Abstract
The high molecular weight melanoma-associated antigen (HMW-MAA) and the cytoplasmic melanoma-associated antigen (cyt-MAA/LGALS3BP) are expressed in melanoma. Their serum levels are increased in melanoma patients and correlate with clinical outcome. We investigated whether these molecules can serve as prognostic markers for neuroblastoma (NB) patients. Expression of cyt-MAA and HMW-MAA was evaluated by flow cytometry in NB cell lines, patients’ neuroblasts (FI-NB), and short-term cultures of these latter cells (cNB). LGALS3BP gene expression was evaluated by RT–qPCR on FI-NB, cNB, and primary tumor specimens. Soluble HMW-MAA and cyt-MAA were tested by ELISA. Cyt-MAA and HMW-MAA were expressed in NB cell lines, cNB, and FI-NB samples. LGALS3BP gene expression was higher in primary tumors and cNB than in FI-NB samples. Soluble cyt-MAA, but not HMW-MAA, was detected in NB cell lines and cNBs supernatants. NB patients’ serum levels of both antigens were higher than those of the healthy children. High cyt-MAA serum levels at diagnosis associated with higher incidence of relapse, independently from other known risk factors. In conclusion, both HMW-MAA and cyt-MAA antigens, and LGALS3BP gene, were expressed by NB cell lines and patients’ neuroblasts, and both antigens’ serum levels were increased in NB patients. Elevated serum levels of cyt-MAA at diagnosis correlated with relapse, supporting that cyt-MAA may serve as early serological biomarker to individuate patients at higher risk of relapse that may require a more careful follow-up, after being validated in a larger cohort of patients at different time-points during follow-up. Given its immunogenicity, cyt-MAA may also be a potential target for NB immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The large majority of tumor markers are developmentally regulated molecules, expressed in specific tissues during fetal development and reexpressed in neoplastic cells after malignant transformation [1]. The number of potential markers is progressively increasing; however, for most malignancies, there are few surrogate serum markers able to predict at diagnosis outcome or tumor relapse.
Two melanoma-associated antigens have been well characterized in the last years by Ferrone et al. [2–9]. The high molecular weight melanoma-associated antigen (HMW-MAA) is a membrane proteoglycan consisting of two subunits of 280 and 440 kDa, expressed in a high proportion of melanomas [5, 9, 10]. Several immunotherapy schedules have been designed using anti-HMW-MAA monoclonal antibodies [7, 11] or vaccination with HMW-MAA whole protein or peptides [6, 12]. The cytoplasmic melanoma-associated antigen (cyt-MAA), also known as Mac-2-binding protein or galectin-3-binding protein (LGALS3BP) [13, 14], is an antigen composed of four polypeptides of 94, 75, 70, and 25 kDa expressed in the cytoplasm of normal cells [2, 4]. Its expression has been found increased in activated normal cells [4], as well as in metastatic melanoma and in other malignancies [8, 9]. It has previously been demonstrated that cyt-MAA is secreted by neuroblastoma cells and has a role in cancer progression, stimulating IL-6 release in bone marrow stromal cells [15].
Soluble HMW-MAA and cyt-MAA have been detected in sera from normal individuals [3, 16], and concentration of both antigens is higher in sera from metastatic melanoma patients than in healthy subjects [9]. High serum levels of cyt-MAA, but not of HMW-MAA, correlate with a higher incidence of relapse and a poorer clinical outcome in metastatic melanoma patients. Moreover, concentration of cyt-MAA in sera is a good marker of immunotherapy response, and patients whose cyt-MAA serum levels remained elevated during treatment were three times more likely to recur or progress [8]. IFN-α and IFN-β increased serum levels of cyt-MAA in melanoma patients [17] and augmented the release of cyt-MAA by melanoma cell lines in vitro [2].
Neuroblastoma (NB) shares a neuroectodermal origin with metastatic melanoma [18], as well as the expression of some tumor-associated antigens, such as MAGE [19], GAGE [20], PRAME [21] and NY-ESO-1 [22].
NB is a neuroectodermal tumor originating from the sympathetic nervous system, and it is the most common extracranial solid malignancy in childhood [23–25]. NB patients’ clinical course is heterogeneous, ranging from localized and well-differentiated tumor with good prognosis (stage 1 and 2) or spontaneous regression (stage 4S), to fatal progression, characterized by metastasis and associated with tumor relapse (stages 3–4) [26].
Accurate risk estimation of the patients is mandatory to choose the most adequate therapy. The clinical variables currently employed are the following: (1) stage of disease, (2) age of patient at diagnosis, and (3) genetic alterations, such as amplification of MYCN oncogene and loss of the long arm of chromosome 11 [26, 27].
Since no surrogate serum biomarkers for risk estimation at diagnosis are available for NB, except lactate dehydrogenase (LDH), whose prognostic value is still controversial [28, 29], we evaluated (1) the expression, secretion, and shedding of cyt-MAA and HMW-MAA in NB cell lines, NB primary tumors, and metastatic neuroblasts, (2) serum levels of both antigens in NB patients compared with those in age-matched healthy subjects, and (3) correlations between altered serum levels of both antigens and clinical outcome of NB patients.
Materials and methods
Patients
The study was approved by the Ethical Committee of the G. Gaslini Institute, Genoa, Italy. Samples were collected at diagnosis from 47 patients with different stages of NB disease, namely 10 stage 1, 6 stage 2, 11 stage 3, 16 stage 4, and 4 stage 4S, according to the International Neuroblastoma Staging System [26]. All patients were untreated at study entry.
