Abstract
Background and aims
Dendritic cell (DC)-based vaccination can induce antitumor T cell responses in vivo. This clinical pilot study examined feasibility and outcome of DC-based tumor vaccination for patients with advanced pancreatic adenocarcinoma.
Methods
Tumor lysate of patients with pancreatic carcinoma was generated by repeated freeze–thaw cycles of surgically obtained tissue specimens. Patients were eligible for DC vaccination after recurrence of pancreatic carcinoma or in a primarily palliative situation. DC were generated from peripheral blood mononuclear cells (PBMC), loaded with autologous tumor lysate, stimulated with TNF-α and PGE2 and injected intradermally. All patients received concomitant chemotherapy with gemcitabine. Disease response was the primary endpoint. Individual immunological responses to DC vaccination were analyzed by T cell-based immunoassays using pre- and post-vaccination samples of non-adherent PBMC.
Results
Twelve patients received DC vaccination and concomitant chemotherapy. One patient developed a partial remission, and two patients remained in stable disease. Median survival was 10.5 months. No severe side effects were observed. Tumor-reactive T cells could be detected prior to vaccination. DC vaccination increased the frequency of tumor-reactive cells in all patients tested; however, the degree of this increase varied. To quantify the presence of tumor-reactive T cells, stimulatory indices (SI) were calculated as the ratio of proliferation-inducing capacity of lysate-loaded versus -unloaded DC. The patient with longest overall survival of 56 months had a high SI of 6.49, indicating that the presence of a pre-vaccination antitumor T cell response might be associated with prolonged survival. Five patients survived 1 year or more.
Conclusion
DC-based vaccination can stimulate an antitumoral T cell response in patients with advanced or recurrent pancreatic carcinoma receiving concomitant gemcitabine treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic carcinoma represents the fifth leading cause of cancer death in the western world [1]. Chemotherapy with gemcitabine has been the reference treatment for patients with advanced pancreatic carcinoma since 1997 [2, 3]. Recent trials have failed to show significant improvement in survival when gemcitabine was combined with other chemotherapeutic drugs [4]. Novel therapeutic options are urgently warranted. Immunotherapy with tumor antigen-loaded dendritic cells (DC) may represent a promising approach to cancer treatment [5]. Recently, Sipuleucel-T, an autologous active cellular immunotherapy, has shown efficacy in treatment of men with metastatic castration-resistant prostate cancer [6].
Dendritic cells are professional antigen-presenting cells (APC) capable of activating T cell responses against tumor antigens [7]. However, DC in a tumor environment may be functionally defective due to the induction of immunosuppressive cytokines, e.g., transforming growth factor-β (TGF-β) and IL-10, by the tumor [8, 9]. This limitation can be overcome by generating DC from peripheral blood precursors and by the activation of these DC with proinflammatory stimuli in vitro.
In vitro studies indicate that subtype and maturation status of the DC, as well as the source of tumor antigen, determines the efficacy of DC-based vaccination [10, 11]. As opposed to single peptides or proteins, whole cell preparations, such as tumor cell lysate, apoptotic tumor cells or tumor cell-DC hybrids contain the unaltered spectrum of known as well as unknown tumor antigens that are unique to the patient’s tumor. In preparation for a clinical trial, we previously demonstrated that DC pulsed with tumor cell lysate and activated with TNF-α and PGE2 can stimulate tumor-reactive CTL in vitro. Loading DC concomitantly with lysate and the highly immunogenic protein KLH enhanced the T cell response against tumor cells [12].
Recent evidence suggests that the combination of chemotherapy with immunotherapy can mediate synergistic effects [13]. In mice, gemcitabine reduces the frequency of myeloid suppressor cells [14] and enhances antitumor T cell responses in vivo through increased cross-presentation of tumor antigens by DC [15]. Moreover, we could show that pancreatic carcinoma cells exposed to gemcitabine are sensitized toward T cell-mediated killing [16]. In a murine pancreatic cancer model, DC-based vaccination was combined with gemcitabine to treat orthotopic pancreatic tumors. A combination of the two strategies significantly increased survival of tumor-bearing mice [17]. Recently, a Japanese group reported on the combination of gemcitabine with immunotherapy consisting of DC vaccination and infusion of lymphokine-activated killer cells in five pancreatic cancer patients [18].
In this pilot trial, we evaluated the feasibility, toxicity, and clinical outcome of autologous DC-based vaccination in patients with advanced pancreatic carcinoma receiving concomitant gemcitabine treatment. The immune response in individual patients was analyzed prospectively and was correlated with clinical outcome.
