Abstract
In this study, we developed a unique in vitro model to mimic the endogenous tumor microenvironment to understand the effect of immunotherapy with activated T-cells (ATC) armed with anti-CD3 × anti-Her2 bispecific antibody (aATC) on antibody response by naive immune cells. This model contained a co-culture of naïve peripheral blood mononuclear cells (PBMC), breast cancer cells (SK-BR-3), ATC or aATC and CpG ODNs. Culture supernatants were tested at various time points for anti-SK-BR-3 antibodies by ELISA, Western blot and flow cytometry. PBMC cocultured with non-irradiated aATC or irradiated (*) aATC showed significant increases in anti-tumor antibody production at day 14 (P < 0.0001) in the presence of CpG-ODN compared to unstimulated PBMC cultures (n = 9). Antibody specificity was confirmed by ELISA, Western blot and flow cytometry. Co-cultures containing *aATC and CpG showed significantly enhanced levels of IgG2 (P < 0.001) and cytokines that promote IgG2 synthesis including IL-13 (P < 0.02), IFNγ (P < 0.01) and GM-CSF (P < 0.05) compared to unstimulated PBMC control (n = 3). We show that aATC targeting and lysis of tumor cells induces an anti-tumor antibody response in our in vitro model. This model provides a unique opportunity to evaluate the interactions of T-cells, B-cells, and antigen-presenting cells leading to specific anti-tumor antibody responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the induction of tumor-specific cytotoxic T-cell responses is considered the gold standard for immunotherapy, there is precedence in murine and human vaccination models for the induction of specific anti-tumor antibody responses that lead to clinically significant treatment responses [1, 2]. For optimal tumor-specific cytotoxic cellular and humoral responses, immunotherapeutic approaches must overcome tumor-induced suppression of vaccine responses. Coordinated interactions between antigen-presenting cells (APC), T-helper cells, and B-cells are required for an optimal immune response to tumor antigens [3]. Therapeutic strategies that improve target-specific killing with concomitant release of tumor-associated antigens (TAA) and promote a favorable microenvironment for generating a humoral response may lead to effective in vivo immunization. In our phase I clinical trial involving stage IV breast cancer patients who received activated T-cells (ATC) armed with anti-CD3 × anti-Her2/neu bispecific antibody (Her2Bi), high levels of specific anti-SK-BR-3 cytotoxicity by PBMC and circulating tumoricidal cytokines were observed. These findings suggest that Her2Bi-armed ATC (aATC) infusions “vaccinated” the endogenous immune system against patient’s own TAAs [4]. Our studies suggested that aATC-mediated target cell killing in the presence of endogenous immune cells leads to the development of both in vivo and in vitro tumor antigen-specific immune responses.
The best examples of successful anti-tumor antibody therapy are the use of Herceptin® (anti-HER2) or Rituxan® (anti-CD20) to treat HER2/neu positive breast cancers and CD20+ hematologic malignancies, respectively [5–7]. Antibodies synergize with cytotoxic T-cells by promoting uptake and cross-presentation of antibody opsonized TAA by APC. Antibodies are known to sensitize tumors for complement and antibody-dependent cytotoxicity [8–11], and presence of anti-tumor antibodies in vaccine trials have been correlated with improved survival [1, 2, 12], and delayed tumor progression [13, 14]. These studies provide evidence that humoral antibody responses play key roles in inducing clinically effective anti-tumor immunity.
To the best of our knowledge, this is the first study to show an in vitro anti-tumor antibody synthesis model to dissect the development of in vivo clinical immune responses. In order to consistently induce anti-tumor antibody production, we used CpG oligonucleotides (ODNs). CpG ODNs are known to activate B-cells, dendritic cells (DCs), and natural killer (NK) cells. CpG ODNs induce proliferation and generation of plasma cells [15] for polyclonal immunoglobulin (Ig) synthesis [16–18], particularly the synthesis of Th1-type IgG2 anti-tumor antibodies [19]. Moreover, CpG are being used as an immune adjuvant in clinical trials [20–22].
Our experiments were designed to determine: (1) the optimal conditions for inducing in vitro primary anti-tumor antibody synthesis in cultured PBMC from normal subjects; (2) the amount, Ig allotype, and specificity of antibody produced; (3) whether the antibodies mediate antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC); (4) subpopulations of T-cells; (5) the cytokine profiles of antibody-producing co-cultures; and (6) whether DC loaded with tumor lysate (produced by co-cultures of tumor cell and aATC) can present TAAs to naïve PBMC to induce specific anti-SK-BR-3 antibodies. Application of such an in vitro model will be a powerful tool for probing the interactions of T-cells, B-cells and APC in normal subjects as well as patients undergoing cancer immunotherapy.
Materials and methods
Cell lines and blood
The human breast cancer cell lines, SK-BR-3 and MCF-7 were cultured in RPMI 1640 (Lonza Inc., Allendale, NJ) and MDA-MB-231 in DMEM/F-12 (Lonza) supplemented with 10% FCS (Valley Biomedical) containing, 2 mM l-glutamine (Lonza Inc., Allendale, NJ), 50 units/ml penicillin-,and 50 μg/ml streptomycin (Lonza Inc., Allendale, NJ) at 37°C and 5% CO2. Human PBMC were isolated from the heparinized whole blood of normal healthy donors using lymphocyte separation solution. The Wayne State University Institutional Review Board approved research protocols for blood collection. All normal donors signed consent forms. The cultures for antibody synthesis were performed in RPMI 1640 supplemented with 10% FCS, l-glutamine (Lonza), and antibiotics.
Oligodeoxynucleotides
The ODNs used included CpG-B (TCG TCG TTT CGT CGT TTT GTC GTT), CpG-B control DNA (TGC TGC TTT TGT GCT TTT GTG CTT), and CpG DNA (TCG TCG TTT TCG GCG CGC GCC G) (Cooley Pharmaceutical group, Wellesley, MA). The optimal ODN concentration (5 μg/ml) was determined in a dose–response curve based on the optimal antibody responses of peripheral blood B-cells (data not shown). Unless otherwise mentioned, all of the co-cultures contained CpG. Cultures without CpG consistently produced background levels of anti-SK-BR-3 antibody.
