Abstract
Dendritic cells (DCs) have the ability to generate peptide epitopes for MHC class I molecules derived from apoptotic tumour cells for direct recognition by cytotoxic T cells. This function has lead to DCs being used in vaccine strategies. In this study, we investigate the effect of inducing apoptosis in tumour cell lines using IFN-γ and poly(I:C), the subsequent maturation of the endocytosing DC and its ability to direct the resulting T cell response. We show that uptake of poly(I:C)-induced apoptotic tumour cells leads to DC maturation and activation with a Th1 cell polarising capacity. In contrast, these effects are not seen by DCs loaded with γ-irradiated apoptotic tumour cells. We propose that the manner in which tumour cells are induced to die can have a profound effect on the endocytosing DC and the resulting T cell response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apoptosis is a genetically programmed form of cell death driven by an intracellular signal transduction pathway leading to disposal of the cell without causing damage to neighbouring cells [1]. During apoptosis, a cell undergoes a series of defined events that results in the condensation of the nucleus and the breakdown of the cellular components into discrete apoptotic bodies. Apoptotic bodies can be efficiently endocytosed by a number of cell types without the release of inflammatory cytokines [2], or indeed with the active production of anti-inflammatory cytokines, such as IL-10 [3], TGF-β [4] and PGE2 [5]. Virally infected cells, however, can also die by apoptosis in response to viral dsRNA generated as a by-product of the replication cycle of many DNA and RNA viruses [6]. A number of intracellular pathways have been described, which detect these viral intermediates including Toll-like receptor (TLR) 3, dsRNA-activated protein kinase (PKR), melanoma differentiation-associated gene 5 (MDA5) and retinoic acid-induced gene 1 (RIG-1). Some studies have demonstrated cytokine production in DCs in response to poly(I:C) [7, 8], and dsRNA activation of PKR has been associated with the induction of TNF-α and IL-1β in human lung epithelial cells [9]. Such proinflammatory cytokines have been shown to be capable of maturing DCs [10].
Immature monocyte-derived DCs (iDCs) are characterised by high endocytic and macropinocytic activity [11, 12]. These cells have been shown to efficiently phagocytose apoptotic cells aided by an array of receptors such as the scavenger receptor CD36, αvβ3 and αvβ5 integrins [13, 14], CD68, Fcγ receptors and complement receptors (CR) [15]. Once apoptotic material has been endocytosed, DCs can generate peptide epitopes for MHC class I molecules [16], a process referred to as cross-presentation.
After encountering antigen, and in conjunction with additional signals, iDCs upregulate surface expression of MHC molecules and co-stimulatory molecules (CD80, CD86 and CD40), a process called ‘DC maturation’ [17]. Mature DCs migrate to the tumour-draining lymph nodes within 4–48 h [18] where they interact with T cells, influencing their differentiation and consequently the type of immune response generated. The signals received during the DC maturation stage may profoundly influence the resulting T cell response. It has been reported that DCs can generate Th1 or Th2 responses and also regulatory cells if the signals so dictate [19–21].
The use of DCs in cancer immunotherapy aims to stimulate the immune system to target and destroy tumour cells. Observations made during human DC-based vaccine trials have shown that ex vivo-derived antigen-pulsed immature DCs can induce regulatory cells, while in their mature form they are immunogenic, inducing antigen-specific Th1 responses [22, 23]. Therefore, optimal maturation of DCs is considered essential for DCs in clinical applications [24]. An effective maturation cocktail, consisting of pro-inflammatory cytokines and prostaglandin, has been described by Jonuleit et al. [10] and has been used as the ‘gold standard’ DC maturation cocktail by many groups. Treatment of immature DCs with cytokine combinations, however, has been shown to induce only partial (without the release of IL-12) and reversible DC maturation [25–27]. More recently, full maturation of DCs capable of inducing pro-inflammatory cytokine production has been associated with microbial products (the evolutionarily conserved pathogen-associated molecular patterns, PAMPs) recognised by pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) [28]. Viral dsRNA, which can be mimicked by its synthetic analogue poly (I:C), has been shown to induce the stable maturation and antigen-specific cytotoxic T cells (CTL) activating capacity of DCs [25, 29]. Cell death that stimulates the endocytosing DC towards the activation of an inflammatory response has been referred to as inflammatory apoptosis [30] or immunogenic cell death [31]. The development of an inflammatory apoptosis could depend on the initiating factor, the cell type or the presence of particular inflammatory mediators such as type I IFNs [30], PAMPs [32] or the recently described damage-associated molecular patterns (DAMPs) such as Calreticulin (CRT), heat shock proteins (Hsp) and high-mobility group box-1 (HMGB-1) [31, 33].
Since the identification of tumour-associated antigens (TAAs), numerous immunisation strategies have been developed aimed at inducing TAA effector cells capable of killing transformed cells [34]. Clinical studies have shown that favourable responses to DC vaccination correlate with immunological responses to more than one antigen [35]. The use of whole tumour cells in the loading of DCs offers a wide variety of TAA to the DC for presentation, unlike peptide or gene transfection methods where knowledge of the antigen is essential. Tumour cells are commonly prepared for DC loading by freeze/thaw lysis [36–38], resulting in necrotic cells, or by irradiation that induces cell death via apoptosis [39, 40]. Using whole tumour cells, however, could potentially induce either tolerance [41] or immunity [42, 43]. It is of key importance therefore to induce an immunogenic tumour cell death that has the potential to activate DCs and at the same time facilitate presentation and cross-presentation of TAAs to efficiently stimulate antigen-specific T cells.
We have investigated the effect of poly(I:C) on IFN-γ pre-treated tumour cells (IFN-γ/poly(I:C) tumour cells) in terms of cell death and its impact on the maturation and activation of the endocytosing DCs. We compared this to γ-irradiation, the conventional way of inducing cell death in tumour cells used in clinical dendritic cell vaccines [40]. We found that IFN-γ pre-treatment followed by poly(I:C)-induced apoptosis in a number of tumour cell lines. Ingestion of IFN-γ/poly(I:C) tumour cells by DC-induced higher expression of the maturation markers MHC class II, CD80 and CD86 than ingestion of irradiated tumour cells. Furthermore, only those DCs that had endocytosed IFN-γ/poly(I:C) tumour cells were capable of stimulating IFN-γ producing T cells. Our data suggest that under the appropriate apoptosis inducing conditions tumour material can effectively provide maturation signals to DCs leading to a Th1 polarised adaptive immune response.
Materials and methods
Tumour cell culture
The MJT3, MJT1, MJT5 and KM melanoma cell lines were previously established from biopsies and characterised at St George’s Hospital [44] (and unpublished data). Melanoma cells were grown in RPMI (Sigma) supplemented with 10% foetal calf serum (FCS) (Invitrogen), 1% Glutamine (G) and 1% Penicillin/Streptomycin (P/S) (Sigma) [RPMI complete medium (c.m)] at 37°C in a 5% CO2 humidified incubator. Hep2 and A549 epithelial cell lines were grown in (Dulbecco’s Modified Eagle Medium) DMEM (Sigma) supplemented with 10% FCS, 1% G and 1% P/S. Tumour cells were cultured in T75 tissue culture flasks (Nunc) and split 1:5 twice a week using 1× trypsin–EDTA (Invitrogen) to detach cells from the flasks.
Induction of cell death in melanoma cells
Cell death was induced in tumour cells by γ-irradiation (150 Gy) or poly(I:C) (Amersham). MJT3 cells were plastic adhered at 2.5 × 105/ml RPMI c.m for 24 h and 100 μg/ml poly(I:C) (as pre-determined by titration) was added to the culture supernatant and cells were incubated for 4, 24, 48 and 72 h. Alternatively, apoptosis was induced in MJT3 cells by γ-irradiation (150 Gy) after 24 h of plastic adherence. Interferon-gamma (IFN-γ) (PeproTech) pre-treatment of tumour cells was performed by the addition of 1,000 U/ml IFN-γ 24 h prior to irradiation or poly(I:C) pulsing. Untreated MJT3 tumour cells were incubated for the above time points and used as negative controls. In some tumour cell lines, cell death was induced by the forced loading (transfection) of poly(I:C) using the cationic liposome-based reagent, TransFast (Promega) as directed by the manufacturer’s instructions. Tumour cell lysates were prepared with 4 freeze/thaw cycles and checked for cell death using trypan blue (Sigma) exclusion.