Materials and methods
Study design and patient recruitment
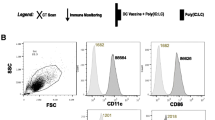
This was a single-institution study performed at the University of Munich between 07/2002 and 12/2009. The study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki and approved by the local ethics committee. After informed consent, tumor samples of patients with suspected pancreatic carcinoma were obtained during Whipple operation. Tumor cell lysate was prepared from fresh tumor samples and cryopreserved. After histological confirmation of the disease, patients were regularly followed up in the oncology department of the Medizinische Klinik Innenstadt, University of Munich, and monitored for development of metastases or local recurrence (Fig. 1a). Patients found not to be curatively operable during surgery proceeded directly to chemotherapy and DC vaccination within a few weeks after surgery. All patients included into the study received concomitant chemotherapy with gemcitabine at a standard dosage of 1,000 mg/m2 body surface in the Oncology Department of the Medizinische Klinik Innenstadt. To limit the number of visits required, DC vaccination was applied immediately after chemotherapy. Eligibility criteria for DC vaccination included age 18 or older, a Karnofsky index of 60% or greater, and an assumed life expectancy of at least 3 months. Exclusion criteria included pregnancy, severe mental or cardiopulmonary disorders, HIV infection or hepatitis, treatment with corticosteroids or other immunosuppressive agents, impossibility to take part in follow-up investigations, as well as the presence of a secondary malignoma.
Recruitment scheme, study protocol, and illustration of DC generation. a Tumor samples of patients with confirmed pancreatic carcinoma were stored for future vaccinations (step 1). In the follow-up phase (step 2), patients were monitored for recurrence of disease. After recurrence (or in case of a primary palliative situation), DC vaccination was initiated (step 3). b The vaccination protocol consisted of two vaccinations in the first month and subsequent monthly vaccinations. Immunological assays and staging CTs were performed after 2, 4, 6, and 12 months as indicated. c Adherent PBMC were cultured in the presence of IL-4 and GM-CSF for 6 days, then loaded with KLH and tumor lysate, resulting in cells termed immature DC. Maturation of DC was induced by stimulation with TNF-α and PGE2. Purity of tumor lysate-loaded DC was 89.63% in median (range 64.89 to 95.49%). Mature DC were CD14 negative (median 0.61%, range 0.18 to 8.18%), CD80 positive (median 84.48%, range 82.27 to 92.57%), CD83 positive (median 70.87%, range 51.37 to 83.69%), and expressed high levels of MHCII (median 97.02%, range 84.48 to 98.23%). d Stimulation with TNF-α and PgE2 led to an increase in IL-12 production. Error bars indicate SEM
Generation of the DC vaccine, treatment protocol, and follow-up
PBMC were derived by density gradient centrifugation from 100 ml of venous blood. Non-adherent cells were cryopreserved and stored at minus 80°C. Adherent cells were cultured with GM-CSF (Cellgenix, Freiburg, Germany, 1,000 U/ml) and IL-4 (Cellgenix, 500 U/ml) to generate DC. After 6 days, DC were incubated with autologous tumor cell lysate generated by repeated freeze–thaw cycles of tumor fragments (50–150 μg/ml) and with keyhole limpet hemocyanin (KLH, EMD Chemicals, Gibbstown, NJ, USA, 25 μg/ml) for 4 h. After stimulation with TNF-α (Cellgenix, 1,000 U/ml) and PGE2 (Pharmacia and Upjohn, London, UK, 1 μM), cells were counted and resuspended in 50 μl PBS. The number of mature DC was documented, and all cells derived from the DC culture were injected intradermally next to an inguinal lymph node. Patients were treated in biweekly intervals for the first 6 weeks, then once every 4 weeks (Fig. 1b). Quality control of DC included microbiological analysis, FACS analysis of DC phenotype and maturation status (all antibodies derived from BD Biosciences, Heidelberg, Germany), as well as measurement of IL-12(p40) production of stimulated and unstimulated DC (BD OptEIA human IL-12(p40) ELISA Kit, BD Biosciences). Clinical follow-up on the patients included CT scans 4, 6, and 12 months after the start of the experimental protocol. At the end of the one-year experimental protocol staging, CTs were performed irregularly. None of the vaccinated patients were lost in the course of follow-up.
Clinical response, toxicity assessment, and delayed-type hypersensitivity (DTH) skin testing
Disease response was defined as the primary study endpoint according to the recent RECIST criteria [19]. Adverse events were recorded according to WHO toxicity criteria. For DTH skin testing, tumor lysate (10–50 μg) was injected intradermally into the forearm. KLH (20 μg) was injected into the contralateral forearm. The DTH response was evaluated 48 h after injection. A skin induration equal or greater than 5 mm was considered positive.