Twenty patients with localized NB (stages 1, 2 and 4s) received only surgery. Eight patients were enrolled in Localised Neuroblastoma European Study (LNESG1) [30], 6 patients were enrolled in multicenter study in Europe for infants (INES) [31], and 6 patients were enrolled in Italian Neuroblastoma protocol NB 92 [32]. Twenty-seven patients with metastatic NB (stages 3 and 4) were subjected to (1) only surgery (5 patients, LNESG1 or INES protocols) or (2) surgery, chemotherapy, and autologous stem cell transplantation (22 patients, European protocol NB-AR-01 and Italian protocol NB 85 and 97).
NB patients’ characteristics and clinical features at diagnosis and at follow-up are summarized in Table 1. The median of follow-up length was 14.37 months (range, 1.87–88.3 months).
Cell lines and NB cell cultures
GI-LI-N, GI-ME-N, IMR-32, LAN-5, SH-SY-5Y NB cell lines and M14 melanoma cell line were purchased from American Type Culture Collection (ATCC, Rockville, MD). Tumor cell lines were maintained in DMEM (Sigma–Aldrich, St.Louis, MO, USA) supplemented with 10% fetal bovine serum (GIBCO, Invitrogen, Carlsbad, CA, USA), HEPES buffer, non-essential amino acids, and antibiotics (Cambrex Bio Science Verviers, Verviers, Belgium).
Fresh neuroblasts were obtained at diagnosis from bone marrow (BM) samples of five stage 4 NB patients. Erythrocytes were lysed using erythrocyte lysis buffer (Qiagen, Hilden, Germany) following manufacturer’s protocol. Cells were then treated with Fc-blocking reagent (Miltenyi Biotech, Bergisch Gladbach, Germany) and stained with the murine anti-GD2 G2a mAb (IgG2a, obtained from 14.G2a hybridoma cell line, kindly donated by Dr. Mirco Ponzoni, Genoa, Italy) [33, 34] for 30 min at 4°C. Cells were then washed with washing buffer (PBS 0.5% BSA, 2 mM EDTA) and treated with Rat anti-mouse IgG2(a + b) microbeads (Miltenyi Biotech) following manufacturer’s protocol and washed with washing buffer. GD2 + cells were then separated using magnetic columns (Miltenyi Biotech), counted, and plated in DMEM 20% FBS. In some experiments, fresh neuroblasts were cultured for few passages. Cells were detached using Trypsin–EDTA solution (Celbio, Milano, Italy) when culture reached the confluence and expanded in DMEM 20% FBS.
Serum samples
Samples were obtained from the serum bank of the Clinical Pathology Laboratory, G. Gaslini Children’s Hospital. Together with samples from the 47 NB patients, 38 samples collected from age-matched healthy children, admitted at the Emergency Department of Gaslini Hospital for accidental traumatic injuries, were studied as controls. Samples were collected between 1997 and 2005, following written informed consent of the patients or legal guardians and stored at −80°C until use.
Monoclonal antibodies
The 465.125 monoclonal antibody (mAb), which recognizes a repeated epitope of cyt-MAA, and the TP41.2 and 763.74 mAbs, which recognize distinct and spatially distant determinants of HMW-MAA, were developed and characterized as previously described [3, 15]. The 465.125 mAb was used in assays as both capture and detection reagent. For detection, the 465.125 and 763.74 mAbs were biotinylated using AnaTag protein biotinylation kit (AnaSpec Inc., San Jose, CA, USA), following manufacturer’s protocol.
Flow cytometry
Intracellular and surface staining of NB cell lines was performed as previously described [35]. Briefly, cells were detached using Trypsin–EDTA solution (Celbio) and washed in PBS 1% FBS. For HMW-MAA detection, cells were incubated for 30 min at 4°C with 1 μg/106 cells of TP41.2 mAb, washed with PBS 1% FBS, and then incubated with goat F(ab)2 anti-mouse IgG1 PE-conjugated secondary antibody (Southern Biotech, Birmingham, AL, USA) for 30 min at 4°C.
For cyt-MAA detection, cells were fixed and permeabilized using BD Perm/Wash (BD Biosciences, Franklin Lake, NJ, USA) following manufacturer’s protocol and then incubated for 30 min at 4°C with 1 μg/106 cells of 465.125 mAb. Cells were then washed with Perm/wash 1× buffer and incubated with goat F(ab)2 anti-mouse IgG2a PE-conjugated secondary antibody (Southern Biotech) for 30 min at 4°C.
In both cases, cells were subsequently washed with PBS 1% FBS and then subjected to flow cytometry using FACScalibur (BD Biosciences). Cell Quest software (BD Biosciences) was used for data analysis. Results are expressed as percentage of positive cells or as mean relative fluorescence intensity (MRFI) obtained as a ratio between mean fluorescence intensity (MFI) of cells stained with specific mAb and MFI obtained with isotype control.
Soluble cyt-MAA and HWM-MAA ELISA
ELISA for soluble cyt-MAA and HMW-MAA was performed using MaxiSorp Nunc-Immuno 96-microwell plates (Nunc A/S, Roskilde, Denmark) coated overnight at 4°C with 465.125 or TP41.2 mAbs, respectively (0.1 μg/ml in carbonate/bicarbonate buffer, pH 9.6, 100 μl/well). After three washes with PBS 0.05% Tween 20 (washing buffer), plates were blocked with 200 μl/w of PBS 0.3% Tween 20 (blocking buffer) for 1 h at room temperature (RT).