Detection of apoptosis
Apoptosis of tumour cells was detected using DIOC6 (dihexyloxacarbocyanine iodide) (Sigma–Aldrich) that accumulates in the mitochondria of viable cells and is lost with the change in membrane permeability associated with apoptosis. Tumour cells were detached from the plastic culture vessels using trypsin, and 1 × 105 cells were incubated with 35 nM DIOC6 for 15 min at 37°C. Cells were washed and stained with 5 μl Propidium Iodide (PI) (BD) in 100 μl Dulbecco’s phosphate-buffered saline (PBS) (Sigma) for 15 min at room temperature (RT) in the dark. Flow cytometric analysis was performed using a FACSCalibur (BD Pharmingen) within 1 h of staining. Cells negative for DIOC6 and PI were considered apoptotic. Cells that became necrotic (primary necrosis) or proceeded from apoptosis to necrosis (late apoptosis or secondary necrosis) stained positive for PI.
Detection of Calreticulin on the cell surface of dying tumour cells
In order to determine whether IFN-γ/poly(I:C) or γ-irradiation induce the expression of the immunogenicity marker Calreticulin (CRT) in the tumour cells, 1 × 105 tumour cells were stained with 4 μg/ml non-conjugated mouse anti-human Calreticulin (CRT) antibodies (Abcam) and incubated at 4°C for 30 min. Alternatively, cells were stained with 4 μg/ml mouse IgG1 negative control antibodies (Serotec). Cells were washed with 2 ml FACS buffer [1× PBS (made from tablet by Sigma), 10 mg/ml Bovine serum albumin (Sigma), 5% Sodium azide (Sigma)] and centrifuged at 500g at 4°C for 5 min. Cells were resuspended in 50 μl FACS buffer, and 1 μg/ml FITC conjugated rabbit anti-mouse IgG (Serotec) was added to each tube. Cells were incubated for 30 min at 4°C in the dark, washed as before and resuspended in 200 μl CellFix (BD). Calreticulin expression was quantified by flow cytometry.
Determination of gene expression by apoptotic tumour cells
Total RNA was isolated using RNeasy minikit (Qiagen) following manufacturer’s instructions. RNA was quantified using a Nanodrop 1000A spectrophotometer (ThermoScientific) and an Agilent 2100 Bioanalyser (Agilent Technologies) used to determine the quality of the sample. RNA was amplified using the Illumina total prep kit following manufacturer’s instructions. Samples were hybridised to human HT12 arrays (Illumina) and data analysed using Genespring software (Agilent Technologies). Differential expression was defined as twofold increase or decrease in irradiated or IFN-γ/poly(I:C)-treated cells over non-treated samples.
Generation of human monocyte-derived dendritic cells
Immature dendritic cells (iDCs) were generated from peripheral blood mononuclear cells (PBMCs) isolated by Ficoll density centrifugation from buffy coats of healthy donors (National Blood Service) following standard protocols [26, 45]. Briefly, blood was diluted 1:1 with HBSS and layered onto 15 ml of Ficoll-Hypaque (Invitrogen) in a 50 ml tube and centrifuged at 800g, for 25 min at 20°C without deceleration. Peripheral blood mononuclear cells were removed from the interface and washed twice in HBSS. Cells were pelleted by centrifugation at 300g for 10 min to ensure a loose pellet. PBMCs were re-suspended in c.m at 3 × 106/ml for 2 h to allow the monocytes to adhere to the plastic. Non-adherent cells were removed and cryopreserved at 7.5 × 106 cells/ml by slow freezing in cryogenic vials in freezing medium [44% RPMI + 44% FCS + 12% dimethyl sulfoxide (DMSO) (Sigma)] and stored in liquid nitrogen for later use as autologous responder T cells. Adherent monocytes were gently washed twice with 10 ml of warm HBSS. Monocytes were cultured in c.m. for 7 days and differentiated into DC by the addition of 100 ng/ml GM-CSF (PeproTech) and 50 ng/ml IL-4 (PeproTech) on days 0, 2 and 5. In this experimental system, DCs were grown in the presence of 10% FCS as it has been shown that DCs grown in the presence of human serum lack the CD1a DC marker and show a relative resistance to maturation [46] (and unpublished results). Dendritic cells were harvested on day 7. DC purity was determined by flow cytometry by the percentage of CD11c positive cells using APC-labelled anti-human CD11c monoclonal antibody (BD).
Phagocytosis assays
To investigate whether immature DCs can endocytose apoptotic tumour cells, two approaches were taken. Firstly, carboxyfluorescein succinimidyl ester (CFSE) (Sigma) stained DCs and CellTrace™ Far Red DDAO (Invitrogen) stained tumour cells were co-cultured for 24 h. Tumour cells at 1 × 106/ml cell concentration were stained after apoptosis induction with 10 μM DDAO red fluorescent dye following manufacturer’s protocol. Immature DCs were stained with 1 μM CFSE green dye. Stained DCs and tumour cells were co-cultured at 1:1 ratio for 24 h at 37 or 4°C. Double positive staining indicated DCs that had internalised tumour cells as detected by flow cytometry. Secondly, uptake of apoptotic tumour cells was confirmed by confocal microscopy. MJT3 tumour cells were labelled with PKH67 (Sigma) according to the manufacturer’s protocol and cultured with or without IFNγ for 24 h. Cells were treated with 100 μg/ml poly(I:C) or γ-irradiated and cultured for a further 24 h. PKH67 stained tumour cells were co-cultured with DCs at a tumour cell: DC ratio of 1:1 for 24 h. DCs that had been co-cultured with tumour cells were then transferred to wells containing the poly-l-lysine (Sigma)-coated coverslips and allowed to adhere for a minimum of 4 h at 37°C. Adherent DCs were fixed in 4% w/v paraformaldehyde/3% w/v sucrose in PBS warmed to 37°C for 20 min. Following 3 further washes in PBS, DCs were stained with CD11c conjugated to alexa-647 (AbD Serotec) for 30 min. After a final 3 washes, coverslips were attached to microscope slides using Vectashield (Vectorlabs) with DAPI (4′,6-Diamidino-2-phenylindole) (Sigma) and stored at 4°C. Cells were examined using a LSM confocal microscope using a 40× oil objective. Fluorescent images were obtained using LSM image browser.
Determination of DC maturation by tumour cells
Immature DCs were co-cultured with viable (untreated) or apoptotic allogeneic tumour cells (irradiated or IFN-γ/poly(I:C) tumour cells) at a 1:1 ratio for 24 h. To negate any effect of residual extracellular IFN-γ or poly(I:C) activating DCs, tumour cells were washed 3 times after induction of apoptosis and re-suspended in fresh culture medium before co-culture. Maturation of DCs in response to apoptotic tumour cells was determined by flow cytometric analysis. HLA class I, HLA class II, CD1a, CD11c, CD14, CD80 and CD86 surface markers were detected using anti-human monoclonal CD1a-FITC, CD11c-APC, CD14-PE, HLA class I-FITC, HLA class II-PE, CD80-PE, CD86-PE, CD83-PE and CD40-PE antibodies (all from BD). Isotype-matched mouse anti-human IgG antibodies were used as negative controls (IgG1-PE, IgG1-FITC, IgG1-APC, IgG2a-PE (all from BD). Cells (1 × 105) were stained with appropriate amount of the above antibodies in 100 μl volume of FACS buffer. Cells were incubated at 4°C for 30 min, washed once in 2 ml FACS buffer and fixed with 300 μl CellFIX (BD). Flow cytometry was performed using a FACSCalibur System. Routinely, 1 × 104 events were collected using a gate on the forward scatter/side scatter (FSC/SSC) profile to exclude debris. Tumour cells were excluded from the analysis by gating on the CD11c positive DC population. The flow cytometric data are presented as the median of fluorescence intensity (MFI) of DCs, representing a semi-quantitative measure of expression level. Analysis was carried out using FCS Express Version 3 software. Some DCs were matured with the ‘gold standard’ maturation cocktail containing 10 ng/ml IL-1β, 10 ng/ml TNF-α, 1,000 U/ml IL-6 and 1 μg/ml PGE-2 (all from PeproTech) [10] for 24 h and then immunophenotyped. In some experiments, DCs were loaded with tumour cell lysates for 24 h and matured with the ‘gold standard’ maturation cocktail for a further 24 h. To exclude the possibility of DC maturation occurring as a result of poly(I:C) adhering to the surface of dying tumour cells, maturation of DCs was also determined in the presence of Benzonase nuclease (BN) (Novagen). DCs were co-cultured with untreated, γ-irradiated or IFN-γ/poly(I:C) MJT3 melanoma cells in the presence or absence of 50 U/ml BN. DCs treated with 100 μg/ml poly(I:C) in the presence of BN was used as positive control for poly(I:C) digestion by BN.
