Abstract
Systemic high-dose interleukin-2 (IL-2) treatment achieves long-term survival in a subset of advanced patients with melanoma. As we reported previously, intratumoral IL-2 induced complete local responses in more than 60% of melanoma patients. This study aimed to analyze the long-term outcome of 72 patients treated in two prior trials. Melanoma patients (49 stage III, 23 stage IV) with injectable metastases received intratumoral IL-2 injections thrice weekly at individually escalated doses (median duration, 6.5 weeks; median total IL-2 dose, 72 MIU; median number of injected metastases, 10). The observed 2-year overall survival rates were 95.5% for stage III patients with cutaneous metastases only (stage IIIB), 72% for those with combined cutaneous and lymph node involvement (stage IIIC), 66.7% for stage IV patients with disease limited to distant soft-tissue metastases (stage IV M1a), and 9.1% for those with visceral metastases (stage IV M1b and stage IV M1c). Thirty patients who reported recurrence of unresectable distant metastases subsequently received chemotherapy in the further course of disease and showed an overall response rate of 36.7% (16.7% complete responses, 20% partial responses). A high total dose of IL-2 and a dacarbazine/temozolomide-based chemotherapy regimen were variables correlated with a clinical response. In conclusion, patients with cutaneous metastasis without lymph node involvement in stage III and with soft-tissue metastasis without visceral involvement in stage IV showed unexpected favorable survival rates after intratumoral treatment with IL-2. Furthermore, the intratumoral IL-2 treatment seemed to be associated with increased complete and partial responses in subsequent chemotherapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgery is the treatment of choice in stage III melanoma and also in stage IV disease, provided the patient can be rendered free of disease at all known metastatic sites. Surgery seems to be associated with clear survival benefit in 10–20% of patients with stage IV disease [1].
If surgery is not feasible, e.g., because of a high number of lesions or repetitive early recurrences, isolated limb perfusion can be applied if the disease is limited to one extremity [2]. Whenever metastatic melanoma is unresectable, prognosis is unfavorable and palliative systemic treatment options are limited [3, 4]. Therefore, alternative direct treatment options are needed if disease is not curable by surgery.
Intratumoral application of drugs is an appealing therapeutic concept, as high concentrations can be achieved within the tumor, which may be essential to attain the desired therapeutic effect. Furthermore, systemic concentrations on local treatment are low in contrast to systemic treatments with the same agent, resulting in comparably low toxicity.
Interleukin 2 (IL-2) has been used as a high-dose systemic immunotherapy since the early 1980s. It was approved in 1998, as a first-line treatment for melanoma by the FDA based on a meta-analysis of 8 clinical trials [5].
The first report describing regression of melanoma metastases after intratumoral IL-2 treatment was published in 1994 [6]. Based on these findings, we conducted two clinical trials with the aim of investigating IL-2 as an alternative local treatment option besides surgery [7, 8]. In both trials, we observed a high local efficacy with complete responses of all treated metastases in over 60% of patients. In contrast to systemic high-dose therapy, intratumoral application was well tolerated and is applicable on an outpatient basis [8]. In the second trial, we found that the treatment was also feasible for patients with many tumor lesions. Factors associated with response were the absence of visceral metastases, stage III disease and dermal rather than subcutaneous localization [7]. We concluded that intratumoral IL-2 may be regarded as a promising local treatment option for patients with soft-tissue metastases not curable surgically.
The present investigation was conducted to analyze long-term survival after treatment with intratumoral IL-2 and to study possible interactions with subsequent treatments. All patients from both the previous studies were included in this retrospective analysis with up to 11 years of current follow-up.
Materials and methods
Intratumoral IL-2 treatments
All patients described here were enrolled into one of the intratumoral IL-2 studies conducted between 1998 and 2002 [7] or 2002 and 2009 [8]. Both study protocols were approved by the local ethics committees and patients were treated only after receiving written informed consent. Inclusion criteria comprised histopathologically proven malignant melanoma, presence of injectable dermal or subcutaneous metastases either in clinical stage III or stage IV. Patients requiring systemic chemotherapeutic treatment for metastatic disease were excluded but patients who underwent prior systemic chemotherapy was allowed. Both trials had the design of a prospective phase II study. Patients were enrolled at the Center for Dermatooncology, Tübingen, Germany (both trials) and at the Department of Dermatology, Homburg, Germany (second trial only). IL-2 (Proleukin®, Novartis) was injected intratumorally with single doses varying between 0.3 and 6 MIU, depending on the lesion size. Treatment was initiated at 3 MIU IL-2/day and escalated for individual patients if they tolerated. The total daily dose was divided between all injectable lesions. The treatment schedule was 3 times weekly on an outpatient basis, until clinical regression of metastases was evident or if progression occurred that was no longer manageable by ongoing IL-2 injections.