Serum levels of each antigen were calculated from the standard curve. For cyt-MAA, standard curve was performed using purified antigen and expressed in ng/ml. For HMW-MAA, standard curve is expressed in units (U), where 1U equaled the amount HMW-MAA in 1 μg per ml of the standard cell lysate. Standard melanoma cell lysate was prepared from 1 × 107 M14 melanoma cells resuspended in lysis buffer (PBS 0.5% NP-40, with protease inhibitors). The preparation was sonicated and then centrifuged for 15 min at 10,000×g. The supernatant was collected, and the protein concentration was determined by the BCA assay (Pierce, Rockford, Illinois). Serum samples were diluted in blocking buffer 1:1,600 for cyt-MAA and 1:50 for HMW-MAA. One hundred microliters of samples or standards was added to each well and incubated at 4°C overnight. Plates were then washed three times with washing buffer and then incubated with 100 μl/well of biotin-conjugated 465.125 or 763.74 mAbs (0.1 μg/ml in blocking buffer) at 37°C for 2 h. After three washes, plates were incubated with streptavidin–horse radish peroxidase (GE Healthcare, Chalfont St. Giles, UK) 1:4,000 in PBS 0.1% Tween 20, 0.1% BSA, for 1 h at RT. After additional three washes, plates were incubated with the substrate (3′-3′-5′-5′ Tetramethylbenzidine, Sigma) for 5 min at RT. Finally, H2SO4 5 M (100 μl/well) was added, and optical densities were measured at 450 nm.
The assay’s lowest threshold was 4.33 ng/ml for cyt-MAA and 2.5 U/ml for HMW-MAA. Each sample was tested in duplicate.
RNA extraction, reverse transcription, and quantitative real-time PCR (qPCR)
Total RNA was extracted from (1) freshly isolated GD2+ cells obtained following magnetic separation from BM samples of five NB patients with stage 4 disease (FI-NB) and (2) the same cells cultured for a few passages in vitro (CNB), using the RNeasy Micro kit (Qiagen), according to the manufacturer’s procedures. A DNAse I digestion was performed to eliminate contaminating DNA.
Total RNA was extracted from 9 primary NB tumors samples, 5 Schwannian stroma-poor NBs (3 stage 4 and 2 stage 3), and 4 Schwannian stroma-rich neuroblastomas (2 stage 1, 1 stage 3, and 1 stage 4) using PerfectPureTM RNA Tissue Kit (5Prime, Hamburg, Germany), including RNase-free DNase I treatment.
RNA (1 μg) was reverse transcribed using 20 pmol of random hexamers (Eppendorf, Hamburg, Germany) and 200 U of SuperScript II enzyme (Invitrogen Life Technologies, Carlsbad, CA) in a total reaction volume of 20 μl. The mixture was incubated in a Mastercycler epGradient S (Eppendorf) at 25°C for 10 min, 42°C for 60 min, and 85°C for 5 min to stop the reaction. cDNA was diluted 10-fold in molecular biology grade water to a final concentration of 5 ng/μl, assuming 100% reverse transcription efficiency. Reverse transcription–quantitative PCR (RT–qPCR) assays for cyt-MAA (TaqMan Gene Expression Assay (ID: Hs00174774_m1), Applied Biosystems, Foster City, CA, USA) and β2-microglobulin (β2-M) (Applied Biosystems) were set up in 96-white-well Twin.tec real-time plates (Eppendorf) by EpMotion 5070 Liquid Handling Workstation (Eppendorf) and performed in duplicate on Mastercycler RealPlex4 S system (Eppendorf) using 2 μl of cDNA, 2.5× RealMaster Mix Probe (5Prime) and 20× primer/probe mix in a final volume of 10 μl. Negative controls (water as template and RNA reverse transcribed without enzyme) and positive controls (cDNA of GI-LI-N and GI-ME-N cell lines) were run simultaneously. Cycling conditions were as follows: 95°C for 2 min, 40 cycles at 95°C for 15 s and at 60°C for 1 min. RealPlex software v. 2.0 (Eppendorf) was used to determine quantification cycle (Cq). Results of qPCR analysis were expressed as relative values (ΔΔCq method [36]) using β2-M as reference gene and GI-ME-N cell line as calibrator sample.
Statistical analysis
For each NB patient, demographic, clinical, biological, and follow-up data were retrieved from the Italian NB Registry that collects patients’ information at diagnosis, during treatment and yearly after treatment discontinuation.
The median serum levels measured in patients and controls were compared by Student’s t test, using Prism software (GraphPad Software Inc., La Jolla, CA).
To determine the cutoff level of each antigen to be considered elevated, ROC curves [37] were constructed using MedCalc software (Mariakerke, Belgium), using as read-out: (1) NB patients’ sera versus control sera, (2) relapsed versus not-relapsed NB patients, and (3) alive versus dead NB patients.
Relationship between patients’ clinical outcome and MAA serum levels was determined according to the Kaplan–Meier method. Relapse-free and overall survival curves were compared by the log-rank test using MedCalc software. A P value <0.05 was considered as statistically significant.
Multivariate analysis of survival in relationship with serum cyt-MAA levels, age, and MYCN amplification was performed by Cox multiple regression model, using StatPlus Professional software (AnalystSoft Inc., Vancouver, Canada).