Activation of T-cells and arming with BiAbs
Anti-CD3–activated ATC (ATC) were expanded in culture from PBMC for 6–14 days [4, 23]. ATC contained 89.0 ± 7.5% CD3+, 42.8 ± 17.3% CD4+, and 46.7% ± 13.2% CD8+ T-cells [4, 23]. Bispecific antibodies (BiAb) were produced by chemical heteroconjugation of OKT3 (murine IgG2a anti-CD3 monoclonal antibody (mAb), Ortho Biotech, Horsham, PA) and Herceptin® (a humanized anti-Her2 IgG1, Genentech Inc., San Francisco, CA) [24]. ATC were armed with 50 ng of BiAb/106 ATC for 30 min at room temperature with either Her2Bi or irrelevant anti-CD3 × anti-CD20 (CD20Bi) and washed to eliminate unbound BiAb [24].
Development of in vitro model for specific anti-tumor antibody synthesis
To optimize antibody synthesis, co-cultures were performed that contained naïve PBMC, SK-BR-3, ATC or aATC, and CpG ODNs. Schematic diagram 1 shows the role of each component in the co-culture system and the sequence of events that may take place. Details are as follows, PBMC were washed to remove carryover serum antibodies (5–6×) prior to culture. SK-BR-3 and PBMC (1 × 106) were co-cultured with 5 μg/ml CpG-B or control ODNs and 0.25 × 106 autologous 2500 rads irradiated (*) *ATC or *aATC or non-irradiated ATC or aATC in 200 μl medium. Cultures were performed in round-bottomed microtiter plates. Antibody production are expressed as ng/ml/106 cells. The purpose of adding ATC or aATC was to mimic the in vivo condition of TAA release that may occur during aATC immunotherapy by aATC-mediated lysis of tumor cells. aATC and ATC were irradiated to eliminate radiosensitive suppressor T-cells to provide “fixed” T-helper activity to B-cells [25, 26]. In selected experiments, non-irradiated ATC or aATC were used in co-cultures. CpG ODNs were added to stimulate antigen-presenting cells (APCs) and B-cells. Co-cultures were antigen pulsed with SK-BR-3 cells for 48 h and the non-adherent cells were replated on day 2 onto a new plate without SK-BR-3 cells. On day 8, the non-adherent PBMC were reexposed (in vitro boost) to SK-BR-3 cells in the presence of CpG-ODNs overnight, removed, and placed into a final culture for the remainder of culture (7–14 days). Two antigenic exposures consistently induced primary anti-SK-BR-3 antibody synthesis compared to a single 48 h antigenic exposure or continuous antigenic exposure throughout the culture. The cultures for antibody synthesis were performed in RPMI 1640 supplemented with 10% FCS, l-glutamine (Lonza), and antibiotics cells were also supplemented with IL-2 every 3rd day (100 IU/106 cells). Control cultures were kept in medium with SK-BR-3, CpG, and with or without armed/unarmed *ATC. Supernatants were collected after 6, 10, 14, and 21 days of culture and stored at 4°C or −80°C until assayed for SK-BR-3 specific antibodies by enzyme-linked immunosorbent assay (ELISA). Cytokines were measured using a 25-plex cytokine BioPlex assay, and anti-SK-BR-3 killing was measured using 51Cr release assay. Cells from these co-cultures were used for phenotyping at the indicated time points.
Cell-based ELISA
To identify the presence of tumor-specific antibodies (Ab), a cell-based ELISA was used as described [27]. SK-BR-3 cells were plated in flat-bottomed 96-well plates at a concentration of 50,000 cells/well. Cells were allowed to settle and spread at room temperature for 30 min, supernatants were removed to dry the plate, and the plate was stored at −20°C until used. Prior to use, cells on the plates were fixed with absolute ethanol at 4°C for 10 min, blocked with 1% BSA and 1% normal goat serum (NGS) in PBS with 0.05% Tween 20. Serial dilutions of “immunized” supernatants (sups) were placed into the wells, allowed to bind to the fixed cells, and the bound antibody was detected using goat anti-human IgG enzyme conjugate and TMB substrate. The amount of cell-bound antibody was calculated from a standard curve generated by known amounts of Herceptin® bound to a fixed number of SK-BR-3 cells [28]. Culture supernatants containing only PBMC consistently produced <15 ng/ml of anti-SK-BR-3 antibody. In order to determine whether anti-SK-BR-3 antibodies were specific, culture supernatants (100 μl) were adsorbed on 1 × 105 fresh SK-BR-3 at 4°C for 15 min. The supernatants were removed after pelleting the cells by centrifugation and placed onto a fresh batch of SK-BR-3 cells. After 3 consecutive adsorptions, supernatants were tested for residual antibodies directed at SK-BR-3.
Determination of antibody specificity by Western blot
Daudi cells and trypsinized SK-BR-3, MCF-7, MDA-MB-231 cells were washed with cold PBS and lysed. Human recombinant Her2/neu or EGFR (R&D Systems, Minneapolis, MN) proteins (positive controls) or cell lysates were separated by SDS–PAGE, transferred to PVDF membranes and probed with either anti-Her2/neu or anti-EGFR antibodies (Herceptin®, Genentech Inc., San Francisco, CA; Erbitux®, Bristol–Myer Squibb, New York, NY) or culture supernatants to assess the presence of anti-Her2 or anti-EGFR antibodies.
Determination of antibody specificity by Her2/neu-specific ELISA
We used a Her2/neu-specific ELISA to test for presence of Her2/neu-specific antibodies in the co-cultures. Ninety-six-well plates were coated with 100 μl of 2 μg/ml of anti-Her-2 antibody in PBS and incubated overnight at 4°C as previously described [28]. Plates were washed and blocked with 3% BSA in PBST. After blocking, 100 μl of 1 ng/ml human recombinant Her2/neu protein (hr-Her2/neu) was added and plate was incubated for 2 h at room temperature followed by adding Herceptin® (standard) or samples (culture supernatants) for additional 1 h incubation at RT. Her2/neu-specific antibodies were detected by HRP conjugated goat anti-human IgG. The plates were developed and analyzed.
Determination of antibody specificity by flow cytometry
To test the antibody specificity by flow cytometry, SK-BR-3 or MCF-7 cells (1 × 106) were incubated with 10 μl of 10 μg/ml Herceptin® or irrelevant antibody Rituxan®, immune serum from a patient treated with Her2Bi-armed ATC known to be positive for anti-Her2 and anti-EGFR antibodies (10 μl of 1:100 dilution), or culture supernatants at 4°C with for 30 min. The cell lines were washed and probed with FITC goat anti-human IgG (Biosource, CA) and analyzed by flow cytometry using FacsDiva software (BD Biosciences, San Jose, CA).