Lymphocyte proliferation assay
Non-adherent cells (containing T cells) from patients’ blood samples were cocultured with autologous tumor lysate-loaded or -unloaded DC in ratios of 20/1, 40/1, 80/1, 160/1, and 320/1 for 5 days. Subsequently, proliferation was determined by 3H-thymidine incorporation. Results were expressed as increase in proliferation relative to 3H-thymidine incorporation into unstimulated PBMC.
ELISPOT assay
For the in vitro assessment of tumor-specific IFN-γ-secreting T cells, an enzyme-linked immunospot (ELISPOT) assay was used. CD8pos cells were enriched from patients’ PBMC by MACS technology (Miltenyi, Bergisch Gladbach, Germany) and cocultured with DC for 1 week at a ratio of 10/1 (T cells/DC). DC were loaded with tumor lysate or were left unloaded. Cells were restimulated with DC for 24 h before the ELISPOT assay was performed. Assays were performed according to manufacturer’s instructions. 10E5 T cells were loaded on flat-bottom 96-well ELISPOT plates (Millipore, Bedford, MA, USA) precoated with capture antibody (BD Pharmingen, Heidelberg, Germany). The plate was incubated for 20 h at 37°C. After a washing step, detection antibody was added to each well and the plates were incubated for 2 h at room temperature. Avidin-horseradish peroxidase conjugate was added, and the plate was developed with 3-amino-9-ethyl-carbazole substrate reagent. Spots were quantified using an automated ELISPOT reader (Carl Zeiss, Berlin, Germany).
Statistics
Student’s t test was applied to reveal significant differences in cytokine production, T cell proliferation, and IFN-γ production in the ELISPOT assay. A value of P = 0.05 was accepted as the level of significance. Association between immune response and survival time was determined by using Spearman’s Rank Correlation Test.
Results
Patient characteristics, DC vaccination, and toxicity
Patient characteristics, the number of vaccinations, and immunological as well as clinical responses are summarized in Table 1. Twelve patients received DC-based vaccination, seven of them in a primarily palliative situation and five (patients 40, 47, 51, 56, and 61) after Whipple operation and subsequent recurrence of disease. Four of these five patients with an assumed tumor-free interval had received adjuvant radiochemotherapy (patients 47, 51, 56, and 61). Purity of tumor lysate-loaded DC was 84.84 ± 5.77% (mean ± SEM). Median surface expression of CD83 was 70.77 ± 5.70%, of CD80 87.19 ± 2.70%, of CD14 1.95 ± 1.45%, and of HLA-DR 93.65 ± 3.21% (Fig. 1c). Unloaded DC had a purity of 90.57 ± 4.55%. Surface expression of CD83 was 73.48 ± 6.64%, of CD80 82.23 ± 3.30%, of CD14 0.35 ± 0.17%, and of HLA-DR 94.40 ± 3.90%. A total of 27 pairs of immature and mature DC from six patients were investigated for their capacity to produce IL-12(p40). All DC pairs showed a significant increase in IL-12(p40) production after stimulation (Fig. 1d). Patients received between two and twelve vaccinations. Patients 44, 45, 56, and 61 received only 2, 2, 4, and 3 vaccinations, respectively, due to rapid progression of disease and death (patient 45) or individual decision to discontinue participation in the study as regular visits to the study clinic became too straining in the context of disease progression. The total number of DC given to individual patients ranged from 3 × 10E5 in two vaccinations to 51 × 10E6 in six vaccinations. No severe side effects according to WHO toxicity criteria ≥2 were observed following DC vaccination. Patients showed side effects common to gemcitabine therapy, nausea being the most frequent one. All patients received gemcitabine as the first line therapy after diagnosis of advanced disease. Two patients received additional oxaliplatin upon diagnosis of progressive disease during the ongoing vaccination trial (patients 52 and 53). Four patients (52, 53, 56, and 62) received second-line therapy with the biological erlotinib.
Histology and clinical results
Twelve patients with pancreatic carcinoma were included into the vaccination protocol. Of these, eleven patients had ductal adenocarcinoma (supp. fig. 1, supplementary materials are available on-line). The tumor of one patient (patient 52) was initially classified as ductal adenocarcinoma and graded as G3, but then classified as acinar cell carcinoma after immunohistochemistry demonstrated α1-AT and CK19 positivity (supp. table 1). One out of the twelve patients treated with DC vaccination and concomitant chemotherapy achieved a partial remission demonstrated by the decrease of a hepatic marker lesion after 4 months of therapy. Two patients showed stable disease. Five patients had survival of 1 year or more since diagnosis of advanced disease. Patient 52 (who had acinar cell carcinoma) survived 56 months. The survival times of patients 47, 53, 54, and 56 were 38, 12, 51, and 14 months, respectively. The median survival of all vaccinated patients was 10.5 months (Fig. 2a).