Detection of cytokines
Cytokines including TNF-α, IL-6, IL-8, IL-10, IL-12p70 and IL-1β was quantified in tumour cell culture supernatants by Bio-Plex Cytokine Assay, while IFN-β was detected by ELISA (PBL). CD40 ligand (CD40L) transfected J558 mouse fibroblast cell line (a kind gift from Dr P Lane) [47] was used to mimic the in vivo DC–T cell interaction via the CD40-CD40L pathway. Non-loaded or tumour cell loaded DCs were co-cultured with CD40L transfected J558 cells at 1:1 DC to J558 cell ratio for 24 h. IL-12p70 and IL-10 were quantified in co-culture supernatants using the Bio-Plex Cytokine Assay (BioRad) following manufacturers’ instructions.
Co-culture of tumour cell loaded DCs with non-adherent PBMCs
Tumour cell loaded DCs were co-cultured with the non-adherent fraction of autologous PBMCs at 1:5 ratio in 2 ml RPMI c.m. To reduce the amount of apoptotic or dead tumour cell material in the loaded DC preparation, DCs at 5 × 105 cells/ml RPMI c.m were layered over a ficoll gradient and centrifuged at 800g for 25 min at 20°C with no deceleration. The percentage of DCs was determined by FACS staining using CD11c-APC (BD) to ensure that equal amounts of DCs and PBMCs were co-cultured. PBMCs were super-stimulated at day 4 or day 7 with 1 μg/ml PMA (Sigma) and 10 ng/ml Ionomycin (Sigma) in the presence of 10 μg/ml Brefeldin A (Sigma). PBMCs were harvested and immunophenotyped for CD3, CD8 and CD4 markers using specific PerCP-, FITC- and APC-labelled monoclonal antibodies (BD), respectively. T cells were gated on FSC/SSC and double gated on FL3 to exclude CD3-ve cells from the analysis. The percentages of IFN-γ, IL-2 and IL-10-producing CD4 and CD8 were determined by intracellular cytokine staining by PE-labelled antibodies (BD) using the Cytofix/Cytoperm kit (BD) according to manufacturer’s instructions. PE-labelled mouse IgG1 was used as isotype-matched control of anti-human IFN-γ and IL-2 monoclonal antibodies, and rat IgG1 was used as isotype-matched control of anti-human IL-10 monoclonal antibody. In some experiments, naïve CD3+ T cells were isolated by negative magnetic bead selection using the T cell negative isolation kit (Dynal) as directed by the manufacturer’s instructions. This kit depletes B cells, NK cells, monocytes, platelets, DCs, granulocytes, erythrocytes and activated (HLA class II positive) T cells. The amount of IFN-γ, IL-2, TNF-α and IL-10 was detected in supernatants of DC/non-adherent PBMC co-cultures by Bio-Plex Cytokine Assay (BioRad) following manufacturers’ instructions.
Induction of antigen-specific response by apoptotic tumour cell loaded DCs
Immature DCs were loaded with 10 μg/ml influenza matrix (M1) peptide (GILGFVFTL) (ProImmune). Two hours later, γ-irradiated or IFN-γ/poly(I:C)-treated MJT3s were added to the DCs and cultured for 24 h. DCs were harvested and co-cultured with autologous CD3+ T cells isolated from the non-adherent fraction of PBMCs using the Dynabeads Untouched Human T Cells negative isolation kit (Invitrogen) at 1:5 ratio. T cells were re-stimulated with the respective M1 loaded DCs on day 7 and supplied with 40 U/ml IL-2 (PeproTech) 4 days after stimulation or re-stimulation. T cells were harvested on day 14 and stained with matched APC-labelled M1 Pentamer (ProImmune) as instructed by the manufacturer and co-stained with CD3-PerCP (BD) and CD8-PE (BD). M1-specific T cells expanded by M1 loaded cocktail-matured DCs as described above was used as a control.
Statistical evaluation
Statistically significant differences were determined using Student’s paired t test. Data that were not normally distributed were subjected to Wilcoxon’s test using the GraphPad Prism 5 software. All P values were obtained by two-tailed tests, and differences were considered statistically significant when P ≤ 0.05.
Results
Poly(I:C) induces apoptotic cell death in tumour cell lines
MJT3 melanoma cells were treated with poly(I:C), and cell death was determined by dihexyloxacarbocyanine iodide (DIOC6)/Propidium iodide (PI) co-staining. Figure 1a shows the mean percentage of apoptotic (DIOC6 −ve/PI −ve) MJT3 tumour cells induced with γ-irradiation or poly(I:C) in 3 independent experiments. Both γ-irradiation (average = 35%) and poly(I:C) (average = 23%) induced apoptosis in MJT3 cell line, however, poly(I:C)-induced apoptosis was significantly increased by IFN-γ pre-treatment (average = 63%) (Student’s t test P = 0.02). This effect was determined in a number of additional cell lines including the MJT1, MJT5 and KM melanoma cell lines, the Hep2 epithelial cell line and the A549 lung carcinoma cell line (data not shown). In general, tumour cell lines fell into two groups, one group being susceptible to cell death induced in this manner (MJT3, MJT1, MJT5, Hep2) and one group not (KM, A549). All cell lines, however, can be induced to undergo apoptosis if poly(I:C) is introduced into the cell using a transfection reagent.
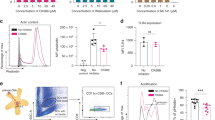
Apoptotic cell death and expression of CRT immunogenicity marker can be induced by the combination of IFN-γ and poly(I:C) in tumour cells. a Apoptotic cell death of MJT3 melanoma cells as determined by DiOC6/PI staining. Tumour cells were untreated (Un) or subjected to 150 Gy γ-irradiation (irr), 100 μg/ml poly(I:C) treatment (pIC) or 24 h IFN-γ pre-treatment followed by 100 μg/ml poly(I:C) treatment (IFN/pIC). The figure shows the mean percentage of apoptotic (DiOC6 negative/PI negative) tumour cells from 3 separate experiments at 48 h. Statistically significant differences are shown (*P < 0.05; **P < 0.01; ***P < 0.001) as determined by Student’s t test using GraphPad Prism 5 data analysis software. b Representative FACS plots of CRT staining. The percentage of CRT expressing tumour cells was detected by flow cytometry using FACSCalibur. Non-specific antibody binding was excluded by gating on cells stained with appropriate isotype control. c CRT expression by apoptotic MJT3s. The graph shows the mean and SD of 3 independent experiments. Statistical analysis was carried out as in a
Calreticulin expression on IFN-γ/poly(I:C)-treated MJT3 tumour cells
Profiling of ‘immunogenic apoptosis’ is thought to have key importance in improving anticancer therapeutics in order to reduce cancer-related mortalities in the future [48]. Cell surface expression of CRT, a damage-associated molecular pattern, has been suggested to be a marker of immunogenic cancer cell death [31, 49]. Gamma irradiation of MJT3 tumour cells induced CRT expression in only a small percentage of cells (20% on average), while there was a marked increase in the percentage of MJT3s with CRT expression when MJT3s were treated with IFN-γ/poly(I:C) (55%) (Fig. 1b, c).
Gene profile of apoptotic tumour cells
In order to investigate other mechanisms that may influence the immunogenicity of apoptotic tumour cells, changes in gene expression of apoptotic tumour cells against non-treated tumour cells were determined by microarray analysis. Relevant changes in gene expression were defined as twofold increase or decrease. The data indicate that the changes in gene expression in tumour cells undergoing apoptosis as a result of IFN-γ/poly(I:C) is markedly different to that seen in irradiated tumour cells (Table 1). Of particular interest is the difference in the two groups of chemokines and cytokines, and the marked increase in expression of these in the IFN-γ/poly(I:C)-treated cells. Conversely, the expression of heat shock proteins is decreased in IFN-γ/poly(I:C)-treated cells but increased in irradiated cells. These data suggest that profoundly different immune responses might be initiated by these two apoptosis inducing modalities. Genes upregulated by either IFN-γ or poly(I:C) alone are shown in bold. Only a small fraction of the genes upregulated by the combination of IFN-γ/poly(I:C) was expressed when the treatments were used alone. In addition, the fact that apoptosis is only induced by the combination of IFN-γ and poly(I:C) emphasises the necessity of using the two reagents together.