Flow cytometry for T-cell sub-populations
Co-cultured PBMCs (1 × 105) were stained for 30 min at 4°C with CD4-Cy5, CD8-FITC, and CD25-PE (BD Biosciences San Jose, CA). Three or two-color data acquisition was performed with a flow cytometer (BD Biosciences, San Jose, CA). Data analysis was performed using FacsDiva software and viable lymphocytes were sized via forward versus side-scatter gating.
Measurement of cytokine secretion
Cytokines were analyzed in supernatants collected from co-cultures using 25-plex human cytokine Luminex Array (Invitrogen, Carlsbad, CA) and the BioPlex system (Bio–Rad Lab., Hercules, CA) according to the manufacturer’s instructions. The BioPlex panel includes interleukin (IL)-1β, IL-1 receptor antagonist (RA), IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-8, IL-13, IL-17, tumor necrosis factor (TNF-α), interferon-alpha (IFN-α), IFN-γ, granulocyte macrophage colony-stimulating factor (GM-CSF), macrophage inhibitory protein (MIP)-1α, MIP-1β, interferon-inducible protein (IP)-10, MIG, Eotaxin, regulated on activation normal T cell expressed and secreted (RANTES) and monocyte chemotactic protein (MCP)-1. The multiplexed, particle-based, flow cytometric assay quantifies multiple analytes from a single sample as previously described [23].
Antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity assays
ADCC or CDC was determined by chromium (51Cr) release assays. Culture supernatants (100 μl/well), anti-Her2 (Herceptin®) or irrelevant anti-CD20 (Rituxan®) at 2 μg/well was added to SK-BR-3 followed by adding effector cells (PBMC). Herceptin® was added to Daudi and Rituxan® to SK-BR-3 cells to test for ADCC directed at irrelevant targets. PBMC were washed and plated at 10:1 effector to target ratio (E/T) in 96-well plates containing SK-BR-3 cells with or without culture supernatants, Herceptin® or Rituxan®. All cells were mixed together at the same time. Experiments were repeated thrice in quadruplicates. 51Cr release was measured after 18 h and percent cytotoxicity was calculated using the following formula (experimental cpm -spontaneous cpm)/(maximum cpm–spontaneous cpm) × 100.
For the CDC assay, SK-BR-3 cells were plated at 4 × 104 cells/well in serum-free (SF)-RPMI and 50 μl of supernatant, anti-Her2 or anti-CD20 control antibodies, and incubated on ice for 30 min. Ten microliters of rabbit complement (Sigma, St. Louis, MO) was added for a final concentration of 10% followed by culture at 37°C for 18 h. Each treatment was performed in quadruplicate.
Generation, loading, and co-culture of DC with naïve PBMC
PBMC were adhered to 6-well, flat-bottom plates for 18 h in RPMI 1640 with 10% FBS. Non-adherent cells were removed and the adherent cells were cultured in X-VIVO 15 (Bio Whittaker) with 10% FBS, IL-4 (500 IU/ml, PeproTech Inc., Rocky Hill, NJ) and granulocyte macrophage colony-stimulating factor (GM-CSF) (50 ng/ml) for 7 days. On day 7, DCs (1 × 105/ml) were exposed to either (a) the SK-BR-3 cell lysate produced by co-culturing armed or unarmed ATC with SK-BR-3 cells for 18 h or (b) the SK-BR-3 cell lysate prepared by freeze/thaw cycles, where SK-BR-3 cells were suspended in balanced salt solution and lysed by 5 freeze (methanol cooled in dry ice) and thaw cycles. Total cell disruption was validated microscopically. Lysed cells were centrifuged at 14,000×g for 30 min at 4°C and supernatant containing tumor cell lysate was used immediately for loading DCs or stored at −80°C. After 24 h of exposure to lysate, DC were washed and co-cultured with fresh PBMC (1 × 106/ml) in X-VIVO-15 with antigenic restimulation occurring after 7 days. For ELISA supernatants were collected after two rounds of restimulation at day 21, respectively.
Statistical analysis
Quantitative data are presented as the mean of at least three or more independent experiments ± standard deviation. A one-way ANOVA was used to determine whether there were statistically significant differences within each experiment. Differences between groups were tested via an unpaired, two-tailed t test.
Results
Induction of in vitro specific anti-SK-BR-3 antibody synthesis
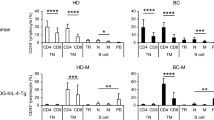
A time-course study was performed to determine the kinetics of anti-SK-BR-3 antibody synthesis in cultures containing naive PBMC from 9 healthy subjects, SK-BR-3, autologous *ATC or *aATC ranging from 6 to 14 days of co-culture (Fig. 1a). Our results show induction of significant anti-SK-BR-3 antibody levels by day 14 (P < 0.0001) in SK-BR-3 exposed PBMC co-cultured with *ATC or *aATC compared to controls or unstimulated PBMC (Fig. 1a). The range of in vitro anti-SK-BR-3 antibody synthesis is shown in Fig. 1b in normal healthy subjects (n = 9).
Co-culture of PBMC exposed to SK-BR-3 cells with autologous *ATC or armed *ATC with one repeated antigenic stimulation-induced specific antibody responses. a Shows the kinetics of the development of in vitro antibody by PBMC in cultures from 9 healthy donors at day 6, 10, and 14 under the indicated co-culture conditions. The total mean amount of anti-SK-BR-3 antibodies reported as SK-BR-3 specific antibodies in ng/ml/1 × 106 cells cultured. All values have been corrected for background (<5 ng/ml); b shows the range of in vitro antibody synthesis in 9 normal subjects at day 14; c shows the proportions of IgG1 and IgG2 antibody subtypes (n = 9); and d shows the effect of unirradiated ATC or aATC on primary antibody synthesis (n = 3)
Co-cultures containing *aATC enhance anti-SK-BR-3 IgG2 antibody subtype synthesis
When we determined the proportions of IgG1 and IgG2 anti-SK-BR-3 antibody synthesis (n = 9), co-cultures of SK-BR-3 exposed to PBMC in the presence of *ATC showed enhanced levels of IgG1 anti-SK-BR-3 antibodies (P < 0.001) whereas co-cultures of PBMC with *aATC showed increased levels of IgG2 anti-SK-BR-3 antibodies (P < 0.02). This result suggests a dominant Th1 microenvironment that was induced by targeting and lysis by *aATC (Fig. 1c).