Results
NB cell lines and neuroblasts from NB patients express HMW-MAA and cyt-MAA
First, surface expression of HMW-MAA and intracellular expression of cyt-MAA were evaluated by flow cytometric analysis of five NB cell lines (GI-ME-N, GI-LI-N; SH-SY-5Y, IMR-32, and LAN-5). As shown in Fig. 1a, cyt-MAA expression was detected in all five NB cell lines tested (black bars), whereas HMW-MAA (grey bars) was expressed on the surface of three out of five NB cell lines (GI-ME-N, GI-LI-N, and IMR-32). The expression of both melanoma-associated antigens in NB samples was much lower than that detected on the M14 melanoma cell line, tested as positive control. Mean of three different experiment carried out ±SD is shown.
Expression of cyt-MAA and HMW-MAA. a FACS analysis of cyt-MAA (black bars) and HMW-MAA (grey bars) expression in 5 NB cell lines (GI-LI-N, GI-ME-N, IMR-32, LAN-5, SH-SY-5Y). M14 melanoma cell line was tested as positive control. Results are expressed as MRFI (mean relative fluorescence intensity = mean fluorescence intensity obtained with specific mAb/mean fluorescence intensity obtained with an irrelevant isotype-matched antibody). Mean of three different experiments carried out ± SD is shown. b FACS analysis of cyt-MAA (black bars) and HMW-MAA (grey bars) expression in three freshly isolated neuroblasts from patients’ BM (FI-NB) and in five short-term culture of freshly isolated neuroblasts from five patient’s BM (cNB). Results are expressed as MRFI (mean relative fluorescence intensity = mean fluorescence intensity obtained with specific mAb/mean fluorescence intensity obtained with an irrelevant isotype-matched antibody). c Statistical analysis of cyt-MAA and HMW-MAA expression in freshly isolated (FI-NB) and short-term cultured neuroblasts (cNB) from patients’ BM. Scatter plots indicate MRFI values obtained by flow cytometric analysis of MAAs expression on FI-NB (N = 3) and cNB (N = 5). Horizontal bars indicate medians. P value is indicated where the difference is statistically significant
Next, the expression of cyt-MAA and HMW-MAA was assessed on metastatic GD2+ neuroblasts isolated from the bone marrow (BM) of five stage 4 NB patients, either freshly isolated (FI-NB) or cultured in vitro for few passages (cNB). As shown in Fig. 1b, both cyt-MAA (black bars) and HMW-MAA (grey bars) were expressed in 3 out of 5 FI-NB and in all 5 cNB samples. A statistical analysis was performed to compare the expression of both antigens in FI-NB and cNB. As shown in Fig. 1c, the expression of cyt-MAA was significantly higher in cNB samples than in FI-NB samples (median MRFI: 14.8 vs. 1.1, P = 0.035). Conversely, the expression of HMW-MAA was similar between FI-NB and cNB samples.
Expression of cyt-MAA/LGALS3BP gene in neuroblastoma samples
The expression of cyt-MAA encoding gene, LGALS3BP, was then evaluated by RT–qPCR. HMW-MAA gene expression could not be evaluated since specific RT–qPCR probes are not available so far.
LGALS3BP gene expression was evaluated on (1) nine primary NB tumor samples (five stroma-poor and four stroma-rich NB frozen tissue samples), (2) five FI-NB samples, and (3) five cNB samples.
As shown in Fig. 2a, LGALS3BP gene was highly expressed in primary NB tumors, regardless of tumor histology. Moreover, LGALS3BP gene was expressed in metastatic neuroblasts, and its expression was significantly higher in cNB samples than in FI-NB samples (mean of −2−ΔCCt values: 0.13 ± 0.03 vs. 0.04 ± 0.03, P = 0.002). These results were in accordance with the flow cytometry data obtained in the same cell preparations.
Expression of LGALS3BP gene and release of soluble cyt-MMA. a LGALS3BP gene expression was evaluated by RT–qPCR analysis using mRNA extracted from (1) nine NB tumor samples, (2) five FI-NB samples, and (3) five cNB samples. Scatter plot indicated 2−ΔCCt values from each sample. Horizontal bars indicated medians. P values are indicated where the difference is statistically significant. b Quantification of soluble cyt-MAA in supernatants from 5 NB cell lines (GI-LI-N, GI-ME-N, IMR-32, LAN-5, and SH-SY-5Y) and 3 cNB samples. M14 melanoma cell line supernatant was tested as positive control. Results are expressed as μg/ml. Mean of three different experiments carried out ± SD is shown
Investigation of soluble cyt-MAA and HMW-MAA in supernatants of NB cell lines
The release of the soluble form of both cyt-MAA and HMW-MAA was then evaluated by specific ELISA on 5 NB cell lines supernatants collected after 48 h culture. Supernatant from M14 melanoma cell line was tested as positive control. As shown in Fig. 2b, soluble cyt-MAA was detected in supernatants from all five NB cell lines (range 0.06–2.75 μg/ml) and in supernatants from 3 out of 5 cNB samples (range 1.18–1.91 μg/ml). Conversely, soluble HMW-MAA was undetectable in all samples (data not shown).
Serum levels of soluble cyt-MAA and HMW-MAA are increased in NB patients
Serum levels of soluble cyt-MAA and HMW-MAA were then tested in sera from 47 NB patients with different stages of disease (10 stage 1, 6 stage 2, 11 stage 3, 16 stage 4, and 4 stage 4s) and from 38 age-matched healthy children.
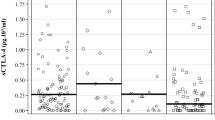
As shown in Fig. 3a, the concentration of soluble cyt-MAA was significantly higher in sera from NB patients (median 99.2 ± 64.9 ng/ml) than in sera from healthy children (median 39.78 ± 47.19 ng/ml, P = 0.0005). The concentration of soluble HMW-MAA (Fig. 3b) was also significantly higher in sera from NB patients (median 2.65 ± 1.2 U/ml) than in sera from healthy children (median 0.86 ± 0.15 U/ml, P = 0.035). However, serum levels of both cyt-MAA and HMW-MAA were similar among patients with different NB stages (Fig. 3c, d, respectively).