Immature monocyte-derived DCs can endocytose both irradiated and IFN-γ/poly(I:C) tumour cells
In order to investigate tumour cell uptake by DCs, DDAO (red) stained untreated, irradiated or IFN-γ/poly(I:C)-treated MJT3s were co-cultured with CFSE (green)-stained DCs. Phagocytosis of tumour cells by DCs was defined by the percentage of double positive cells. Interferon-γ/poly(I:C) and irradiated tumour material were internalised by DCs (Fig. 2). Apoptotic tumour cell uptake was abrogated by incubation of DCs in the presence of IFN-γ/poly(I:C)-treated tumour cells at 4°C indicating that the uptake occurred by an active mechanism (data not shown). To confirm ingestion of tumour material by DCs, PKH67-stained tumour cells were co-cultured with DCs, which were then stained with CD11c-alexa 647 and analysed by confocal microscopy. Results demonstrate the ingestion of apoptotic tumour material supporting the flow cytometric results (Supplementary Fig. 1). The morphology of DCs that had ingested irradiated tumour cells was somewhat different from those that ingested IFN-γ/poly(I:C) tumour cells. DCs loaded with irradiated tumour cells appeared more adherent (Supplementary Fig. 1a) than those loaded with IFN-γ/poly(I:C) tumour cells (Supplementary Fig. 1b), which could be due to loss of adhesive structures that accompanies DC maturation [17] or dedifferentiation of DCs into macrophages as a result of ingestion of γ-irradiated tumour cells.
Monocyte-derived immature DCs are capable of endocytosing both irradiated and IFN-γ/poly(I:C)-treated tumour cells. CFSE-stained DCs are shown where CFSE/DDAO double positive DCs represent the percentage of DCs that had endocytosed DDAO-stained MJT3 tumour cells. DDAO positive CFSE negative cells were gated out to exclude any unendocytosed tumour cells from the values. Representative figures of 4 individual experiments
IFN-γ/poly(I:C)-treated tumour cells induce DC maturation
Expression of antigen presentation and co-stimulatory molecules by DCs is essential for their interaction with T cells. Expression of DC maturation markers was determined by the median fluorescence intensity (MFI) of CD11c positive cells after 24 h of culture with or without treated tumour cells. Immature monocyte-derived DCs (Mo-DCs) showed characteristic expression of CD11c, CD1a and MHC class II and lack of CD14 expression (Supplementary Fig. 2a). Figure 3a shows the MFI of DCs cultured alone, with non-treated, irradiated MJT3 or IFN-γ/poly(I:C)-treated MJT3 cells. DCs loaded with either irradiated or IFN-γ/poly(I:C)-treated MJT3s showed higher expression of MHC class II, CD80, CD86 than non-loaded DCs indicating DC maturation. The increase in the levels of MHC class I, MHC class II, CD83, CD80, CD86 and CD40 expression of DCs loaded with IFN-γ/poly(I:C)-pulsed MJT3s were significant when compared to DCs loaded with γ-irradiated MJT3s (P < 0.0001, P = 0.0005, P = 0.0005, P = 0.0005, P = 0.0005 and P = 0.0052, respectively). Statistical significance was obtained between DC loaded with untreated MJT3 and DC loaded with IFN-γ/poly(I:C)-treated MJT3 for MHC class I, MHC class II, CD80, CD86 and CD40, which is not shown for clarity of graphs but indicated as fold increase in Supplementary Fig. 2b. The difference between DC loaded with untreated MJT3 and DC loaded with irradiated MJT3 was only significant for CD40. The relative expression levels of maturation markers between different donors varied, which explains the magnitude of error bars generated for the 15 individuals investigated, as has been observed by others [50]. Data expressed as fold increase relative to non-loaded DCs also showed significant differences between DCs loaded with IFN-γ/poly(I:C) tumour cells compared to DCs loaded with γ-irradiated tumour cells (P = 0.0006, P = 0.0005, P = 0.0005, P = 0.0005, P = 0.0005, P = 0.0042) (Supplementary Fig. 2b).
Phenotype of dendritic cells. a Changes in surface phenotype of DCs loaded with apoptotic tumour cells. Immature DCs were cultured for 24 h alone, with untreated, irradiated or IFN-γ/poly(I:C)-treated MJT3s at 1:1 DC to TC ratio. The MFI of markers expressed on CD11c positive gated DCs was analysed in FCS Express. The data show the MFI obtained from 15 individuals. The error bars indicate standard deviation (SD). Statistically significant differences are shown (*P < 0.05; **P < 0.01; ***P < 0.001) as determined by paired t test using GraphPad Prism 5 data analysis software. Data failing normality test was subjected to Wilcoxon signed-rank test. Statistical data are shown between non-loaded and tumour cell loaded DCs and between DCs loaded with γ-irradiated MJT3s and DCs loaded with IFN-γ/poly(I:C)-treated MJT3s. b Comparison of surface phenotype of DCs loaded with IFN-γ/poly(I:C) tumour cells and DCs matured with the ‘gold standard’ maturation cocktail. Results show the MFI of 5 independent experiments. Statistical analysis was carried out as in a. c Dendritic cells loaded with IFN-γ/poly(I:C)-treated tumour cells exhibit a Th1-biased phenotype. IL-12p70 and IL-10 release were detected in culture supernatants of non-loaded or tumour cell loaded DCs after CD40 ligation. The data show the ratio of IL12p70 to IL-10 of 3 independent experiments using PBMCs of different individuals. The error bars indicate standard deviation (SD). Statistically significant differences are shown (*P < 0.05) as determined by paired t test using GraphPad Prism 5 data analysis software
Although statistically significant differences were obtained, there was only a small increase in CD83 and CD40 expression by DCs loaded with IFN-γ/poly(I:C) tumour cells. This observation was surprising since these molecules are recognised as markers of maturation in DCs. Therefore, we compared CD83, CD80, CD86 and CD40 upregulation seen with IFN-γ/poly(I:C)-treated tumour cells to those seen with the ‘gold standard’ maturation cocktail (Fig. 3b). As expected, the standard maturation cocktail upregulated CD83, CD80, CD86 and CD40 on DCs validating the detection method and the quality of DCs generated in our laboratory (P = 0.0191, P = 0.0087, P = 0.0217, P = 0.0019). The expression level of CD83 was several folds lower than CD86 on cocktail-matured DCs; however, this has been observed by others [50]. Although CD83 and CD40 expression of cocktail-matured DCs were higher than those co-cultured with MJT3 IFN-γ/poly(I:C), it should be noted that CD86 upregulation, the costimulatory molecule which is thought to have major role in initial T cell priming, was similar in both cases [51]. Furthermore, expression of CD80 and CD86 by cocktail-matured DCs was further enhanced when they were pre-loaded with IFN-γ/poly(I:C) tumour cells (Fig. 3b), which was not seen when DCs were pre-loaded with lysates (data not shown). This indicates that tumour cell death induced by IFN-γ/poly(I:C) has no inhibitory properties on DC maturation. Furthermore, this approach has the potential to deliver additional DC maturation signals compared with tumour lysates, a commonly used whole tumour cell loading strategy for DC-based vaccination [52, 53].
Co-culture of DCs with IFN-γ/poly(I:C) MJT3s in the presence of Benzonase Nuclease (BN) confirmed that DC maturation was not caused by residual poly(I:C) (Supplementary Fig. 2c). To investigate whether tumour cells transfected with poly(I:C) had similar properties, we immunophenotyped DCs that had internalised IFN-γ pre-treated and poly(I:C)-transfected KMs and found DC maturation (Supplementary Fig. 2d). KMs that were unresponsive to IFN-γ/poly(I:C) pulsing did not cause DC maturation indicating that DC maturation was not caused by IFN-γ or poly(I:C) carry over via the tumour cells. Untreated or irradiated KMs did not mature DCs. Furthermore, IFN-γ treatment of MJT3 cells alone did not induce DC maturation to such extent as IFN-γ/poly(I:C) treatment (Supplementary Fig. 2e). In conclusion, tumour cell lines when induced to die by effective loading of poly(I:C), either by IFN-γ/poly(I:C) pulsing or transfection, exhibit a greater ability to drive DC maturation then their γ-irradiated counterparts.