Evidence for a radiosensitive suppressor ATC
In order to determine whether there are radiosensitive regulatory T-cells that would inhibit the specific antibody production, we co-cultured unirradiated and irradiated (2500 rads) autologous ATC and aATC (n = 3). Co-cultures containing unirradiated ATC and SK-BR-3 produced significantly less (P < 0.0001) anti-SK-BR-3 antibodies than co-cultures containing unirradiated aATC and SK-BR-3 (Fig. 1d). These data show that anti-SK-BR-3 antibody synthesis was inhibited by unirradiated ATC whereas co-cultures containing unirradiated aATC provide helper activity for anti-SK-BR-3 antibody synthesis.
Specificity of antibodies directed at Her2/neu and EGFR
Western blotting confirmed anti-Her2/neu and anti-EGFR antibodies in culture supernatants bound to human recombinant Her2/neu or EGFR proteins (positive control) or Her2/neu or EGFR proteins present in the cell lysates of SK-BR-3, MCF-7 or MDA-MB-231 cells. No binding was detected against Daudi (an irrelevant CD20+ cell line) lysate (Fig. 2a).
a Left panel Western blot analysis showing a Her2 protein band (185 kD) in a positive control lane containing human recombinant Her2 protein or tumor lysates of SK-BR-3, MDA-MB-231, and Daudi cells using either anti-Her2 antibody or culture supernatants from indicated culture conditions; Right panel Western blot analysis showing an EGFR protein band (170 kD) in a positive control lane containing human recombinant EGFR protein or tumor lysates of SK-BR-3, MCF-7, and Daudi cells using either anti-EGFR antibody or culture supernatants from indicated culture conditions. b Shows detection of anti-Her2 antibodies against human recombinant Her2 protein in culture supernatants stimulated with SK-BR-3 (upper panel) or Daudi cells (lower panel) from the indicated culture conditions by ELISA. Herceptin® was used as a positive control and anti-CD20 antibody as a negative control; c Immunostaining of SK-BR-3, MCF-7 or Daudi cells using anti-Her2 antibody, immune serum from a stage IV metastatic breast cancer patient or culture supernatants from indicated culture conditions. Staining with irrelevant controls was negative when Rituxan® was used to stain SK-BR-3 cells or when Herceptin® was used to stain Daudi cells, and d Shows the anti-SK-BR-3 antibody levels by whole cell ELISA after 3-serial absorptions of culture supernatants to SK-BR-3 cells (n = 4)
Anti-Her2/neu antibodies were also detected by ELISA against human recombinant Her2/neu antigen (n = 3). Culture supernatants from co-cultures containing *ATC or *aATC or unirradiated aATC showed significantly higher (P < 0.003) anti-Her2/neu antibody levels ranging from 4,000 to 7,000 pg/ml compared to control supernatants without target cells (Fig. 2b, upper panel). Culture supernatants from cultures containing B-cell line Daudi showed no reactivity against human recombinant Her2/neu antigen (Fig. 2b, lower panel) confirming the specificity of antibodies raised in our in vitro model.
Flow cytometry using Herceptin® as a detection antibody for Her2/neu expression on SK- BR-3 and MCF-7 cells revealed 97.87% positivity on SK-BR-3 cells and 14.5% positivity on MCF-7 cells. Culture supernatants from co-cultures containing *ATC or *aATC showed 15.5 or 15.2% positivity for binding antibodies, respectively, against SK-BR-3 cells; experiment was repeated 3 times and a representative experiment is shown in Fig. 2c. *ATC or *aATC supernatants were 3.5 or 3.65% positive against low Her2/neu expressing MCF-7 cells. The irrelevant control (Daudi) cells showed less than 1% positive staining with either immune sera or culture supernatants from co-cultures containing *ATC or *aATC (Fig. 2c). The irrelevant control antibodies showed no staining when Rituxan® was used to stain SK-BR-3 cells or when Herceptin® was used to stain Daudi cells (Fig. 2c).
There were decreased amounts of anti-SK-BR-3 antibody in culture supernatants after serial absorptions to SK-BR-3 whereas absorptions to irrelevant targets (Daudi, B-cell lymphoma line) did not decrease anti-SK-BR-3 antibody levels (data not shown). Absorbed supernatants showed a 20–50% reduction of SK-BR-3 specific antibody levels compared to unabsorbed supernatants (Fig. 2d).
Anti-SK-BR-3 antibody mediates CDC and ADCC
Since IgG1 and IgG2 subclasses of specific anti-SK-BR-3 antibodies were detected in co-cultures, we tested whether the antibodies could mediate CDC and ADCC in Cr51 release assays (n = 3). Culture supernatants were tested for specific binding to SK-BR-3 cells and cytotoxicity directed at SK-BR-3 in the presence or absence of PBMC as effectors (E:T of 10:1) in the ADCC assay. The anti-SK-BR-3 antibodies in cultures containing PBMC stimulated with SK-BR-3, *ATC, *aATC or unirradiated aATC exhibited significant lysis (P < 0.02) compared to the other conditions (Fig. 3a). Similarly, CDC was significantly enhanced (P < 0.05) in supernatants from cultures containing PBMC, SK-BR-3, *aATC or unirradiated aATC compared to control PBMC and CpG alone or PBMC, SK-BR-3 (Fig. 3a, top panel). Although background killing by culture supernatants from control conditions against SK-BR-3 cells was high for both CDC and ADCC, this was most likely due to polyclonal antibody in the supernatants. Although background killing by culture supernatants from control conditions against SK-BR-3 cells and irrelevant target B-cell line Daudi was high for both ADCC and CDC, this was most likely due to polyclonal antibodies in the supernatants. Similar levels of CDC and ADCC were observed against Daudi cells as seen by control supernatants (Fig. 3a, lower panel).