Kaplan–Meier analysis of patients treated with DC and gemcitabine and correlation of survival with pre-vaccination immune response. a Three out of twelve patients were long-term survivors with survival times of more than 2 years. The median survival of all vaccinated patients was 10.5 months. One-year survival of the 12 patients receiving DC vaccination was 42%. b The capacity of lysate-loaded versus -unloaded DC to induce proliferation of T cells was used as a marker for the presence of a pre-vaccination antitumor immune response and expressed as stimulatory index (SI). Higher SIs correlated significantly with prolonged survival (P = 0.0036)
Pre-existing tumor-reactive T cells and survival
T cell proliferation assays were performed with PBMC derived prior to DC vaccination of eight out of twelve patients. From four patients, the required blood samples for immunological analyses could not be obtained due to the severity and progressive course of disease associated with relevant anemia, leucopenia, or other significant clinical conditions (patients 44, 45, 61, 56, compare Table 1). Proliferation of T cells upon activation with lysate-pulsed DC was used as a marker of a pre-vaccination antitumor immune response and expressed as stimulatory index (SI) in comparison to proliferation of T cells cocultured with unloaded DC. The presence of a pre-vaccination pool of tumor-reactive T cells could be correlated with survival time. Patient 52 showed a pre-vaccination SI of 6.49 and survived 56 months (Fig. 2b). Using Spearman’s Test, a significant correlation between the detection of tumor-reactive T cells before vaccination and survival time could be observed (r = 0.8862, P = 0.0036).
Antitumoral immune responses despite concomitant chemotherapy
Proliferation assays, ELISPOT assays, and DTH skin testing were used to assess tumor-reactive T cells in patients’ peripheral blood before, during, and after DC vaccination. The number of tumor-reactive T cells increased during the course of DC vaccination, despite concomitant chemotherapy with either gemcitabine only or gemcitabine plus oxaliplatin (Table 1, supp. fig. 2). The three columns “Percent increase over baseline in ELISPOT after vaccination,” “Stimulatory index before vaccination,” and “Stimulatory index after vaccination” of Table 1 allow estimation of individual patient’s immune response to vaccination.
The increase in tumor-reactive T cells after DC-based vaccination was not merely an epiphenomenon caused by reduction of tumor load in response to chemotherapy or—the other way around—by progression of tumor disease, as demonstrated by immunomonitoring results of patient 47, who showed a consistent increase of antitumor immune response compared to pre-vaccination baseline during phases of stable disease as well as progression (Fig. 3, supp. fig. 3). Patient 47 received chemotherapy and DC vaccination after diagnosis of metastasized tumor disease. Proliferation and ELISPOT assays indicated that the patient had a small pool of pre-vaccination tumor-reactive T cells which increased significantly during the course of vaccination, confirming results of DTH skin testing. However, staging CT scans performed after 16 and 24 weeks did not show a relevant objective tumor response. Immunomonitoring at week 52 confirmed a long-term increase of tumor-reactive T cells.
DC-induced expansion of tumor-reactive T cells and DTH skin testing in patients with concomitant gemcitabine therapy. a Proliferative capacity of T cells stimulated with tumor lysate-loaded DC was compared to T cells stimulated with unloaded DC. The four graphs represent T cells derived from a representative patient (47) prior to, as well as 16, 27, and 52 weeks after, the start of DC vaccination. The clinical course of the disease is noted in the figure headlines. Y-axis depicts increase in non-adherent cell proliferation induced by lysate-loaded versus -unloaded DC relative to proliferation of unstimulated NAC, expressed as index over baseline. b Tumor lysate was injected subcutaneously into the forearm. Forty-eight hours after injection, patients were examined for the presence of a skin induration. Whereas no skin induration was found initially, a reddish and indurated skin reaction appeared after the three initial DC vaccinations. Skin reactivity increased over time. c T cells derived at weeks 0, 19, and 52 after start of therapy were stimulated with unloaded versus lysate-loaded DC. The number of IFN-γ-producing T cells was quantified in an ELISPOT assay. Error bars indicate SEM, asterisks indicate P ≤ 0.05 in Student’s t test
ELISPOT assays confirmed that DC vaccination led to an increase in antitumor T cells. Specificity of the ELISPOT assay for detection of tumor antigen-induced IFN-γ production of T cells was demonstrated by stimulating samples with combinations of unloaded and lysate-loaded DC for activation and restimulation (Fig. 4a). As demonstrated for patient 47, ELISPOT assays showed an increase in tumor-reactive T cells during the course of vaccination for patient 52 (Fig. 4b, supp. fig. 4) and patient 54 (Fig. 4c).