Endocytosis of IFN-γ/poly(I:C) tumour cells drives DCs towards a Th1-biased phenotype
Apart from antigen presentation (‘signal 1’) and costimulatory interaction (‘signal 2’), efficient polarisation of T cells requires a third signal delivered by other means, such as inflammatory cytokines, environmental factors, and interaction between CD40 on the DC and the rapidly induced CD40L on the activated antigen-specific Th cells [54–56]. Mature DCs capable of directing a Th1 response are capable of releasing both IL-12p70 and IL-10 but are biased towards the production of IL-12p70 [57]. In order to achieve a Th1 response, it is believed that DCs need to be able to release IL-12p70 especially at the phase of DC/T cell interaction [58]. Therefore, we investigated IL-12p70 release relative to IL-10 from DCs loaded with apoptotic tumour cells after interaction with CD40 ligand transfected cells, used to mimic DC/T cell interaction. We show that uptake of IFN-γ/poly(I:C)-treated tumour cells induce DCs to release significantly higher levels of IL-12p70 relative to IL-10 after CD40 ligation when compared to non-loaded DCs, unlike DCs loaded with γ-irradiated tumour cells (Fig. 3c).
Induction of apoptosis by IFN-γ/poly(I:C) tumour cells primes DCs to direct type 1 T cell differentiation
In order to compare T cell activation by the DCs loaded with irradiated or IFN-γ/poly(I:C) tumour cells, loaded DCs were co-cultured with autologous non-adherent PBMCs. To make the readout clinically relevant, in these experiments, the IFN-γ/poly(I:C) tumour cells were also irradiated prior to DC loading. This did not affect apoptosis of the tumour cells and maturation by the phagocytosing DC (data not shown). We compared the cytokine response of autologous T cells to DCs loaded with apoptotic tumour cells and to tumour lysate-loaded DCs matured with a standard cytokine cocktail. Cytokine quantification showed that the levels of IFN-γ and IL-2 increased in the culture supernatants of PBMCs that were co-cultured with either DCs loaded with the irradiated or the IFN-γ/poly(I:C)-treated MJT3s at day 4 (Fig. 4). However, only the PBMCs cultured with DCs that had been loaded with IFN-γ/poly(I:C)-treated MJT3s continued to release IFN-γ, which lead to a significant increase in IFN-γ release at day 7, when compared to non-loaded DCs or DCs loaded with γ-irradiated MJT3s. This release of IFN-γ was not detected when MJT3s were co-cultured with DCs or PBMCs indicating that it was due to the interaction of tumour cell loaded DCs with PBMCs (data not shown). Importantly, very low levels of IL-10 were detected (<40 pg/ml) in all DC/PBMC co-culture supernatants and IL-4 levels were under the detection level of the bioplex assay (data not shown). Similar results were observed when loaded DCs were co-cultured with CD3+ naïve T cells (Supplementary Fig. 3a) indicating that the increased IFN-γ release was due to T cells activated by the tumour cell loaded DCs. Although the extent of DC maturation was greater in lysate-loaded cocktail-matured DCs, DCs that were loaded with the IFN-γ/poly(I:C)-treated tumour cells, irrespective of the lower expression of maturation markers, enhanced the production of IFN-γ from PBMC to a much greater extent (Supplementary Fig. 3b). In addition, the ability of such apoptotic tumour cell preparation to induce Th1-biasing cytokine release can be further enhanced with the standard maturation cocktail.
DCs loaded with IFN-γ/poly(I:C)-treated MJT3s induce higher levels of IFN-γ release from PBMCs than DCs loaded with γ-irradiated MJT3s. The graphs show the mean and SD of the data obtained in 8 individual experiments using PBMCs of different individuals. The data were analysed by Wilcoxon signed-rank test in GraphPad Prism5. IL-10 and IL-2 concentrations were below the detection limit of the Luminex assay (<30 pg/ml) by day 7; therefore, they were excluded from statistical analysis. Statistically significant data are indicated (*P < 0.05; **P < 0.01)
In order to investigate whether the IFN-γ release measured in the co-culture supernatants was due to type 1 biased CD4+ T helper cells, the percentage of IFN-γ producing CD4+ T cells were identified by intracellular cytokine staining. Paired t test analysis showed that the percentage of both IFN-γ and IL-2 producing CD4+ T cells was significantly higher when PBMCs were co-cultured with DCs loaded with IFN-γ/poly(I:C)-treated tumour cells compared to those loaded with γ-irradiated tumour cells at both day 4 (P = 0.0070 and P = 0.0107, respectively) and day 7 time points (P = 0.0180 and P = 0.0140, respectively) (Fig. 5a). At the same time, the percentage of IL-10 producing CD4+ T cells was low. This induction of Th1 cell polarisation was not seen by the DCs that had ingested γ-irradiated tumour cells. Indeed, a reduction in IFN-γ producing CD4+ T cells was observed when PBMCs were co-cultured with irradiated MJT3 loaded DCs compared to non-loaded DC-stimulated PBMCs (data not shown). Similar cytokine profiles were seen by the CD8+ T cell population suggesting enhanced cross-presenting ability of those DCs that had ingested IFN-γ/poly(I:C) MJT3s (Fig. 5b). Tumour cell lines that were rendered apoptotic by poly(I:C) transfection also had the ability to skew DCs to direct a type 1 T cell response as opposed to their irradiated counterparts. Three independent experiments showed induction of IFN-γ producing CD4+ and CD8+ T cell responses when PBMCs were stimulated with DCs that had ingested IFN-γ/poly(I:C) KMs, while irradiated KMs did not stimulate such responses (Fig. 5c).
Dendritic cells loaded with IFN-γ/poly(I:C) tumour cells can direct the development of a Th1 type of immune response. a DCs loaded with IFN-γ pre-treated poly(I:C)-pulsed tumour cells have a greater ability to induce IFN-γ and IL-2 positive CD4+ T cells than DCs loaded with γ-irradiated tumour cells. DCs loaded with γ-irradiated (filled triangle) or IFN-γ/poly(I:C) (filled circle) MJT3 cells were co-cultured with autologous PBMCs for 4 or 7 days. Cells positive for both CD3 and CD4 were analysed for cytokine production in FCS express. Paired t test analysis was carried out throughout using GraphPad Prism 5 software. Statistically significant increase or decrease in the percentage of cytokine producing CD4+ T cells is shown (*P < 0.05, **P < 0.01) obtained from 8 individual experiments. b DCs loaded with IFN-γ/poly(I:C) MJT3s are more potent inducers of IFN-γ and IL-2 producing CD8+ T cells than DCs loaded with γ-irradiated MJT3s. c DCs loaded with IFN-γ pre-treated and poly(I:C)-transfected tumour cells are also more potent at inducing both CD4+ and CD8+ type 1-biased T cells than those loaded with the irradiated counterparts. The comparison of non-loaded DCs (DC) (open square), DCs loaded with irradiated KMs (KM irr) (filled triangle) and DCs loaded with IFN-γ/poly(I:C) KMs (KM IFN/pIC) (filled circle) are shown at day 7 time point
Dendritic cells matured with IFN-γ/poly(I:C)-treated tumour cells have an enhanced ability to induce antigen-specific CD8+ T cells
In order to investigate whether DCs loaded with IFN-γ/poly(I:C)-treated MJT3s had elevated potential to expand antigen-specific T cells compared to DCs loaded with γ-irradiated MJT3s, we pre-loaded DCs with M1 peptide and determined their ability to generate M1 specific CD8+ T cells. In the three donors investigated, M1 loaded DCs matured with IFN-γ/poly(I:C)-treated MJT3s consistently induced a greater number of M1 specific CD8+ T cells when compared to irradiated MJT3s or DCs matured with the standard maturation cocktail (Fig. 6a, b).