a Culture supernatants were tested for the ADCC or CDC, anti-Her2/neu antibody (2 μg/well) was used as a positive control, Rituxan® (anti-CD20 antibody, 2 μg/well) as an irrelevant or negative control and Daudi cells + Herceptin® were used as Her2/neu lacking targets. For ADCC, PBMC were added at 10:1 effector-to-target ratio, SK-BR-3 (upper panel) Daudi (lower panel). For CDC, rabbit complement was added to a final concentration of 10%. The x-axis shows anti-SK-BR-3 antibody-mediated ADCC and CDC in the culture supernatants from the following culture conditions: 1 contains PBMC; 2 contains PBMC, SK-BR-3 cells; 3 and 4 contain PBMC + SK-BR-3 + with *ATC or *aATC, respectively; 5 and 6 contain PBMC + SK-BR-3 with unirradiated ATC or aATC, respectively. b Phenotyping for T-cell subsets at day 6, 10, and 14 of co-cultures showing different proportions of CD4+, CD8+, CD4+/CD25+ and CD8+/CD25+ T-cells. Co-cultures containing PBMC + SK-BR-3 with *aATC showed significantly lower CD4+ T-cells (P < 0.0001) at day 6, significantly higher proportion of CD8+ T-cells (P < 0.02) at day 10 and day 14 (P < 0.0001). CpG was added to all culture conditions
Phenotyping of co-cultures containing *ATC and *aATC
Since antibody synthesis differed significantly among the various co-culture conditions, we determined whether this difference in antibody production was associated with different ratio of T-helper and/or T-regulatory cells in the cultured population of PBMC (n = 3). The armed or unarmed ATC were irradiated to prevent proliferation and/or eliminate suppressor cells sensitive to irradiation so the contribution of surviving ATC would be constant. FoxP3+ cells gated on CD4/CD25 positive cells showed reduced percentage of FoxP3+ cells in irradiated cells compared to non-irradiated cells (non-irradiated ATC-3.4% and aATC-3.3% versus irradiated ATC-2.0% and aATC-2.1%). Immunostaining for CD4, CD8, and CD25 after 6 days of culture showed significantly higher percentages of CD4+ cells in cultures containing PBMC, PBMC + SK-BR-3, and PBMC + SK-BR-3 + *ATC compared to PBMC + SK-BR-3 with *aATC (P < 0.0001) (Fig. 3b, day 6). Similarly, the pattern of other cells was different in cultures containing *aATC (PBMC + SK-BR-3) from the rest of the conditions.
By day 10, the proportion of both CD4+ and CD4+CD25+ cells in cultures containing *aATC were lower than the rest of the cultures conditions. On the other hand, the proportion of CD8+ (P < 0.02) and CD8+CD25+ (P < 0.001) cells were significantly higher in cultures containing *aATC compared to other conditions (Fig. 3b, day 10).
By day 14, there were no differences in the proportions of CD4+, CD4+CD25+, CD8+, or CD8+CD25+ cells in cultures containing PBMC + SK-BR-3 with or without *ATC (Fig. 3b, day 14). However, in cultures containing *aATC, PBMC, and SK-BR-3, the proportion of CD8+ cells were significantly increased (P < 0.001) compared to all other culture conditions. The proportions of CD4+CD25+ and CD8+CD25+ positive cells were decreased significantly (P < 0.045) compared to the proportions detected at day 10. Overall cultures containing PBMC + SK-BR-3 + *aATC showed an entirely different pattern that had significantly higher percentages of CD8+ and CD8+CD25+ positive cells and significantly lower percentages of CD4+ and CD4+CD25+ positive cells throughout the culture period compared to the rest of the culture conditions.
Co-cultures containing *aATC induce a Th1 pattern
Supernatants from SK-BR-3 exposed PBMC co-cultures containing *aATC showed significantly increased levels of Th1 cytokines especially IFNγ (P < 0.01) and GM-CSF (P < 0.05) as well as significantly higher levels of IL-13, a Th2 cytokine (P < 0.02), compared to co-cultures containing *ATC (Fig. 4a, Top and Middle panels). Similarly, chemokines MIP-1α (P < 0.0001) and MIP-1β (P < 0.0001) were significantly higher in supernatants from *aATC compared to all other conditions (Fig. 4a, Bottom panel). No difference was observed for IL-6 or IL-10 levels in co-cultures containing either *ATC or *aATC. Supernatants from control co-cultures showed low to undetectable levels of cytokines or chemokines. The mean Th1 (IL-2 + IFNγ)/Th2 (IL-4 + IL-10) ratio show a Th1-type response and the average ratio of Type 1 (IL-2 + IL-12 + IFNγ+TNF-α)/Type 2 (IL-4 + IL-5 + IL-6 + IL-10 + IL-13) also show a Type 1 response in co-cultures containing either *ATC and/or *aATC compared to control conditions without *ATC or *aATC (n = 3; Fig. 4b). Type 1 (Th1-like) and Type 2 (Th2-like) cytokines can be produced by all immune cell types rather than only CD4 + T-cells.
Cytokine profile of co-cultures of PBMC, briefly exposed to SK-BR-3 cells, and autologous *ATC or *aATC in the presence of CpG. The cytokine array represents results of two independent experiments. a Top panel show Th1 cytokines IL-2, IFN-γ, IL-12p40p70, IL-17, and GM-CSF. Middle panel, show Th2 cytokines IL-5, IL-6, IL-10, and IL-13. Bottom panel shows chemokines RANTES, MIP-1α, MIP1β, IP-10, and MIG at day 14. b shows the Th1:Th2 ratio and Type 1:Type 2 ratio at day 14 from the indicated culture conditions
In vitro immunization of naïve PBMCs by antigen loaded DCs
In order to confirm whether targeting of SK-BR-3 by Her2Bi armed ATC can induce in vitro primary responses, we loaded DC with tumor lysates. DC loading experiments were performed using IL-4 and GM-CSF generated DC that showed phenotypically CD80+, CD86+, and CD14− at day 7. DC were loaded with lysates prepared by (a) overnight incubation of SK-BR-3 and *aATC or (b) by freeze/thaw cycles of SK-BR-3 cells. Analysis of the cytokine profile of lysate resulting from the overnight killing of SK-BR-3 by *aATC, which were used to “load” DC, showed enhanced IFN-γ and TNF-α synthesis (Fig. 5a). Likewise, DC loaded with *aATC-generated lysate when co-cultured with fresh PBMC showed higher levels of IFN-γ, TNF-α, and IL-12 compared to lysate generated by freeze–thaw cycles (Fig. 5b). Co-cultures of naive PBMC from normal donors (n = 3) with DC either loaded with *aATC-generated lysates or lysates generated by freeze–thaw cycles showed significantly higher levels of anti-SK-BR-3 antibody synthesis after 21 days of culture with antigenic restimulation compared to various control conditions (Fig. 5c, upper panel), no antibody response was measured against irrelevant Daudi targets (Fig. 5c, lower panel). These data show that the SK-BR-3 lysates produced by *aATC cytotoxicity can induce in vitro and humoral responses. Furthermore, Th1 and Th2 cytokines produced by *aATC may support DC maturation, enhanced antigen presentation and activation, and proliferation of B-cells leading to enhanced antibody synthesis (Fig. 6).
