of HLA-A*0201 patients with different (other than NSCLC) types of cancer, and we analyzed and further characterized the immune responses induced by the Vx-001 vaccine. The findings of the current study confirm our previous observations since the administration of Vx-001 induced specific CD8+ T cells against TERT peptides, which exhibited in vitro effector functions including IFN-γ and perforin production.
Since many tumor antigens are normal non-mutated “self-proteins,” “dominant” peptides derived from these proteins are often affected by T-cell tolerance; therefore, “cryptic” peptides derived from these proteins could be better candidates for inducing an anti-tumor immune response since, due to their low affinity and recognition efficiency, they are not affected by tolerance and/or ignorance. In previous studies, we have shown that the substitution of an arginine by a tyrosine at the position 1 of the “native” TERT572 peptide significantly increases the immunogenicity of the “cryptic” peptide [5]; such modified peptides have also been used by other groups in order to induce a stronger T-cell activation than that induced by the native peptides [16–19].
In the present study, patients were vaccinated with two doses of the “optimized” peptide (TERT572Y) followed by four doses with the “native” peptide (TERT572). This vaccination schedule was based on in vivo preclinical studies which have shown that vaccination of HLA-A*0201 transgenic HHD mice with the optimized TERT572Y followed by the native TERT572 peptide induced CTLs with higher avidity and stronger anti-tumor efficacy than serial vaccinations with the optimized TERT572Y peptide alone [11]. This could be explained by the hypothesis that the optimized TERT572Y peptide first generates peptide-specific T cells and then the stimulation with the native TERT572 peptide selects among T cells those with the highest specificity for the native TERT572 peptide that is presented by tumor cells. Indeed, the presented data demonstrate that TERT-specific immune responses could be induced in 51 and 70% of the vaccinated patients after the 2nd and 6th vaccinations, respectively, as assessed by both IFN-γ ELISpot and intracellular cytokine staining assays (Fig. 1); these findings clearly indicate that our vaccination strategy efficiently circumvented the presumed immune tolerance against TERT [8, 10]. Furthermore, the specific T cells present in blood after the completion of the 6 vaccinations were of high TCR avidity, relative to the native peptide, compared to those present after the 2nd vaccination, which were of low avidity (data under publication).
The induction of TERT572-specific immune response was independent of the stage of disease or the disease clinical status at enrollment; moreover, the kinetics of immune response varied from patient to patient since some patients required more than two doses of the vaccine in order to mount a detectable immune response (Fig. 4a), as already reported with other vaccines [20]. Furthermore, boost vaccinations with the native peptide resulted in the maintenance of specific immune response that had been induced by the 6-vaccination schedule (Fig. 4b, c). These TERT-specific T cells were functional, since they specifically released perforin following stimulation with TERT peptides (Fig. 2a); it is well established that the perforin ELISpot correlates with cytotoxicity assays [21]. hTERT572Y-tetramer+ CD8+ T cells from one vaccinated patient were able to kill TERT-expressing tumor cells (Fig. 2b).
In the current study, we observed that in some patients the vaccine-induced TERT-reactive T cells detected in the blood after the 2nd vaccination disappeared after the completion of the 6-vaccination protocol (Fig. 1a); this observation clearly suggests that either the CTLs migrated to the tumor sites and therefore became undetectable in the blood or were subjected to cell death. It has been previously reported by two different groups that tumor-reactive T cells could be easily detected in the skin and lymph node biopsies but not in the blood of patients after vaccination [22, 23]. Moreover, Zaks et al. [24] reported that the re-stimulation of T cells at the peak of their expansion or activation may cause activation-induced cell death. Recent studies have proposed that one mechanism of immune escape used by the tumors is the production of immunosuppressive type II cytokines at the tumor sites [25–27]. Alternatively, we cannot exclude an involvement of other homeostatic mechanisms such as enhanced expression of surface CTLA-4 (cytolytic T lymphocyte-associated antigen 4) molecule, which has higher affinity and effectively competes with CD28 for B7.1 and B7.2 binding, thus inducing inhibitory signals to effector T cells [28–33] or an increased expansion of T regulatory cells (Tregs) that can suppress effector T cells [34–38]. In addition, the decreased immune response observed in a proportion of patients who completed the six vaccinations could be due to initial stimulation of the immune response by the modified peptide that was subsequently faded off due to further stimulation by the native peptide. Hence, considering all the above possible mechanisms, those responsible for explaining our findings need to be investigated further. This could not be achieved in the present study due to the complete utilization of the biological material collected from patients.