Dendritic cells loaded with IFN-γ/poly(I:C)-treated MJT3 have the ability to induce antigen-specific CD8+ T cells. a Representative figures showing M1+ CD8+ T cell induction by differentially matured M1 loaded DCs. M1 peptide loaded DCs (DC/M1) were cultured alone, with γ-irradiated or IFN-γ/poly(I:C) pulsed MJT3s or in the presence of ‘gold standard’ maturation cocktail (mat.co) for 24 h. Autologous T cells were stimulated with the DCs and re-stimulated on day 7. T cells were stained with APC-conjugated M1 Pentamers on day 14. b Induction of M1+ CD8+ T cells by M1 loaded DCs matured with apoptotic tumour cells as in three independent experiments carried out from PBMCs of three individuals
Discussion
Loading of DCs with TAAs is a crucial component of vaccine production to ensure that specific cytotoxicity against tumour cells is induced. Loading of DCs with apoptotic tumour cells could provide DCs with multiple tumour antigens for cross-presentation. In this paper, we demonstrate that tumour cells undergo apoptosis following culture with IFN-γ and poly(I:C). Virally infected cells can be triggered to die by apoptosis in response to viral dsRNA generated during the replication cycle of many DNA and RNA viruses [6]. Synthetic dsRNA (poly I:C), presumably mimicking viral dsRNA, has been shown to induce cell death in various cell types such as Cama/1 breast cancer [59] and Me260 melanoma cells [60], while interferons (IFNs) have been suggested as sensitisers to cell death [60–62]. Our data demonstrate that IFN-γ can augment cell death induced by poly(I:C) possibly due to the upregulation of the receptor for dsRNA, TLR3, that has been reported by others [63]. Tumour cells that do not respond to poly(I:C) can be induced to die via apoptosis when they are loaded with the poly(I:C) using a cationic lipid-based transfection reagent. The need for internalisation of poly(I:C) in order to induce apoptosis has recently been demonstrated by others [64]. This suggests that not all tumour cells are capable of internalising poly(I:C), but once inside, poly(I:C) is capable of inducing cell death in all tumour cells tested. The enhanced Th1 polarising ability of DCs loaded with poly(I:C) electroporated leukaemia cells has been demonstrated compared to their pulsed counterparts [65]. Our study, however, clearly indicates that tumour cell lines susceptible to the apoptotic effect of poly(I:C) when pre-sensitised with IFN-γ does not require transfection. A clinical trial has demonstrated significant survival benefit associated with using IFN-γ-treated autologous tumour cell loaded DCs in patients with advanced melanoma [66]. Therefore, in our system, IFN-γ may not only act as sensitiser to the apoptotic effect of poly(I:C) but may also induce more potent DCs. Furthermore, our study compares this treatment with γ-irradiation, which is important since irradiation is the conventional way of inducing apoptosis in tumour cells used for DC loading [50]. It has been documented that apoptotic tumour cell death induced by irradiation only partially matures DCs, rendering them insufficient for CTL priming without additional signals [4]. We also show here that γ-irradiation induces tumour cell apoptosis to a lesser extent in all cell lines tested during the time course investigated than IFN-γ/poly(I:C).
Gene array analysis of apoptotic tumour cells induced by IFN-γ/poly(I:C) treatment showed marked differences in gene expression compared to those subjected to irradiation. The major finding of these studies is that IFN-γ/poly(I:C) treatment increased the expression of immunomodulatory chemokines and cytokines. These include IL-6, IL-8 and TNF-α, confirmed by our protein bead array studies (data not shown) but also chemokines such as CCL3, CCL5 and CCL20. These three chemokines have recently been implicated in the ability of exosomes isolated from heat stressed tumour cells to activate dendritic cells and T cells [67]. An unexpected finding of this part of the study was that a range of heat shock protein families were decreased in IFN-γ/poly(I:C) tumour cells, and a member of the HSP70 family is increased in irradiated cells. This finding appears to be counter-intuitive in the light of evidence that HSP can increase the immunogenicity of tumour cells [68]. Our study suggests that chemokine/cytokine signals might override HSP signals. Indeed, one reported function of HSP is to induce cytokine secretion [69]. One additional finding is the upregulation of mRNA in the IFN-γ/poly(I:C) tumour cells for IL-28, IL-29 and IFNω. IL-28 and 29 are both identified as members of the newly termed IFNλ family that shares antiviral activity with type 1 interferons and can be stimulated by TLR3 activation [70].
In the context of cancer therapy, to achieve a potent DC vaccine, DCs need to be able to drive the differentiation of a type 1-biased immune response that is capable of recognising and eliminating the targeted tumour cells. Dendritic cells are the most potent antigen-presenting cells unique in their ability to cross-present exogenous antigens acquired from apoptotic cells in the context of MHC class I molecules for direct activation of CD8+ T cells [16, 42]. Dendritic cells have a central role in both innate and adaptive immune responses, and therefore, they have been extensively used in therapeutic cancer vaccines. The rapid engulfment of apoptotic cells in vivo is considered an immunologically silent event [5]. We show here that this is not the case for apoptotic tumour cells obtained by IFN-γ/poly(I:C) pulsing or transfection, because DCs that had endocytosed such apoptotic tumour cells were able to generate a Th1-biased T cell response. Uptake of IFN-γ/poly(I:C) tumour material may provide poly(I:C) to the endocytosing DC ligating TLR3 which, in turn, can lead to maturation. The delivery of antigen and TLR ligand has previously been shown to result in the optimal maturation of DCs where the efficiency of presenting antigens from phagocytosed cargo is dependent on the presence of TLR ligands within the cargo [71]. Immunisation of mice with virus-infected cells or cells containing synthetic dsRNA has been shown to result in CTL cross-priming against cell-associated antigens via TLR3 triggering [72].
Phenotypic maturation of DCs involving the upregulation of co-stimulatory molecules is essential to achieve induction of immunity. It has been shown by others that apoptotic tumour cells in the absence of danger signals can induce only partial maturation of DCs [73] and is also dependent on the phase of apoptosis of neoplastic cells [4]. Consistent with these observations, our data shows that IFN-γ/poly(I:C) tumour cells are capable of driving DCs towards a mature phenotype more effectively than γ-irradiated tumour cells, despite ingestion of both by DCs. These data suggest that tumour cells induced to die by IFN-γ and poly(I:C) can deliver maturation signals to the endocytosing DC. The extent of maturation is often used as a measure of T cell activating capacity of DCs; however, DCs with various maturation states have been shown to activate or suppress immune responses [74, 75]. In this study, the accepted surface phenotypic maturation of DCs does not seem to directly correlate with their ability to prime T cells.
According to current views, DCs capable of driving a type 1-biased immune response release pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β and in particular IL-12p70, while secreting lower amounts of immunosuppressive cytokines, such as IL-10 [76]. We observed TNF-α and IL-6 release (detected by Luminex) when DCs were co-cultured with IFN-γ/poly(I:C)-induced apoptotic tumour cells although this was partially due to the production of cytokines by the tumour cells as they continued to release these cytokines even after extensive washing after apoptosis induction (data not shown). We saw no IL-12p70 or IL-10 secretion, from these DCs or from the tumour cells. Similar levels of TNF-α and IL-6 were observed by Smits et al. [65] in supernatants collected after 24 h co-culture of DCs with leukaemia cells with no or very low levels of IL-12p70. A recent publication by Longhi et al. [77] suggested the importance of IFN-β in the skewing of DCs towards a Th1-biasing phenotype when poly(I:C) is used as adjuvant. IFN-γ/poly(I:C) treatment of MJT3 cell line leads to IFN-β release peaking at 8 h (1,055 pg/ml), which is reduced by 24 h (185 pg/ml) (data not shown). However, by 48 h, there is no detectable IFN-β production; therefore, factors other than cytokines, such as apoptotic bodies containing poly(I:C) or exosomes, may be important contributors to DC differentiation in our system. Dendritic cells loaded with IFN-γ/poly(I:C)-treated apoptotic MJT3s were able to produce IL-12p70 after CD40 ligation and showed a stronger bias towards IL-12p70 rather than IL-10 when compared to DCs loaded with γ-irradiated MJT3s. This agrees with the observation that little or no biologically active IL-12 is released from TLR-stimulated DCs unless they are cultured with activated T cells or their products [78] in contrast to that reported by others [79]. As long as mature DCs retain the ability to produce sufficient IL-12p70 at the time of interaction with T cells, they are thought to be able to induce the differentiation of a Th1 phenotype [74, 80].