ELISPOT analysis of tumor-reactive T cells. a To validate ELISPOT analysis, T cells were stimulated with unloaded or lysate-loaded DC. Only after 7 days of coculture and an additional 24-hour restimulation with lysate-loaded DC, IFN-γ-specific T cells could be observed. b The antitumor immune response of patient 52, treated with gemcitabine plus oxaliplatin, in the course of vaccination was examined by measuring the T cell response to tumor lysate 0, 17, 23, 34, 54, and 68 weeks after start of DC vaccination by ELISPOT assay. c Tumor-reactive T cells of a representative patient (54) receiving concomitant gemcitabine therapy before and after vaccination were quantified by ELISPOT assay. Error bars indicate SEM. Asterisks indicate P ≤ 0.05 in Student’s t test, in a in comparison to the unloaded situation, in b in comparison to IFN-γ production at week 0
Six patients were included into DTH skin testing using tumor lysate. Of these, four developed a positive response to tumor lysate after a mean of 5.5 vaccinations. Taken together, concomitant chemotherapy did not prevent the expansion of pre-existing tumor-reactive T cells or the induction of a de novo immune response by DC-based vaccination.
Correlation of clinical and immunological response
In an attempt to correlate individual pre- and post-vaccination immunological responses with clinical outcome, it is necessary to divide patients into four groups based on the detection of tumor-reactive T cells before and after vaccination: group 1, patients with pre-vaccination tumor-reactive T cells and immunological response to vaccination; group 2, patients with pre-existing tumor-reactive T cells, but without immunological response to vaccination; group 3, patients without pre-existing tumor-reactive T cells, but with immunological response to vaccination; and group 4, patients without any detectable immunological response. It is difficult to define strict criteria of post-vaccination immune response or even perform statistical analyses based on the low number of patients included into this study. However, some findings seem notable. Four patients (47, 52, 53, and 54) showed pre-vaccination tumor-reactive T cells that were expanded by DC vaccination. These patients had survival times between 12 and 56 months. Interestingly, both patients with stable disease (47 and 52) belonged to this group.
The one patient (patient 51) with a partial response had no relevant pre-existing tumor-reactive T cells, but developed an immunological response to vaccination. The CT scan at 4 months showed a regression of the hepatic marker lesion from 2.3 cm in diameter to 1.5 cm. Reduction of CA19-9 levels confirmed the reduction of tumor cell mass. Before Whipple operation, the patient had a CA19-9 of 1,010 U/ml that decreased to 30 U/ml after R0 surgery (normal range <37 U/ml). The patient showed a recurrence of disease 7 months later with hepatic metastases and a CA19-9 of 747 U/ml. After the start of combined DC vaccination and gemcitabine therapy, CA19-9 decreased to values constantly below 200 U/ml. This favorable development was associated with a positive DTH skin test and an increase in T cell proliferation to autologous tumor lysate (SI increasing from 1.28 to 2.14 at week 22, the biggest increase of all patients). However, overall survival after diagnosis of advanced disease was only 7 months (14 months after surgery) when the patient died of systemic deterioration in an outside hospital after having not shown signs of disease progression upon the last visit to our hospital.
Two other patients without a pre-vaccination pool of tumor-reactive T cells developed low to moderate antitumor responses during vaccination (42 and 62). Both these patients had progressive disease and a survival time of 8 months.
Proliferation assays after DC vaccination were performed on material from seven of the twelve patients and ELISPOT assays on material from three patients. The magnitude of DC-induced immune responses varied. SIs after vaccination were found to lie between 1.78 and 8.48, representing increases between 14 and 118%, compared to the situation before vaccination. There was no correlation of increased T cell proliferation to better survival. However, ELISPOT assays demonstrated significant vaccination responses in three patients (47, 52, and 54) with T cell counts 241, 354, and 153% over the pre-vaccination baseline. All three patients had pre-vaccination responses, and these three were the study participants with longest overall survival with 38, 56, and 51 months, respectively. This might indicate a correlation between vaccination response and survival in patients with pre-vaccination antitumor immune responses.
Discussion
Here, we report a pilot study using autologous tumor lysate-pulsed DC to treat patients with advanced pancreatic carcinoma. We attempted to correlate clinical outcome with the detection of tumor-reactive T cells analyzed from pre- and post-vaccination samples of PBMC. DC preparations of high quality could be reliably generated from the peripheral blood of pancreatic carcinoma patients. Vaccination was tolerated well. DC vaccination led to a robust immune response against the neo-antigen KLH, as demonstrated by DTH skin test that indicates that patients were capable of mounting a DC-induced immune response despite tumor burden and concomitant chemotherapy. Immunomonitoring using DTH testing with autologous tumor lysate, proliferation assays, and ELISPOT analysis indicated that DC-based vaccination could enhance a pre-existing antitumoral immune response or induce such a response de novo. The median overall survival in our pilot study was 10.5 months. This compares to 5.6 months in the study of Burris et al. [2] that led to the approval of gemcitabine. One-year survival was 42% in our study compared to 18% in the Burris trial. A recent metaanalysis on the use of combination therapies in advanced pancreatic carcinoma published between 2004 and 2007 reported an overall survival ranging from 5.0 to 7.1 months in patients treated with gemcitabine monotherapy [20].