Quantification of soluble cyt-MAA and HMW-MAA in NB patients’ sera. a Quantification of soluble cyt-MAA in sera from 38 age-matched healthy children (open circles) and 47 NB patients (closed circles). Results are expressed as ng/ml. b Quantification of soluble HMW-MAA in sera from 38 age-matched healthy children (open circles) and 41 NB patients (closed circles). Results are expressed as arbitrary units (U)/ml. c The quantification of soluble cyt-MAA in patients stratified according to NB stage. d shows the quantification of soluble HMW-MAA in patients stratified according to NB stage. P values are shown were the difference is statistically significant. Horizontal bars indicate means
Relationships between serum levels of cyt-MAA and clinical outcome of NB patients
To evaluate whether serum cutoff levels could discriminate between NB patients and healthy children, ROC curves were obtained. As summarized in Table 2A, the number of NB patients with serum levels above the cutoff levels was significantly higher than that of healthy children, for both cyt-MAA and HMW-MAA. Next, ROC cutoff levels for both antigens were calculated by considering as read-out the NB patients’outcome (relapse-free vs. relapsed and alive vs. dead). As shown in Table 2B, cyt-MAA serum levels above the cutoff level (91.47 ng/ml) significantly correlated with a higher incidence of relapse (9/30 vs. 11/17, P = 0.032), whereas no correlation was found between HMW-MAA serum levels and incidence of relapse. Neither cyt-MAA nor HMW-MAA serum levels correlated with overall survival (Table 2C). Accordingly, as shown in Fig. 4a, Kaplan–Meier analysis confirmed that soluble cyt-MAA serum levels higher than 91.47 ng/ml correlated with a higher incidence of relapse (P = 0.0028), whereas no impact of cyt-MAA serum levels was found on overall survival (Fig. 4b). Kaplan–Meier analysis confirmed that HMW-MAA serum levels had no correlation with either relapse-free or overall survival (data not shown).
Kaplan–Meier analysis of relapse-free survival and overall survival of NB patients. a Relapse-free survival in NB patients with cyt-MAA serum levels above (dashed line) or below (black line) the ROC cutoff level (91.47 ng/ml). Y-axes indicate proportion of relapse-free patients, whereas X-axes indicate time to relapse (months). b Overall survival in NB patients with cyt-MAA serum levels above (dashed line) or below (black line) the ROC cutoff level (40.42 ng/ml). Y-axes indicate proportion of alive patients, whereas X-axes indicate survival time (months)
Next, correlation between cyt-MAA levels below or over the estimated ROC cutoff level (91.47 ng/ml) and different clinical parameters, i.e., gender (M vs. F), MYCN amplification (Yes or No), age at diagnosis (>18 months vs. <18 months), and disease stage (stages 1, 2, 3, and 4s vs. stage 4), was analyzed by Fisher exact test. As shown in Table 2D, cyt-MAA serum levels were independent from all parameters (P > 0.05). To confirm these data, a multivariate analysis using Cox regression model was performed on cyt-MAA serum levels below or above cutoff level and other known prognostic factors. As summarized in Table 2E, cyt-MAA serum levels displayed a significant association with relapse (P = 0.01, HR = 4.11), independently from the other parameters.
Discussion
Serological markers for human tumors may help predicting clinical outcome. Serological markers currently used for human NB are neuron-specific enolase, ferritin, and LDH. These markers, however, with the possible exception of LDH [28, 29] have limited prognostic role.
In the present study, we show that cyt-MAA, as well as surface HMW-MAA, was expressed by NB cell lines and, more importantly, by metastatic neuroblasts isolated from BM samples. These latter cells showed a low expression of cyt-MAA immediately after isolation, which increased upon short-term in vitro culture. In contrast, HMW-MAA expression levels were similar in freshly isolated and cultured metastatic neuroblasts. Cyt-MAA encoding gene LGALS3BP was expressed by metastatic neuroblasts and primary NB tumors, regardless of tumor histology. Soluble cyt-MAA was released in culture supernatants by NB cell lines and freshly isolated metastatic neuroblasts after short-term culture. Indeed, soluble cyt-MAA and soluble HMW-MAA, which are generated through proteolytic cleavage by plasmin and other endogenous protease [15], were detected in sera from NB patients. Concentration of both antigens was higher than in healthy children, regardless of tumor stage. While serum HMW-MAA levels did not correlate with outcome, soluble cyt-MAA serum levels above the cutoff level of 91.47 ng/ml (calculated with ROC curves) significantly correlated with a higher incidence of relapse, independently from other known risk factors, with a high sensitivity and specificity. Conversely, serum cyt-MAA levels did not predict a lower overall survival of NB patients, accordingly the low specificity obtained with ROC curves calculations.
Taken together, these data support that high cyt-MAA/LGALS3BP serum levels at diagnosis may serve as early serological biomarker to identify patients at higher risk of relapse that may require a more careful follow-up. The lack of correlation between cyt-MAA serum levels and tumor stage was in line with other reports concerning both serological and histological markers of NB relapse [38–40].