Dendritic cells can induce antigen-specific CTL responses via Th1 activation and via cross-presentation of apoptotic cell-derived antigenic peptides to CD8+ cytotoxic lymphocytes. To achieve an anti-tumour immune response, the induction of a Th1 immune response is required, which can support CTLs to kill targeted tumour cells. Our data demonstrates that uptake of IFN-γ/poly(I:C) tumour cells prime DCs to induce the development of a type 1 polarised T cell response and indicate cross-presentation of tumour-derived antigens by the loaded DCs to CD8+ T cells. The increase in the percentage of IFN-γ producing CD4+ T cells while maintaining low numbers of IL-10 producing CD4+ T cells suggests that these DCs are capable of driving Th1 activation. Regardless of whether tumour cell apoptosis was induced by pulsing or loading with the poly(I:C), DC maturation and activation were achieved with Th1 priming ability. In addition, DCs loaded with IFN-γ/poly(I:C)-treated tumour cells have an enhanced ability to expand antigen-specific CD8+ T cells when compared to DC loaded with γ-irradiated tumour cells as well as DCs matured with the ‘gold standard’ maturation cocktail.
Gamma irradiation has been shown to induce immunogenic cell death in CT26 colon carcinoma cells [49]. We demonstrate that DCs co-cultured with γ-irradiated MJT3, KM or A549 tumour cells are unable to drive a Th1-biased response. Immunogenicity of tumour cell death has been suggested to be dictated by the exposure of Calreticulin (CRT) [31]. We saw an increase in CRT expression by IFN-γ/poly(I:C) MJT3 when compared to untreated or γ-irradiated MJT3s correlating with their ability to prime DCs towards the induction of a Th1-biased response. A similar expression of CRT was not observed in the KM cell line although a Th1-biased T cell response was suggesting that signals other than CRT may play a role in this effect. New maturation cocktails have been designed recently and those containing poly(I:C) seem to be most efficient at driving Th1-biased responses [50, 81]. Unlike freeze/thaw lysed tumour cells that have been indicated to disrupt the responsiveness of DCs to TLR stimulation [82], IFN-γ/poly(I:C)-induced apoptotic tumour cells do not inhibit DC activation and show immunogenic potential enhancing T cell activating capacity of cocktail-matured DC. In agreement with Pietra et al. [4], our data indicate that irradiated tumour cells only induce partial DC maturation not capable of driving Th1 differentiation, while IFN-γ/poly(I:C)-treated tumour cells induce DC maturation with the ability to drive Th1 as well as antigen-specific CD8+ T cells responses.
In conclusion, we found that tumour cell lines can respond to poly(I:C) by undergoing apoptosis, which is enhanced by IFN-γ pre-treatment. All cell lines tested were susceptible to apoptosis if poly(I:C) was introduced by forced loading. Unlike γ-irradiated tumour cells, IFN-γ/poly(I:C) tumour cells had the ability to induce DC activation that can drive the development of a type 1 immune response. These data suggest that whole cell vaccine preparations may benefit from using dying cells for DC loading that have been modified to contain ‘danger signals’, such as the synthetic analogue of dsRNA, to enhance their immunogenic potential.
Abbreviations
- IFN-γ:
-
Interferon-gamma
- dsRNA:
-
Double stranded RNA
- IFN-γ/poly(I:C) tumour cell:
-
IFN-γ pre-treated and poly(I:C) pulsed or loaded tumour cell
References
Hacker G (2000) The morphology of apoptosis. Cell Tissue Res 301:5–17
Kurosaka K, Takahashi M, Watanabe N, Kobayashi Y (2003) Silent cleanup of very early apoptotic cells by macrophages. J Immunol 171:4672–4679
Savill J, Dransfield I, Gregory C, Haslett C (2002) A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2:965–975
Pietra G, Mortarini R, Parmiani G, Anichini A (2001) Phases of apoptosis of melanoma cells, but not of normal melanocytes, differently affect maturation of myeloid dendritic cells. Cancer Res 61:8218–8226
Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101:890–898
Jacobs BL, Langland JO (1996) When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology 219:339–349
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732–738
Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105
Meusel TR, Kehoe KE, Imani F (2002) Protein kinase R regulates double-stranded RNA induction of TNF-alpha but not IL-1 beta mRNA in human epithelial cells. J Immunol 168:6429–6435
Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH (1997) Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol 27:3135–3142
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Sallusto F, Cella M, Danieli C, Lanzavecchia A (1995) Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med 182:389–400
Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N (1998) Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med 188:1359–1368
Rubartelli A, Poggi A, Zocchi MR (1997) The selective engulfment of apoptotic bodies by dendritic cells is mediated by the alpha(v)beta3 integrin and requires intracellular and extracellular calcium. Eur J Immunol 27:1893–1900
Skoberne M, Somersan S, Almodovar W, Truong T, Petrova K, Henson PM, Bhardwaj N (2006) The apoptotic-cell receptor CR3, but not alphavbeta5, is a regulator of human dendritic-cell immunostimulatory function. Blood 108:947–955
Albert ML, Sauter B, Bhardwaj N (1998) Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86–89
Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K (2000) Immunobiology of dendritic cells. Annu Rev Immunol 18:767–811
van Wilsem EJ, Breve J, Kleijmeer M, Kraal G (1994) Antigen-bearing Langerhans cells in skin draining lymph nodes: phenotype and kinetics of migration. J Invest Dermatol 103:217–220
Blander JM (2007) Signalling and phagocytosis in the orchestration of host defence. Cell Microbiol 9:290–299
Kapsenberg ML, Hilkens CM, Wierenga EA, Kalinski P (1999) The paradigm of type 1 and type 2 antigen-presenting cells. Implications for atopic allergy. Clin Exp Allergy 29(Suppl 2):33–36
Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA (2006) Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology 117:433–442
Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N (2001) Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med 193:233–238
Schuler-Thurner B, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, Bender A, Feuerstein B, Fritsch PO, Romani N, Schuler G (2002) Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med 195:1279–1288
Moller I, Michel K, Frech N, Burger M, Pfeifer D, Frommolt P, Veelken H, Thomas-Kaskel AK (2008) Dendritic cell maturation with poly(I:C)-based versus PGE2-based cytokine combinations results in differential functional characteristics relevant to clinical application. J Immunother 31:506–519
Verdijk RM, Mutis T, Esendam B, Kamp J, Melief CJ, Brand A, Goulmy E (1999) Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J Immunol 163:57–61
Sallusto F, Lanzavecchia A (1994) Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179:1109–1118
Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N (1996) Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods 196:121–135
Reis e Sousa C, Diebold SD, Edwards AD, Rogers N, Schulz O, Sporri R (2003) Regulation of dendritic cell function by microbial stimuli. Pathol Biol (Paris) 51:67–68
Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P (2004) Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res 64:5934–5937
Restifo NP (2000) Building better vaccines: how apoptotic cell death can induce inflammation and activate innate and adaptive immunity. Curr Opin Immunol 12:597–603
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G (2007) Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 13:54–61
Medzhitov R, Janeway CA Jr (2000) How does the immune system distinguish self from nonself? Semin Immunol 12:185–188 (discussion 257–344)
Ullrich E, Bonmort M, Mignot G, Kroemer G, Zitvogel L (2008) Tumor stress, cell death and the ensuing immune response. Cell Death Differ 15:21–28
Stevanovic S (2002) Identification of tumour-associated T-cell epitopes for vaccine development. Nat Rev Cancer 2:514–520
Banchereau J, Palucka AK (2005) Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol 5:296–306
Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D (1998) Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 4:328–332
Redman BG, Chang AE, Whitfield J, Esper P, Jiang G, Braun T, Roessler B, Mule JJ (2008) Phase Ib trial assessing autologous, tumor-pulsed dendritic cells as a vaccine administered with or without IL-2 in patients with metastatic melanoma. J Immunother 31:591–598
Adams M, Navabi H, Jasani B, Man S, Fiander A, Evans AS, Donninger C, Mason M (2003) Dendritic cell (DC) based therapy for cervical cancer: use of DC pulsed with tumour lysate and matured with a novel synthetic clinically non-toxic double stranded RNA analogue poly [I]:poly [C(12)U] (Ampligen R). Vaccine 21:787–790