In vivo immunization a Cytokine profile of the SK-BR-3 lysate produced by overnight killing of SK-BR-3 by ATC or aATC; b Supernatants from the co-cultures of loaded DC with naïve PBMC under indicated culture conditions (n = 3). c Co-cultures of naïve PBMC with DC loaded with SK-BR-3 lysate were tested for anti-SK-BR-3 antibodies against SK-BR-3 (upper panel) or irrelevant targets Daudi (lower panel; n = 3). All of the cultures have been corrected for background
A proposed model of aATC immunotherapy induced in vivo immunization of patient’s endogenous immune system: aATC-mediated killing of tumor cells releases cytokines and tumor antigens; cytokines secreted as a function of aATC induce maturation and activation of monocytes to become DC and Ag-primed DCs present antigen to naïve T-cells. Primed T-cell and B-cell interactions leads to B-cell proliferation and differentiation into tumor-specific antibody-producing cells
Discussion
To our knowledge, this is the first report demonstrating that primary antibody responses to tumor cells can be induced in PBMC from normal donors. This novel co-culture system provides a powerful tool for interrogating the ability of normal and patient PBMC to produce specific anti-tumor antibodies. More importantly, it is a sensitive probe for monitoring the effects of aATC immunotherapy or vaccine therapies and helps to identify and dissect the regulatory interactions of T-cells and B-cells that lead to tumor-specific antibody responses in vitro and in vivo. Consistent with our hypothesis that immunotherapy with aATC may vaccinate patients against their own tumors, we detected significantly increased levels of in vitro anti-SK-BR-3 antibodies by PBMC in this co-culture model. This approach may provide an innovative method for assessing adaptive humoral immune responses in patients undergoing immunotherapy.
To assess the specificity of antibodies produced in our co-culture system, we focused on two antigens (Her2/neu and EGFR) that are expressed on SK-BR-3 cells. The induction of antibodies directed at Her2/neu and EGFR were confirmed by Western blot, ELISA, flow cytometry, and target-specific absorption (Fig. 2a–d). All the assays confirmed the presence of anti-Her2 and anti-EGFR antibodies in supernatants from cultures containing *ATC, *aATC or aATC whereas anti-Her2 and anti-EGFR antibodies were not detected in control supernatants. Both ADCC and CDC directed at SK-BR-3 targets provide additional evidence of functional in vitro anti-SK-BR-3 antibody synthesis. Both IgG1 and IgG2 antibody subclasses were produced with significantly higher levels of IgG2 anti-SK-BR-3 antibody in co-culture supernatants containing *aATC than those detected in the co-cultures containing *ATC. These results show that primary IgG2 anti-tumor antibody response can be induced by *aATC and this response can augment ADCC and CDC. Irradiation of ATC or aATC prior to co-cultures consistently provided helper activity for antibody synthesis. Enhanced antibody synthesis occurred using unirradiated aATC, suggesting that the Th1 cytokine pattern produced by aATC can overcome inhibitory signals from radiosensitive regulatory T-cells.
The mechanism for in vitro immunization was confirmed by co-culturing naïve PBMC with DCs loaded with tumor lysates. Anti-SK-BR-3 antibodies were produced in 21-day cultures containing the PBMCs and DCs that were loaded with aATC-generated tumor lysate. Tumor lysates generated by overnight exposure of SK-BR-3 with aATC showed high levels of IFN-γ and TNF-α, suggesting that these cytokines may contribute to DC maturation and efficient antigen presentation to T-cells. Since optimal antigen presentation by DC depends upon the mode of DC loading [29], our DC loading experiments show that targeting with aATC not only kills tumor, but also promotes a microenvironment that facilitates in situ vaccination of endogenous immune cells [30–32].
To determine whether enhanced levels of IgG2 antibody were driven by Th1 cytokines, cytokines were measured in co-cultures containing *aATC or *ATC. Co-cultures containing *aATC showed significantly increased levels of the Th1 cytokines IFNγ, IL-2, and GM-CSF as well as high levels of a Th2 cytokine IL-13. On the other hand, supernatants from *ATC showed significantly lower levels of IFNγ, GM-CSF, and IL-13 but levels of IL-6 comparable to those seen in *aATC co-cultures. IFNγ is known to induce IgG2 synthesis and antagonizes IL-6 to inhibit IgG1 synthesis in B-cells [33]. Th2 cytokines IL-6 and IL-13, are multifunctional cytokines that play roles in the maturation of B-lymphocytes into antibody-producing plasma cells and induction of IgG1 antibody synthesis [34–38]. Relative ratios of IL-6, IL-13, and IFN-γ are likely responsible for relative proportions of IgG1 and IgG2 synthesis in the presence of *ATC or *aATC. An increased Th1 bias would occur in the presence of *aATC leading to the IgG2 anti-tumor antibody subclass. Enhanced IgG2 antibody production and increased levels of IFN-γ and GM-CSF can synergistically enhance the anti-tumor cellular and humoral immune responses [33]. In our model, aATC-tumor cell interactions trigger release of cytokines that induce the maturation and activation of monocytes in naïve PBMC to APC and generate a specific humoral response. Furthermore, enhanced IL-13 secretion may induce the maturation of peripheral monocytes into DC [39, 40]. These data suggest that targeting with ATC or aATC can induce an immunologic milieu favoring immunization of naïve PBMC in the presence of CpG ODNs (Fig. 6).