The role of cyt-MAA/LGALS3BP in cancer is not entirely understood, and LGALS3BP has been shown to have multiple functions. In addition to metastatic melanoma [8, 9], cyt-MAA/LGALS3BP is expressed in numerous cancer cell types, including non-small cell lung cancer, astrocytoma, and colon cancer, where it is a predictor of poor survival [41–43]. Cyt-MAA/LGALS3BP stimulates the secretion of many cytokines in peripheral blood mononuclear cells, including tumor necrosis factor-α (TNF-α), IL-1, and IL-6 [44], which contribute to inflammation and tumor progression. Moreover, cyt-MAA/LGALS3BP binds to multiple proteins of the extracellular matrix [45], which are critical during tumor cell invasion and migration. Finally, the engagement of galectin-3 receptor, which binds to cyt-MAA/LGALS3BP, leads to the release of elastin-derived peptides VGVAPG and VAPG, which are chemotactic for tumor cells and stimulate the expression of CXCR4, CXCL12 and metalloproteases MMP-2 and MMP-3, thus facilitating the spreading of tumor cells [46].
Expression of cyt-MAA antigen was low to absent in freshly isolated metastatic NB and similar to primary tumor cells after short-term in vitro culture, suggesting that factors present in the BM microenvironment may suppress cyt-MAA expression. Fukaya et al. have previously reported that cyt-MAA was detectable in conditioned medium of NB cell lines. The soluble cyt-MAA, following interaction with the Galectin-3 receptor on stromal cells was shown to stimulate the release of IL-6 [15], which in turn induced proliferation of NB cells, creating a favorable microenvironment for progression of metastatic NB [47]. Elevated soluble cyt-MAA levels were found in NB patients, regardless of tumor stage, suggesting that soluble cyt-MAA was secreted either by the primary NB tumor cells or by normal cells [2, 4], stimulated by factors released by NB cells.
Ozaki et al. have previously demonstrated that cyt-MAA/LGALS3BP is highly immunogenic, since HLA-A2 restricted epitopes of this antigen are recognized by specific T lymphocytes [48] and anti-cyt-MAA/LGALS3BP-specific antibodies are elevated in cancer patients [49]. Our finding that NB cells express high levels of cyt-MAA/LGALS3BP may be very interesting in the view of a possible use of this antigen as target for T-cell-mediated immunotherapy of NB. Moreover, since cyt-MAA/LGALS3BP is also recognized by B lymphocytes, it would be interesting to evaluate in further studies whether serum levels of anti-cyt-MAA/LGALS3BP antibodies are elevated in NB patients, as previously demonstrated in patients with lung cancer [49]. In addition, a possible inverse correlation between serum levels of these antibodies and incidence of relapse may be analyzed.
In conclusion, the quantification of soluble cyt-MAA in serum samples at diagnosis may help NB patients’ risk stratification. Validation in a larger cohort of NB patients and at different time-points during follow-up is required in a translational perspective, also in view of its possible use as surrogate marker of treatment response, as observed in metastatic melanoma patients [8]. Moreover, our study paves the way to further investigations on cyt-MAA as target for NB immunotherapy.
References
Labdenne P, Heikinheimo M (2002) Clinical use of tumor markers in childhood malignancies. Ann Med 34(5):316–323
Giacomini P, Gambari R, Barbieri R, Nistico P, Tecce R, Pestka S, Gustafsson K, Natali PG, Fisher PB (1986) Regulation of the antigenic phenotype of human melanoma cells by recombinant interferons. Anticancer Res 6(5):877–884
Giacomini P, Veglia F, Cordiali Fei P, Rehle T, Natali PG, Ferrone S (1984) Level of a membrane-bound high-molecular-weight melanoma-associated antigen and a cytoplasmic melanoma-associated antigen in surgically removed tissues and in sera from patients with melanoma. Cancer Res 44(3):1281–1287
Giacomini P, Viora M, Tecce R, Knowles DM, Natali PG, Ferrone S (1987) A cytoplasmic human melanoma associated antigen as a marker of activation in lymphoid cells. Cancer Res 47(19):5175–5180
Goto Y, Ferrone S, Arigami T, Kitago M, Tanemura A, Sunami E, Nguyen SL, Turner RR, Morton DL, Hoon DS (2008) Human high molecular weight-melanoma-associated antigen: utility for detection of metastatic melanoma in sentinel lymph nodes. Clin Cancer Res 14(11):3401–3407
Luo W, Hsu JC, Tsao CY, Ko E, Wang X, Ferrone S (2005) Differential immunogenicity of two peptides isolated by high molecular weight-melanoma-associated antigen-specific monoclonal antibodies with different affinities. J Immunol 174(11):7104–7110
Luo W, Ko E, Hsu JC, Wang X, Ferrone S (2006) Targeting melanoma cells with human high molecular weight-melanoma associated antigen-specific antibodies elicited by a peptide mimotope: functional effects. J Immunol 176(10):6046–6054
Reynolds SR, Vergilis IJ, Szarek M, Ferrone S, Bystryn JC (2006) Cytoplasmic melanoma-associated antigen (CYT-MAA) serum level in patients with melanoma: a potential marker of response to immunotherapy? Int J Cancer 119(1):157–161