O’Rourke MG, Johnson MK, Lanagan CM, See JL, O’Connor LE, Slater GJ, Thomas D, Lopez JA, Martinez NR, Ellem KA, Schmidt CW (2007) Dendritic cell immunotherapy for stage IV melanoma. Melanoma Res 17:316–322
Delirezh N, Moazzeni SM, Shokri F, Shokrgozar MA, Atri M, Kokhaei P (2009) Autologous dendritic cells loaded with apoptotic tumor cells induce T cell-mediated immune responses against breast cancer in vitro. Cell Immunol 257:23–31
Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L (2005) Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+ CD25+ regulatory T cell proliferation. J Exp Med 202:919–929
Russo V, Tanzarella S, Dalerba P, Rigatti D, Rovere P, Villa A, Bordignon C, Traversari C (2000) Dendritic cells acquire the MAGE-3 human tumor antigen from apoptotic cells and induce a class I-restricted T cell response. Proc Natl Acad Sci USA 97:2185–2190
Goldszmid RS, Idoyaga J, Bravo AI, Steinman R, Mordoh J, Wainstok R (2003) Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. J Immunol 171:5940–5947
Kovalcsik E, John J, Turner M, Birchall L, Sage D, Whittle R, Dalgleish A, Pandha H (2004) Differential expression of melanoma-associated antigens and molecules involved in antigen processing and presentation in three cell lines established from a single patient. Melanoma Res 14:463–471
Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G (1996) Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods 196:137–151
Kalinski P, Wieckowski E, Muthuswamy R, de Jong E (2010) Generation of stable Th1/CTL-, Th2-, and Th17-inducing human dendritic cells. Methods Mol Biol 595:117–133
Lane P, Burdet C, McConnell F, Lanzavecchia A, Padovan E (1995) CD40 ligand-independent B cell activation revealed by CD40 ligand-deficient T cell clones: evidence for distinct activation requirements for antibody formation and B cell proliferation. Eur J Immunol 25:1788–1793
Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P (2009) Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta 1805:53–71
Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, Zitvogel L, Kroemer G (2007) Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ 14:1848–1850
Wieckowski E, Chatta GS, Mailliard RM, Gooding W, Palucka K, Banchereau J, Kalinski P (2010) Type-1 polarized dendritic cells loaded with apoptotic prostate cancer cells are potent inducers of CD8(+) T cells against prostate cancer cells and defined prostate cancer-specific epitopes. Prostate 71:125–133
Denoeud J, Moser M (2010) Role of CD27/CD70 pathway of activation in immunity and tolerance. J Leukoc Biol 89:195–203
Tamir A, Basagila E, Kagahzian A, Jiao L, Jensen S, Nicholls J, Tate P, Stamp G, Farzaneh F, Harrison P, Stauss H, George AJ, Habib N, Lechler RI, Lombardi G (2007) Induction of tumor-specific T-cell responses by vaccination with tumor lysate-loaded dendritic cells in colorectal cancer patients with carcinoembryonic-antigen positive tumors. Cancer Immunol Immunother 56:2003–2016
Hatfield P, Merrick AE, West E, O’Donnell D, Selby P, Vile R, Melcher AA (2008) Optimization of dendritic cell loading with tumor cell lysates for cancer immunotherapy. J Immunother 31:620–632
Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P (2000) Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol 164:4507–4512
Cools N, Ponsaerts P, Van Tendeloo VF, Berneman ZN (2007) Balancing between immunity and tolerance: an interplay between dendritic cells, regulatory T cells, and effector T cells. J Leukoc Biol 82:1365–1374
Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML (1999) T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today 20:561–567
Trepiakas R, Pedersen AE, Met O, Hansen MH, Berntsen A, Svane IM (2008) Comparison of alpha-Type-1 polarizing and standard dendritic cell cytokine cocktail for maturation of therapeutic monocyte-derived dendritic cell preparations from cancer patients. Vaccine 26:2824–2832
Giermasz AS, Urban JA, Nakamura Y, Watchmaker P, Cumberland RL, Gooding W, Kalinski P (2009) Type-1 polarized dendritic cells primed for high IL-12 production show enhanced activity as cancer vaccines. Cancer Immunol Immunother 58:1329–1336
Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T (2006) TLR3 can directly trigger apoptosis in human cancer cells. J Immunol 176:4894–4901
Salaun B, Lebecque S, Matikainen S, Rimoldi D, Romero P (2007) Toll-like receptor 3 expressed by melanoma cells as a target for therapy? Clin Cancer Res 13:4565–4574
Balachandran S, Roberts PC, Kipperman T, Bhalla KN, Compans RW, Archer DR, Barber GN (2000) Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. J Virol 74:1513–1523
Tissari J, Siren J, Meri S, Julkunen I, Matikainen S (2005) IFN-alpha enhances TLR3-mediated antiviral cytokine expression in human endothelial and epithelial cells by up-regulating TLR3 expression. J Immunol 174:4289–4294
Sato A, Iizuka M, Nakagomi O, Suzuki M, Horie Y, Konno S, Hirasawa F, Sasaki K, Shindo K, Watanabe S (2006) Rotavirus double-stranded RNA induces apoptosis and diminishes wound repair in rat intestinal epithelial cells. J Gastroenterol Hepatol 21:521–530
Besch R, Poeck H, Hohenauer T, Senft D, Hacker G, Berking C, Hornung V, Endres S, Ruzicka T, Rothenfusser S, Hartmann G (2009) Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J Clin Invest 119:2399–2411
Smits EL, Ponsaerts P, Van de Velde AL, Van Driessche A, Cools N, Lenjou M, Nijs G, Van Bockstaele DR, Berneman ZN, Van Tendeloo VF (2007) Proinflammatory response of human leukemic cells to dsRNA transfection linked to activation of dendritic cells. Leukemia 21:1691–1699
Cornforth AN, Fowler AW, Carbonell DJ, Dillman RO (2011) Resistance to the proapoptotic effects of interferon-gamma on melanoma cells used in patient-specific dendritic cell immunotherapy is associated with improved overall survival. Cancer Immunol Immunother 60:123–131
Chen T, Guo J, Yang M, Zhu X, Cao X (2011) Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J Immunol 186:2219–2228
Knudsen S, Schardt A, Buhl T, Boeckmann L, Schon MP, Neumann C, Haenssle HA (2010) Enhanced T-cell activation by immature dendritic cells loaded with HSP70-expressing heat-killed melanoma cells. Exp Dermatol 19:108–116
Yokota S, Fujii N (2010) Immunomodulatory activity of extracellular heat shock proteins and their autoantibodies. Microbiol Immunol 54:299–307
Uze G, Monneron D (2007) IL-28 and IL-29: newcomers to the interferon family. Biochimie 89:729–734
Blander JM, Medzhitov R (2006) Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440:808–812
Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, Reis e Sousa C (2005) Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433:887–892
Kacani L, Wurm M, Schwentner I, Andrle J, Schennach H, Sprinzl GM (2005) Maturation of dendritic cells in the presence of living, apoptotic and necrotic tumour cells derived from squamous cell carcinoma of head and neck. Oral Oncol 41:17–24
Felzmann T, Huttner KG, Breuer SK, Wimmer D, Ressmann G, Wagner D, Paul P, Lehner M, Heitger A, Holter W (2005) Semi-mature IL-12 secreting dendritic cells present exogenous antigen to trigger cytolytic immune responses. Cancer Immunol Immunother 54:769–780
Frick JS, Grunebach F, Autenrieth IB (2010) Immunomodulation by semi-mature dendritic cells: a novel role of Toll-like receptors and interleukin-6. Int J Med Microbiol 300:19–24
Schuler G, Schuler-Thurner B, Steinman RM (2003) The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol 15:138–147
Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, Salazar AM, Colonna M, Steinman RM (2009) Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med 206:1589–1602
Abdi K, Singh N, Matzinger P (2006) T-cell control of IL-12p75 production. Scand J Immunol 64:83–92
Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A (2005) Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol 6:769–776
Steinman RM (2003) Some interfaces of dendritic cell biology. Apmis 111:675–697
Jensen SS, Gad M (2010) Differential induction of inflammatory cytokines by dendritic cells treated with novel TLR-agonist and cytokine based cocktails: targeting dendritic cells in autoimmunity. J Inflamm (Lond) 7:37
Tirapu I, Lewis A, Kreutz M, McLinden H, Diebold SS (2008) Freeze-and-thaw-disrupted tumour cells impair the responsiveness of DC to TLR stimulation. Eur J Immunol 38:2740–2750
Acknowledgments
This work was supported by funding from the Fischer Family Trust (1075453), and the Cancer Vaccine Institute. The authors would like to thank Dr Jayne Dennis and Dr Gary Coulton of the SGUL Biomics Unit for technical assistance and helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kovalcsik, E., Lowe, K., Fischer, M. et al. Poly(I:C)-induced tumour cell death leads to DC maturation and Th1 activation. Cancer Immunol Immunother 60, 1609–1624 (2011). https://doi.org/10.1007/s00262-011-1058-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1058-7