Finally, we observed a significant correlation between late (after the 6th vaccination) TERT-specific IFN-γ immune response and overall survival of vaccinated patients who entered the study with progressive disease. Indeed, late immune responders had a significantly better overall survival compared to that of non-responding patients (Fig. 5c). This observation seems to indicate that the failure of induction of immune response at the end of vaccination protocol may define a subgroup of patients who cannot derive a clinical benefit from the vaccination. However, this observation should be interpreted with caution since the present study was not designed to investigate this question and the patient population was very heterogeneous in terms of type of cancer and previous treatments that had been administered. Alternatively, the observed survival difference may merely reflect the overall better clinical status of patients including their ability to mount an immune response following Vx-001 vaccination.
In summary, the results of the current study demonstrate that Vx-001 is able to induce a TERT-specific immune response in vaccinated patients with different types of solid tumors and irrespective of the stage of disease and clinical status. The mechanisms regulating the induction or possible suppression of Vx-001-specific immune response merit to be further investigated as this vaccine is a promising candidate for cancer immunotherapy.
References
Nagorsen D, Scheibenbogen C, Marincola FM, Letsch A, Keilholz U (2003) Natural T cell immunity against cancer. Clin Cancer Res 9:4296–4303
Smyth MJ, Dunn GP, Schreiber RD (2006) Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol 90:1–50
van der Bruggen P, Zhang Y, Chaux P et al (2002) Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev 188:51–64
Blasco MA (2005) Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6:611–622
Kim NW, Piatyszek MA, Prowse KR et al (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011–2015
Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA (1999) Creation of human tumour cells with defined genetic elements. Nature 400:464–468
Hahn WC, Stewart SA, Brooks MW et al (1999) Inhibition of telomerase limits the growth of human cancer cells. Nat Med 5:1164–1170
Robbins PF, Kawakami Y (1996) Human tumor antigens recognized by T cells. Curr Opin Immunol 8:628–636
Van PA, van der BP, Coulie PG et al (1995) Genes coding for tumor antigens recognized by cytolytic T lymphocytes. Immunol Rev 145:229–250
Tourdot S, Scardino A, Saloustrou E et al (2000) A general strategy to enhance immunogenicity of low-affinity HLA-A2. 1-associated peptides: implication in the identification of cryptic tumor epitopes. Eur J Immunol 30:3411–3421
Gross DA, Graff-Dubois S, Opolon P et al (2004) High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J Clin Invest 113:425–433
Scardino A, Gross DA, Alves P et al (2002) HER-2/neu and hTERT cryptic epitopes as novel targets for broad spectrum tumor immunotherapy. J Immunol 168:5900–5906
Hernandez J, Garcia-Pons F, Lone YC et al (2002) Identification of a human telomerase reverse transcriptase peptide of low affinity for HLA A2.1 that induces cytotoxic T lymphocytes and mediates lysis of tumor cells. Proc Natl Acad Sci USA 99:12275–12280
Mavroudis D, Bolonakis I, Cornet S et al (2006) A phase I study of the optimized cryptic peptide TERT(572y) in patients with advanced malignancies. Oncology 70:306–314
Bolonaki I, Kotsakis A, Papadimitraki E et al (2007) Vaccination of patients with advanced non-small-cell lung cancer with an optimized cryptic human telomerase reverse transcriptase peptide. J Clin Oncol 25:2727–2734
Meijer SL, Dols A, Jensen SM et al (2007) Induction of circulating tumor-reactive CD8+ T cells after vaccination of melanoma patients with the gp100 209–2 M peptide. J Immunother 30:533–543