Several in vitro studies have demonstrated T-cell regulation of Ig synthesis in patients and normal subjects [41–43]. T-helper cells and the cytokines produced by them are required for the proliferation and differentiation of antigen-specific B-cells, development of memory B-cells, and antibody affinity maturation [25, 44, 45]. In the presence of CpG or T-cell help, B-cells differentiate into plasma cells by losing CD20 expression and up-regulating CD38 or CD138 expression [15]. Interestingly, co-cultures containing *aATC showed significantly higher numbers of CD8+ and CD8+ CD25+. It is likely that there is rapid activation and proliferation CD8+ T-cells in co-cultures containing *aATC and this may induce a wave of CD8 regulatory T-cells [46]. The identification of the T-cells subsets responsible for regulating anti-tumor antibody synthesis is the subject of future investigations.
Despite the successes of passive antibody therapy, the strategies to enhance active adaptive humoral responses are needed. There is clinical evidence that humoral responses and immune-mediated cancer remissions are dependent upon generating effective tumor-specific CTLs [1, 2, 47, 48]. Studies in animal models show cross-talk between B- and T-cells leading to eradication of Her2/neu expressing tumors [49]; furthermore, the IgG2a isotype of anti-Her2/neu antibody has been reported to protect against Her2/neu expressing tumors [50]. Developing non-toxic immune strategies that augment in vivo antibody formation against TAAs would most likely induce concerted cellular and humoral immune responses against breast cancer or other tumors. This in vitro antibody model provides a unique and novel tool to evaluate our phase I clinical trial patients who are receiving Her2Bi armed ATC. Our immune studies show that treatment with targeted T-cells induces an endogenous immune response that persists up to 4 months in women with metastatic breast cancer [4].
In summary, this study shows that PBMC for normal subjects can be induced to produce in vitro primary specific anti-SK-BR-3 antibody responses. B-cells in PBMC co-cultured with SK-BR-3 and *aATC or *ATC in the presence of CpG-ODN produced significant amounts of anti-SK-BR-3 antibody when compared to cultures of unstimulated PBMC. Our Western blotting, flow cytometry, and adsorption experiments verified that culture supernatants contained anti-Her2/neu and anti-EGFR antibodies. Co-cultures containing *aATC in the presence of CpG had significantly enhanced levels of IgG2 and of IL-13, IFNγ, and GM-CSF which promote IgG2-specific antibody synthesis as well as shift in the cytokine pattern to Th1 in the cultures containing *aATC. This model provides an opportunity to evaluate the immune cell interactions that lead to specific anti-tumor antibody responses and to monitor immune responses in patients undergoing immunotherapy. In addition to testing patient immune responses, this model will also provide an opportunity to dissect the mechanisms for developing clinically relevant humoral immune responses in cancer vaccination strategies.
References
Jinushi M, Hodi FS, Dranoff G (2006) Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci USA 103(24):9190–9195
Wu CJ, Yang XF, McLaughlin S et al (2000) Detection of a potent humoral response associated with immune-induced remission of chronic myelogenous leukemia. J Clin Invest 106(5):705–714
Bhardwaj N (2007) Harnessing the immune system to treat cancer. J Clin Invest 117(5):1130–1136
Grabert RC, Cousens LP, Smith JA et al (2006) Human T cells armed with Her2/neu bispecific antibodies divide, are cytotoxic, and secrete cytokines with repeated stimulation. Clin Cancer Res 12(2):569–576
Cobleigh MA, Vogel CL, Tripathy D et al (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17(9):2639–2648
Vogel CL, Cobleigh MA, Tripathy D et al (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20(3):719–726
Marty M, Cognetti F, Maraninchi D et al (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol 23(19):4265–4274
Clynes RA, Towers TL, Presta LG, Ravetch JV (2000) Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med 6(4):443–446
Dhodapkar KM, Dhodapkar MV (2005) Recruiting dendritic cells to improve antibody therapy of cancer. Proc Natl Acad Sci USA 102(18):6243–6244
Dhodapkar KM, Kaufman JL, Ehlers M et al (2005) Selective blockade of inhibitory Fc gamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc Natl Acad Sci USA 102(8):2910–2915
Dhodapkar MV (2005) Harnessing host immune responses to preneoplasia: promise and challenges. Cancer Immunol Immunother 54(5):409–413
DiFronzo LA, Gupta RK, Essner R et al (2002) Enhanced humoral immune response correlates with improved disease-free and overall survival in American joint committee on cancer stage II melanoma patients receiving adjuvant polyvalent vaccine. J Clin Oncol 20(15):3242–3248
Riethmuller G, Kufer P (1998) Minimal residual cancer: a target for antibody-based strategies. Naunyn-Schmiedebergs. Arch Pharmacol 358(1):R197
Riethmuller G, Holz E, Schlimok G et al (1998) Monoclonal antibody therapy for resected Dukes’ C colorectal cancer: seven-year outcome of a multicenter randomized trial. J Clin Oncol 16(5):1788–1794
Jung J, Yi AK, Zhang X, Choe J, Li L, Choi YS (2002) Distinct response of human B cell subpopulations in recognition of an innate immune signal, CpG DNA. J Immunol 169(5):2368–2373
Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM (1996) CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA 93(7):2879–2883
Krieg AM, Yi AK, Matson S et al (1995) Cpg motifs in bacterial-Dna trigger direct B-cell activation. Nature 374(6522):546–549
Krieg AM (2002) CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 20:709–760
Davis HL, Weeranta R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM (1998) CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol 160(2):870–876
Carpentier AF, Capelle L, Carpentier A et al (2005) Phase I trial of CpG ODN in recurrent glioblastoma. Clin Cancer Res 11(24):9124S
Friedberg JW, Kim H, McCauley M et al (2005) Combination immunotherapy with a CpG oligonucleotide (1018 ISS) and rituximab in patients with non-Hodgkin lymphoma: increased interferon-alpha/beta-inducible gene expression, without significant toxicity. Blood 105(2):489–495
Link BK, Ballas ZK, Weisdorf D et al (2006) Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother 29(5):558–568