Vergilis IJ, Szarek M, Ferrone S, Reynolds SR (2005) Presence and prognostic significance of melanoma-associated antigens CYT-MAA and HMW-MAA in serum of patients with melanoma. J Invest Dermatol 125(3):526–531
Ulmer A, Fierlbeck G (2006) Circulating tumor cells and detection of the melanoma-associated antigen HMW-MAA in the serum of melanoma patients. J Invest Dermatol 126(4):914–915 author reply 915-916
Wagner S, Krepler C, Allwardt D, Latzka J, Strommer S, Scheiner O, Pehamberger H, Wiedermann U, Hafner C, Breiteneder H (2008) Reduction of human melanoma tumor growth in severe combined immunodeficient mice by passive transfer of antibodies induced by a high molecular weight melanoma-associated antigen mimotope vaccine. Clin Cancer Res 14(24):8178–8183
Maciag PC, Seavey MM, Pan ZK, Ferrone S, Paterson Y (2008) Cancer immunotherapy targeting the high molecular weight melanoma-associated antigen protein results in a broad antitumor response and reduction of pericytes in the tumor vasculature. Cancer Res 68(19):8066–8075
Tinari N, D’Egidio M, Iacobelli S, Bowen M, Starling G, Seachord C, Darveau R, Aruffo A (1997) Identification of the tumor antigen 90 K domains recognized by monoclonal antibodies SP2 and L3 and preparation and characterization of novel anti-90 K monoclonal antibodies. Biochem Biophys Res Commun 232(2):367–372
Grassadonia A, Tinari N, Iurisci I, Piccolo E, Cumashi A, Innominato P, D’Egidio M, Natoli C, Piantelli M, Iacobelli S (2004) 90 K (Mac-2 BP) and galectins in tumor progression and metastasis. Glycoconj J 19(7–9):551–556
Fukaya Y, Shimada H, Wang LC, Zandi E, DeClerck YA (2008) Identification of galectin-3-binding protein as a factor secreted by tumor cells that stimulates interleukin-6 expression in the bone marrow stroma. J Biol Chem 283(27):18573–18581
Natali PG, Giacomini P, Russo C, Steinbach G, Fenoglio C, Ferrone S (1983) Antigenic profile of human melanoma cells. Analysis with monoclonal antibodies to histocompatibility antigens and to melanoma-associated antigens. J Cutan Pathol 10(4):225–237
Scambia G, Panici PB, Iacobelli S, Baiocchi G, Battaglia F, Perrone L, Sonsini C, Ferrandina G, Natoli C, Mancuso S (1990) Recombinant alpha-2b-interferon enhances the circulating levels of a 90-kilodalton (K) tumor-associated antigen in patients with gynecologic and breast malignancies. Cancer 65(6):1325–1328
Gray-Schopfer V, Wellbrock C, Marais R (2007) Melanoma biology and new targeted therapy. Nature 445(7130):851–857
Barker PA, Salehi A (2002) The MAGE proteins: emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J Neurosci Res 67(6):705–712
Castelli C, Rivoltini L, Andreola G, Carrabba M, Renkvist N, Parmiani G (2000) T-cell recognition of melanoma-associated antigens. J Cell Physiol 182(3):323–331
Epping MT, Bernards R (2006) A causal role for the human tumor antigen preferentially expressed antigen of melanoma in cancer. Cancer Res 66(22):10639–10642
Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N, Old LJ (2001) Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer 92(6):856–860
Brodeur GM (2003) Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 3(3):203–216
Maris JM, Hogarty MD, Bagatell R, Cohn SL (2007) Neuroblastoma. Lancet 369(9579):2106–2120
Park JR, Eggert A, Caron H (2008) Neuroblastoma: biology, prognosis, and treatment. Pediatr Clin North Am 55(1):97–120
Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, De Bernardi B, Evans AE, Favrot M, Hedborg F et al (1993) Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 11(8):1466–1477
Fischer M, Spitz R, Oberthur A, Westermann F, Berthold F (2008) Risk estimation of neuroblastoma patients using molecular markers. Klin Padiatr 220(3):137–146
Mora J, Gerald WL, Cheung NK (2003) Evolving significance of prognostic markers associated with new treatment strategies in neuroblastoma. Cancer Lett 197(1–2):119–124
Simon T, Hero B, Hunneman DH, Berthold F (2003) Tumour markers are poor predictors for relapse or progression in neuroblastoma. Eur J Cancer 39(13):1899–1903
Cecchetto G, Mosseri V, De Bernardi B, Helardot P, Monclair T, Costa E, Horcher E, Neuenschwander S, Toma P, Rizzo A, Michon J, Holmes K (2005) Surgical risk factors in primary surgery for localized neuroblastoma: the LNESG1 study of the European International Society of Pediatric Oncology Neuroblastoma Group. J Clin Oncol 23(33):8483–8489. doi:10.1200/JCO.2005.02.4661
Rubie H, De Bernardi B, Gerrard M, Canete A, Ladenstein R, Couturier J, Ambros P, Munzer C, Pearson AD, Garaventa A, Brock P, Castel V, Valteau-Couanet D, Holmes K, Di Cataldo A, Brichard B, Mosseri V, Marquez C, Plantaz D, Boni L, Michon J (2011) Excellent outcome with reduced treatment in infants with nonmetastatic and unresectable neuroblastoma without MYCN amplification: results of the prospective INES 99.1. J Clin Oncol 29(4):449–455. doi:10.1200/JCO.2010.29.5196