Rosenberg SA, Yang JC, Schwartzentruber DJ et al (1998) Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med 4:321–327
Parkhurst MR, Salgaller ML, Southwood S et al (1996) Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol 157:2539–2548
Valmori D, Fonteneau JF, Lizana CM et al (1998) Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J Immunol 160:1750–1758
Bercovici N, Haicheur N, Massicard S et al (2008) Analysis and characterization of antitumor T-cell response after administration of dendritic cells loaded with allogeneic tumor lysate to metastatic melanoma patients. J Immunother 31:101–112
Zuber B, Levitsky V, Jonsson G et al (2005) Detection of human perforin by ELISpot and ELISA: ex vivo identification of virus-specific cells. J Immunol Methods 302:13–25
De Vries IJM, Lesterhuis WJ, Barentsz JO et al (2005) Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol 23:1407–1413
Slingluff CL Jr, Petroni GR, Yamshchikov GV et al (2004) Immunologic and clinical outcomes of vaccination with a multiepitope melanoma peptide vaccine plus low-dose interleukin-2 administered either concurrently or on a delayed schedule. J Clin Oncol 22:4474–4485
Zaks TZ, Chappell DB, Rosenberg SA, Restifo NP (1999) Fas-mediated suicide of tumor-reactive T cells following activation by specific tumor: selective rescue by caspase inhibition. J Immunol 162:3273–3279
Aruga A, Aruga E, Tanigawa K, Bishop DK, Sondak VK, Chang AE (1997) Type 1 versus type 2 cytokine release by Vbeta T cell subpopulations determines in vivo antitumor reactivity: IL-10 mediates a suppressive role. J Immunol 159:664–673
Lattime EC, Mastrangelo MJ, Bagasra O, Li W, Berd D (1995) Expression of cytokine mRNA in human melanoma tissues. Cancer Immunol Immunother 41:151–156
Yang AS, Lattime EC (2003) Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res 63:2150–2157
Gabriel EM, Lattime EC (2007) Anti-CTL-associated antigen 4: are regulatory T cells a target? Clin Cancer Res 13:785–788
Hodi FS, Mihm MC, Soiffer RJ et al (2003) Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA 100:4712–4717
Korman A, Yellin M, Keler T (2005) Tumor immunotherapy: preclinical and clinical activity of anti-CTLA4 antibodies. Curr Opin Investig Drugs 6:582–591
Krummel MF, Allison JP (1995) CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 182:459–465
Phan GQ, Yang JC, Sherry RM et al (2003) Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA 100:8372–8377
Sun J, Schiffman J, Raghunath A, Ng TD, Chen H, Sharma P (2008) Concurrent decrease in IL-10 with development of immune-related adverse events in a patient treated with anti-CTLA-4 therapy. Cancer Immun 8:9
Ling KL, Pratap SE, Bates GJ et al (2007) Increased frequency of regulatory T cells in peripheral blood and tumour infiltrating lymphocytes in colorectal cancer patients. Cancer Immun 7:7
O’Mahony D, Morris JC, Quinn C et al (2007) A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin Cancer Res 13:958–964
Sakaguchi S (2000) Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101:455–458
Sakaguchi S (2005) Naturally arising Foxp3-expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 6:345–352
Yamaguchi T, Sakaguchi S (2006) Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol 16:115–123
Acknowledgments
Supported by the Cretan Association for Biomedical Research.
Conflict of interest
K. Kosmatopoulos and J. Menez-Jamet are employees and shareholders of Vaxon Biotech. All the other authors have no potential conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vetsika, EK., Konsolakis, G., Aggouraki, D. et al. Immunological responses in cancer patients after vaccination with the therapeutic telomerase-specific vaccine Vx-001. Cancer Immunol Immunother 61, 157–168 (2012). https://doi.org/10.1007/s00262-011-1093-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1093-4