Selection bias is of major importance for the critical discussion of clinical trials. The twelve patients were included into the study protocol directly after diagnosis of advanced disease. Therefore, selection bias based on the fact that only long-time survivors have a chance to be included into a study protocol is limited. However, we cannot exclude that there is a selection bias toward patients that are actively involved in their treatment plan and prepared to undergo maximal therapy. These patients might not only accept frequent study visits, but also side effects of more aggressive chemotherapy more easily, possibly resulting in better survival.
It is a matter of ongoing debate if long-time survivors of pancreatic cancer are in fact suffering from an adenocarcinoma of the pancreas or from a more benign tumor entity, carrying a false diagnosis [21]. In this study, one patient survived a total of 56 months after diagnosis of advanced pancreatic carcinoma with pulmonary and liver metastases. The histology of this patient’s cancer revealed an acinar cell carcinoma. Prognosis of acinar cell carcinoma is probably better than ductal adenocarcinoma of the pancreas, and rare cases of long-time survival are reported in the literature. Holen et al. [22] reported a median survival of 19 months in their patient population. Most experts suggest an aggressive approach with multimodal chemotherapy [23]. Patient 52 received—after completion of the vaccination study—various combinations of gemcitabine, 5-FU, capecitabine, oxaliplatine, and irinotecan together with bevacizumab, erlotinib, and SIRT therapy. This makes evaluation of the contribution of DC vaccination to clinical outcome difficult. However, particularly long survival of this patient, together with positive immunomonitoring data, might indicate that acinar cell carcinoma responds well to DC vaccination therapy.
We interpreted higher T cell proliferation using lysate-loaded DC in comparison to unloaded DC (resulting in higher SIs) in a coculture assay as indication of a pre-vaccination antitumor T cell response. We cannot formally rule out that lysate loading of DC resulted in higher antigen-unspecific T cell stimulation capacity. However, the phenotype of lysate-loaded and -unloaded DC was homogeneous, indicating that SIs measure biologically relevant differences in the patients’ T cell pools. Most patients demonstrated SIs between one and two. One patient (patient 52) showed a pre-vaccination SI of 6.49. This patient had the longest survival time of all patients included in the study. It is problematic to define a cut off for positive antitumor immune responses in a post-hoc manner. The limited number of patients tested made grouped analysis of immune responders versus non-responders impossible. However, correlation of SIs with survival time was significant, indicating that a pre-vaccination pool of tumor-reactive T cells might be associated with better overall survival.
Patients with particularly long survival tended to show DC-induced expansion of the antitumor T cell response. All patients showing either clinical response and/or survival of 12 months or longer, mounted a relevant antitumoral T cell response after DC vaccination, irrespective of whether a pre-vaccination T cell response was found or not.
We used tumor cell lysate as the antigen source for DC loading. Various tumor-associated antigens, including those belonging to the family of cancer testis antigens, have been described for pancreatic carcinoma [24]. However, clinical investigation of these candidate antigens is still in the very beginning. Although whole cell preparations potentially offer the advantage of a polyvalent vaccine, the lack of a defined epitope restricts the use of immunomonitoring techniques to bulk assays, such as thymidine incorporation and ELISPOT assay. Here, tumor lysate-loaded DC were used for the first time in pancreatic cancer patients to stimulate pre- and post-vaccination samples of the patient’s T cells in vitro. Using semi-quantitative analyses, we were able to show an increase in T cell proliferation and IFN-γ secretion upon activation with lysate-pulsed autologous DC in the course of chemo-/immunotherapy in the majority of patients. Importantly, these responses occurred despite concomitant chemotherapy and were independent from tumor burden.
A concern of concomitant gemcitabine therapy is a negative impact of chemotherapy-mediated immune suppression on the DC-induced T cell response. However, there is good reasoning for combining the two approaches [5, 25, 26]. The release of danger signals by dying tumor cells after chemotherapy may lead to activation of antigen-presenting cells and the subsequent enhancement of T cell-dependent antitumor immune responses. We and others have shown that chemotherapy and DC-based vaccination can be combined to achieve additive therapeutic responses [17]. The initial study protocol would have allowed us to compare a vaccination-only arm with an arm administering gemcitabine parallel to DC vaccination. However, clinical studies published during the past decade demonstrated efficacy of gemcitabine therapy, making the drug the gold standard for treatment of patients with advanced pancreatic carcinoma. Thus, chemotherapy had to be included in the study protocol for ethical concerns. A major obstacle for immunotherapy of pancreatic cancer is tumor-mediated immunosuppression, especially in patients with large tumor burden [27–29]. Thus, we propose that the application of vaccination strategies for pancreatic carcinoma may be particularly effective in an adjuvant setting, potentially in combination with gemcitabine. Moreover, analysis of a pre-vaccination antitumor T cell response could be a promising strategy for select patients who are more likely to benefit from DC therapy.