Olgun N, Kansoy S, Aksoylar S, Cetingul N, Vergin C, Oniz H, Sarialioglu F, Kantar M, Uysal K, Tuncyurek M, Kargi A, Aktas S, Bayol U, Karaca I, Arikan A, Balik E, Aktug T, Elmas N, Kovanlikaya A, Kinay M, Anacak Y, Degirmenci B, Burak Z (2003) Experience of the izmir pediatric oncology group on neuroblastoma: IPOG-NBL-92 protocol. Pediatr Hematol Oncol 20(3):211–218
Brignole C, Pastorino F, Marimpietri D, Pagnan G, Pistorio A, Allen TM, Pistoia V, Ponzoni M (2004) Immune cell-mediated antitumor activities of GD2-targeted liposomal c-myb antisense oligonucleotides containing CpG motifs. J Natl Cancer Inst 96(15):1171–1180
Pastorino F, Mumbengegwi DR, Ribatti D, Ponzoni M, Allen TM (2008) Increase of therapeutic effects by treating melanoma with targeted combinations of c-myc antisense and doxorubicin. J Control Release 126(1):85–94
Chiesa S, Prigione I, Morandi F, Buoncompagni A, Picco P, Bocca P, Martini A, Pistoia V, Gattorno M (2004) Cytokine flexibility of early and differentiated memory T helper cells in juvenile idiopathic arthritis. J Rheumatol 31(10):2048–2054
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408
Zweig MH, Campbell G (1993) Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 39(4):561–577
Corrias MV, Gambini C, Gregorio A, Croce M, Barisione G, Cossu C, Rossello A, Ferrini S, Fabbi M (2010) Different subcellular localization of ALCAM molecules in neuroblastoma: Association with relapse. Cell Oncol 32(1–2):77–86. doi:10.3233/CLO-2009-0494
Granchi D, Corrias MV, Garaventa A, Baglio SR, Cangemi G, Carlini B, Paolucci P, Giunti A, Baldini N (2011) Neuroblastoma and bone metastases: clinical significance and prognostic value of Dickkopf 1 plasma levels. Bone 48(1):152–159. doi:10.1016/j.bone.2010.06.028
Gregorio A, Corrias MV, Castriconi R, Dondero A, Mosconi M, Gambini C, Moretta A, Moretta L, Bottino C (2008) Small round blue cell tumours: diagnostic and prognostic usefulness of the expression of B7–H3 surface molecule. Histopathology 53(1):73–80. doi:10.1111/j.1365-2559.2008.03070.x
Marchetti A, Tinari N, Buttitta F, Chella A, Angeletti CA, Sacco R, Mucilli F, Ullrich A, Iacobelli S (2002) Expression of 90 K (Mac-2 BP) correlates with distant metastasis and predicts survival in stage I non-small cell lung cancer patients. Cancer Res 62(9):2535–2539
Gordower L, Decaestecker C, Kacem Y, Lemmers A, Gusman J, Burchert M, Danguy A, Gabius H, Salmon I, Kiss R, Camby I (1999) Galectin-3 and galectin-3-binding site expression in human adult astrocytic tumours and related angiogenesis. Neuropathol Appl Neurobiol 25(4):319–330
Ulmer TA, Keeler V, Loh L, Chibbar R, Torlakovic E, Andre S, Gabius HJ, Laferte S (2006) Tumor-associated antigen 90 K/Mac-2-binding protein: possible role in colon cancer. J Cell Biochem 98(5):1351–1366
Hajto T, Hostanska K, Frei K, Rordorf C, Gabius HJ (1990) Increased secretion of tumor necrosis factors alpha, interleukin 1, and interleukin 6 by human mononuclear cells exposed to beta-galactoside-specific lectin from clinically applied mistletoe extract. Cancer Res 50(11):3322–3326
Inohara H, Akahani S, Koths K, Raz A (1996) Interactions between galectin-3 and Mac-2-binding protein mediate cell–cell adhesion. Cancer Res 56(19):4530–4534
Pocza P, Suli-Vargha H, Darvas Z, Falus A (2008) Locally generated VGVAPG and VAPG elastin-derived peptides amplify melanoma invasion via the galectin-3 receptor. Int J Cancer 122(9):1972–1980. doi:10.1002/ijc.23296
Ara T, Song L, Shimada H, Keshelava N, Russell HV, Metelitsa LS, Groshen SG, Seeger RC, DeClerck YA (2009) Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res 69(1):329–337
Ozaki Y, Kontani K, Teramoto K, Fujita T, Tezuka N, Sawai S, Watanabe H, Fujino S, Asai T, Ohkubo I (2004) Identification of antigenic epitopes recognized by Mac-2 binding protein-specific cytotoxic T lymphocytes for use in cancer immunotherapy. Biochem Biophys Res Commun 317(4):1089–1095. doi:10.1016/j.bbrc.2004.03.155
Ozaki Y, Kontani K, Hanaoka J, Chano T, Teramoto K, Tezuka N, Sawai S, Fujino S, Yoshiki T, Okabe H, Ohkubo I (2002) Expression and immunogenicity of a tumor-associated antigen, 90 K/Mac-2 binding protein, in lung carcinoma. Cancer 95(9):1954–1962. doi:10.1002/cncr.10899
Acknowledgments
This work has been supported by grants from Ministero della Salute, Progetti di Ricerca Corrente. BC is recipient of a Fondazione Italiana per la Lotta al Neuroblastoma fellowship. SS is recipient of a fellowship from Ministero della Salute/Regione Liguria. We thank Mrs Chiara Bernardini and Camilla Valentino for excellent secretarial assistance, Dr. Mirco Ponzoni, Genoa, for providing us the G2a anti-GD2 antibody and Mrs Barbara Galleni at the Italian NB registry, Genoa, for the excellent work aimed at providing clinical data on NB patients.
Conflict of interest
The authors disclose any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morandi, F., Corrias, M.V., Levreri, I. et al. Serum levels of cytoplasmic melanoma-associated antigen at diagnosis may predict clinical relapse in neuroblastoma patients. Cancer Immunol Immunother 60, 1485–1495 (2011). https://doi.org/10.1007/s00262-011-1052-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1052-0