Reusch U, Sundaram M, Davol PA et al (2006) Anti-CD3 x anti-epidermal growth factor receptor (EGFR) bispecific antibody redirects T-cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin Cancer Res 12(1):183–190
Gall JM, Davol PA, Grabert RC, Deaver M, Lum LG (2005) T cells armed with anti-CD3 x anti-CD20 bispecific antibody enhance killing of CD20(+) malignant B cells and bypass complement-mediated rituximab resistance in vitro. Exp Hematol 33(4):452–459
Lum LG, Orcuttthordarson N, Seigneuret MC, Hansen JA (1982) Invitro regulation of immunoglobulin-synthesis by T-cell sub-populations defined by a new human T-cell antigen (9.3). Cell Immunol 72(1):122–129
Lum LG, Orcuttthordarson N, Seigneuret MC, Storb R (1982) The regulation of Ig synthesis after marrow transplantation.4. T4 and T8 Subset function in patients with chronic graft-vs-host disease. J Immunol 129(1):113–119
Lee JCW, Cevallos AM, Naeem A, Lennard-Jones JE, Farthing MJG (1999) Detection of anti-colon antibodies in inflammatory bowel disease using human cultured colonic cells. Gut 44(2):196–202
Maple L, Lathrop R, Bozich S et al (2004) Development and validation of ELISA for Herceptin detection in human serum. J Immunol Methods 295(1–2):169–182
Groh V, Lil YQQ, Cioca D et al (2005) Efficient cross-priming of tumor antigen-specific T cells by dendritic cells sensitized with diverse anti-MICA opsonized tumor cells. Proc Natl Acad Sci USA 102(18):6461–6466
Chomarat P, Dantin C, Bennett L, Banchereau J, Palucka AK (2003) TNF skews monocyte differentiation from macrophages to dendritic cells. J Immunol 171(5):2262–2269
Iwamoto S, Iwai S, Tsujiyama K et al (2007) TNF-alpha drives human CD14(+) monocytes to differentiate into CD70(+) dendritic cells evoking Th1 and Th17 responses. J Immunol 179(3):1449–1457
Vitale M, la Chiesa M, Carlomagno S et al (2005) NK-dependent DC maturation is mediated by TNF alpha and IFN gamma released upon engagement of the NKp30 triggering receptor. Blood 106(2):566–571
Smith KE, Janelidze S, Visse E et al (2007) Synergism between GM-CSF and IFN gamma: Enhanced immunotherapy in mice with glioma. Int J Cancer 120(1):75–80
Aversa G, Cocks B, Punnonen J et al (1993) Il-13-induced B-cell proliferation and Ige synthesis is blocked by an Il-4 mutant protein—support for a shared component of the Il-4 and Il-13 receptors. J Leukoc Biol 93
Kopf M, Ramsay A, Brombacher F et al (1995) Pleiotropic defects of IL-6-deficient mice including early hematopoiesis, T and B cell function, and acute phase responses. Interleukin-6 Type Cytokines 762:308–318
Kopf M, Herren S, Wiles MV, Pepys MB, Kosco-Vilbois MH (1998) Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J Exp Med 188(10):1895–1906
Lai YH, Mosmann TR (1999) Mouse IL-13 enhances antibody production in vivo and acts directly on B cells in vitro to increase survival and hence antibody production. J Immunol 162(1):78–87
Mckenzie ANJ, Culpepper JA, Malefyt RD et al (1993) Interleukin-13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc Natl Acad Sci USA 90(8):3735–3739
Morse MA, Lyerly HK, Li YW (1999) The role of IL-13 in the generation of dendritic cells in vitro. J Immunother 22(6):506–513
Sato K, Nagayama H, Tadokoro K, Juji T, Takahashi TA (1999) Interleukin-13 is involved in functional maturation of human peripheral blood monocyte-derived dendritic cells. Exp Hematol 27(2):326–336
Lum LG, Seigneuret MC, Storb RF, Witherspoon RP, Thomas ED (1981) In vitro regulation of immunoglobulin synthesis after marrow transplantation I. T-cell and B-cell deficiencies in patients with and without chronic graft-versus-host disease. Blood 58:431–439
Lum LG, Seigneuret MC, Orcutt-Thordarson N, Noges JE, Storb R (1985) The regulation of immunoglobulin synthesis after HLA-identical bone marrow transplantation: VI. Differential rates of maturation of distinct functional groups within lymphoid subpopulations in patients after human marrow grafting. Blood 65:1422–1433
Waldmann TA, Korsmeyer SJ, Hieter PA, Ravetch JV, Broder S, Leder P (1983) Regulation of the humoral immune-response—from immunoglobulin genes to regulatory T-cell networks. Fed Proc 42(8):2498–2503
Amoroso K, Lipsky PE (1990) Frequency of human B-cells that differentiate in response to anti-cd3-activated T-cells. J Immunol 145(10):3155–3161
Lipsky PE (1990) The induction of human B-cell activation, proliferation and differentiation by anti-cd3-stimulated T-cells—a model of T-cell B-cell collaboration. Res Immunol 141(4–5):424–427
Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC (2005) TCR stimulation with modified anti-CD3 mAb expands CD8(+) T cell population and induces CD8(+)CD25(+) Tregs. J Clin Invest 115(10):2904–2913
Sampson JH, Crotty LE, Lee S et al (2000) Unarmed, tumor-specific monoclonal antibody effectively treats brain tumors. Proc Natl Acad Sci USA 97(13):7503–7508
Yang XF, Wu CJ, McLaughlin S et al (2001) CML66, a broadly immunogenic tumor antigen, elicits a humoral immune response associated with remission of chronic myelogenous leukemia. Proc Natl Acad Sci USA 98(13):7492–7497
Reilly RT, Machiels JPH, Emens LA et al (2001) The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu-expressing tumors. Cancer Res 61(3):880–883
Park JM, Terabe M, Sakai Y et al (2005) Early role of CD4(+) Th1 cells and antibodies in HER-2 adenovirus vaccine protection against autochthonous mammary carcinomas. J Immunol 174(7):4228–4236
Acknowledgments
These studies were funded in part by R01 CA 092344 (L.G.L.), R01 CA 140412 (L.G.L), 5P39 CA 022453 from National Cancer Institute, Translational Grants #6066-06 and #6092-09 from the Leukemia and Lymphoma Society (L.G.L), Susan G. Komen Foundation Translational Grant #BCTR0707125 (L.G.L), and Michigan Cell Therapy Center for Excellence Grant from the State of Michigan #1819, and startup funds from the Barbara Ann Karmanos Cancer Institute.
Conflict of interest
The authors have no financial conflict of interest. L.G.L. is a founder of Transtarget, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thakur, A., Norkina, O. & Lum, L.G. In vitro synthesis of primary specific anti-breast cancer antibodies by normal human peripheral blood mononuclear cells. Cancer Immunol Immunother 60, 1707–1720 (2011). https://doi.org/10.1007/s00262-011-1056-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1056-9