In conclusion, DC-based vaccination in patients with advanced pancreatic carcinoma is feasible and safe. Despite concomitant chemotherapy, patients showed an immunological response to DC vaccination. A high SI, indicating the presence of pre-vaccination tumor-reactive T cells, was associated with increased survival after chemo-/immunotherapy in one patient.
Abbreviations
- APC:
-
Antigen-presenting cells
- DC:
-
Dendritic cells
- DOC:
-
Died of other causes
- DOD:
-
Dead of disease
- DTH:
-
Delayed-type hypersensitivity
- ELISPOT:
-
Enzyme-linked immunospot
- KLH:
-
Keyhole limpet hemocyanin
- PBMC:
-
Peripheral blood mononuclear cells
- PMA:
-
Phorbol 12-myristate 13-acetate
- SI:
-
Stimulatory index
- TGF-β:
-
Transforming growth factor-β
References
Ozols RF, Herbst RS, Colson YL, Gralow J, Bonner J, Curran WJ Jr, Eisenberg BL, Ganz PA, Kramer BS, Kris MG, Markman M, Mayer RJ, Raghavan D, Reaman GH, Sawaya R, Schilsky RL, Schuchter LM, Sweetenham JW, Vahdat LT, Winn RJ (2007) Clinical cancer advances 2006: Major research advances in cancer treatment, prevention, and screening–a report from the American society of clinical oncology. J Clin Oncol 25(1):146–162. doi:10.1200/JCO.2006.09.7030
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15(6):2403–2413
Rothenberg ML, Moore MJ, Cripps MC, Andersen JS, Portenoy RK, Burris HA 3rd, Green MR, Tarassoff PG, Brown TD, Casper ES, Storniolo AM, Von Hoff DD (1996) A phase ii trial of gemcitabine in patients with 5-fu-refractory pancreas cancer. Ann Oncol 7(4):347–353
Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, Andre T, Zaniboni A, Ducreux M, Aitini E, Taieb J, Faroux R, Lepere C, de Gramont A (2005) Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a gercor and giscad phase iii trial. J Clin Oncol 23(15):3509. doi:10.1200/JCO.2005.06.023
Dauer M, Schnurr M, Eigler A (2008) Dendritic cell-based cancer vaccination: Quo vadis? Expert Rev Vaccines 7(7):1041–1053. doi:10.1586/14760584.7.7.1041
Kantoff PW, Higano CS, Shore NDE, Berger R, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF (2010) Sipuleucel-t immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411–422
Steinman RM, Banchereau J (2007) Taking dendritic cells into medicine. Nature 449(7161):419–426. doi:10.1038/nature06175
Chaux P, Favre N, Martin M, Martin F (1997) Tumor-infiltrating dendritic cells are defective in their antigen-presenting function and inducible b7 expression in rats. Int J Cancer 72(4):619–624. doi:10.1002/(SICI)1097-0215(19970807)72:4<619:AID-IJC12>3.0.CO;2-6
Troy AJ, Summers KL, Davidson PJ, Atkinson CH, Hart DN (1998) Minimal recruitment and activation of dendritic cells within renal cell carcinoma. Clin Cancer Res 4(3):585–593
Dauer M, Lam V, Arnold H, Junkmann J, Kiefl R, Bauer C, Schnurr M, Endres S, Eigler A (2008) Combined use of toll-like receptor agonists and prostaglandin e(2) in the fastdc model: Rapid generation of human monocyte-derived dendritic cells capable of migration and il-12p70 production. J Immunol Methods 337(2):97–105. doi:10.1016/j.jim.2008.07.003
Dauer M, Obermaier B, Herten J, Haerle C, Pohl K, Rothenfusser S, Schnurr M, Endres S, Eigler A (2003) Mature dendritic cells derived from human monocytes within 48 h: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol 170(8):4069–4076
Schnurr M, Galambos P, Scholz C, Then F, Dauer M, Endres S, Eigler A (2001) Tumor cell lysate-pulsed human dendritic cells induce a t-cell response against pancreatic carcinoma cells: An in vitro model for the assessment of tumor vaccines. Cancer Res 61(17):6445–6450
Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G (2008) Immunological aspects of cancer chemotherapy. Nat Rev Immunol 8(1):59–73. doi:10.1038/nri2216
Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM (2005) Gemcitabine selectively eliminates splenic gr-1 +/cd11b + myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 11(18):6713–6721. doi:10.1158/1078-0432.CCR-05-0883
Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JA, Robinson BW (2003) Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific cd8 t cells. J Immunol 170(10):4905–4913
Dauer M, Herten J, Bauer C, Renner F, Schad K, Schnurr M, Endres S, Eigler A (2005) Chemosensitization of pancreatic carcinoma cells to enhance t cell-mediated cytotoxicity induced by tumor lysate-pulsed dendritic cells. J Immunother 28(4):332–342
Bauer C, Bauernfeind F, Sterzik A, Orban M, Schnurr M, Lehr HA, Endres S, Eigler A, Dauer M (2007) Dendritic cell-based vaccination combined with gemcitabine increases survival in a murine pancreatic carcinoma model. Gut 56(9):1275–1282. doi:10.1136/gut.2006.108621
Hirooka Y, Itoh A, Kawashima H, Hara K, Nonogaki K, Kasugai T, Ohno E, Ishikawa T, Matsubara H, Ishigami M, Katano Y, Ohmiya N, Niwa Y, Yamamoto K, Kaneko T, Nieda M, Yokokawa K, Goto H (2009) A combination therapy of gemcitabine with immunotherapy for patients with inoperable locally advanced pancreatic cancer. Pancreas 38(3):e69–e74. doi:10.1097/MPA.0b013e318197a9e3
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C (2008) Meta-analysis of randomized trials: Evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 8(1):82. doi:10.1186/1471-2407-8-82
Carpelan-Holmstrom M, Nordling S, Pukkala E, Sankila R, Luttges J, Kloppel G, Haglund C (2005) Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the finnish cancer registry. Gut 54(3):385–387. doi:10.1136/gut.2004.047191
Holen KD, Klimstra DS, Hummer A, Gonen M, Conlon K, Brennan M, Saltz LB (2002) Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol 20(24):4673–4678
Butturini G, Pisano M, Scarpa A, D’Onofrio M, Auriemma A, Bassi C Aggressive approach to acinar cell carcinoma of the pancreas: A single-institution experience and a literature review. Langenbecks Arch Surg. doi:10.1007/s00423-010-0706-2
Kubuschok B, Xie X, Jesnowski R, Preuss KD, Romeike BF, Neumann F, Regitz E, Pistorius G, Schilling M, Scheunemann P, Izbicki JR, Lohr JM, Pfreundschuh M (2004) Expression of cancer testis antigens in pancreatic carcinoma cell lines, pancreatic adenocarcinoma and chronic pancreatitis. Int J Cancer 109(4):568–575. doi:10.1002/ijc.20006
Menard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F (2008) Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother 57(11):1579–1587. doi:10.1007/s00262-008-0505-6
Gulley JL, Madan RA, Arlen PM (2007) Enhancing efficacy of therapeutic vaccinations by combination with other modalities. Vaccine 25(Suppl 2):B89–B96. doi:10.1016/j.vaccine.2007.04.091
von Bernstorff W, Voss M, Freichel S, Schmid A, Vogel I, Johnk C, Henne-Bruns D, Kremer B, Kalthoff H (2001) Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res 7(3 Suppl):925s–932s
Hiraoka N, Onozato K, Kosuge T, Hirohashi S (2006) Prevalence of foxp3 + regulatory t cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 12(18):5423–5434. doi:10.1158/1078-0432.CCR-06-0369
Lin Y, Kikuchi S, Tamakoshi A, Obata Y, Yagyu K, Inaba Y, Kurosawa M, Kawamura T, Motohashi Y, Ishibashi T (2006) Serum transforming growth factor-beta1 levels and pancreatic cancer risk: A nested case-control study (japan). Cancer Causes Control 17(8):1077–1082. doi:10.1007/s10552-006-0048-0
Acknowledgments
This work was supported by grants from the Gravenhorst Stiftung (M.D.), the Förderprogramm für Forschung und Lehre (FöFoLe) of the University of Munich (C.B.), the Deutsche Forschungsgemeinschaft (Ba 3824/1-1 to C.B. and Graduiertenkolleg 1202 to M.S., S.E.), and the Deutsche Krebshilfe (M.S.). This work is part of the doctoral thesis of S.S. at the University of Munich.
Conflict of interest
All authors declare that no conflicts of interest exist.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Bauer and M. Dauer both authors have contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bauer, C., Dauer, M., Saraj, S. et al. Dendritic cell-based vaccination of patients with advanced pancreatic carcinoma: results of a pilot study. Cancer Immunol Immunother 60, 1097–1107 (2011). https://doi.org/10.1007/s00262-011-1023-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1023-5