Analysis of overall survival
Overall survival was calculated from the date of the first IL-2 injection until last follow-up or death. Only deaths caused by melanoma were considered as an event. The other patients were censored at the time of the last observation. The last survival update was made for all patients in April 2010. Kaplan–Meier analyses were performed to estimate overall survival.
Response to chemotherapy during follow-up of IL-2-treated patients
All patients who received systemic chemotherapy for unresectable distant metastases after intratumoral IL-2 were considered. During chemotherapy, tumor assessment was performed every 8–12 weeks by whole-body CT scans including the brain. All patients had a measurable disease. For response evaluation upon chemotherapy, the RECIST criteria were used [9].
Time to progression (TTP) was calculated from the first dose of chemotherapy to the time point of progressive disease (PD). All objective responses were reanalyzed for validation by an independent radiologist (D.S.). Differences between subgroups were tested statistically using the Pearson chi-square method. Statistical analyses were conducted using the software PASW Statistics 18 (SPSS Inc., Chicago, USA).
Results
Patients and treatments
In total, 72 patients who enrolled since 1998 and completed IL-2 treatment by December 2007 were evaluable. The baseline data are summarized in Table 1. The median age was 66 years (range 19–88 years). Forty-nine patients were treated in stage III and 23 in stage IV. Among the stage IV patients, 9 had nodal/visceral metastases not accessible to local IL-2 treatment, whereas in the stage III patients and the remaining 14 stage IV patients, all metastases were accessible for injection. Most patients had received extensive previous therapy. In 13 patients, limb perfusion or radiotherapy was performed. Prior systemic treatments comprised interferon-α (21 patients) and chemotherapy (25 patients) before IL-2 treatment was initiated. The median duration of IL-2 treatment was 6.5 weeks, and the applied median total dose was 72 MIU IL-2 (range 9–548.1 MIU). The median number of metastases treated per patient was 10. A complete response of all treated metastases was achieved in 48 of 72 (66.7%) patients. The clinical situation before and the outcome after intratumoral IL-2 is presented for one patient in Fig. 1. Eighteen patients (25%) remained free of any recurrence after intratumoral IL-2, while 54 progressed. Of those 54 patients who progressed, 30 received chemotherapy in the further course of disease, and 24 patients were treated by surgery. Chemotherapy was based on dacarbazine in 11 patients and temozolomide in 12 patients, while the 7 remaining patients received other agents or polychemotherapy. When chemotherapy was initiated, all patients had stage IV disease (M1a: 5, M1b: 8, and M1c: 15 patients).
Clinical situation before initiation of intratumoral IL-2 and outcome 5 years later of a 74 year-old woman. In April and May 2004 (left) approximately 100 cutaneous metastases located on the left leg were treated over 5 weeks, resulting in a complete response. The total IL-2 dose was 105.5 MIU. Two weeks after stopping intratumoral treatment, iliacal lymph node metastases, which were already detected before initiation of IL-2 were excised. Since then the patient remained free of any recurrence (right)
Overall survival
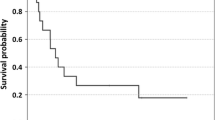
The median follow-up time was 25 months from the start of IL-2 treatment (23 months for 40 patients who died of their disease and 47 months for 32 patients alive at end of follow-up; range 1–131 months). Two-year survival rates were 82.9 and 39.1% for stage III and IV patients, respectively. In stage III patients, the 2-year and 5-year survival rates according to substages were 95.5 and 86.8% for those with cutaneous metastases only (stage IIIB) and 72.0 and 31.4% if there was additional lymph node involvement (stage IIIC). In stage IV patients, the 2-year and 5-year survival rates were 66.7 and 16.7% for those with distant soft-tissue metastases only (stage IV M1a) in contrast to 9.1 and 0.0% for patients with presence or history of additional visceral lesions (stage IV M1b and M1c). Kaplan–Meier curves according to stage are shown in Fig. 2.
Overall survival since intratumoral IL-2 treatment shown for patients with intransit metastases only (stage IIIB, gray continuous line), with additional lymph node involvement (stage IIIC, gray broken line), with distant soft-tissue metastases (stage IV M1a, black continuous line) and with visceral lesions (stage IV M1b or M1c, black broken line)
The long-term outcome did not depend on the number of treated metastases, because the overall survival for 21 patients who had ≥20 metastases was as good as for the other 51 patients who had <20 metastases (data not shown).
Response to first chemotherapy during follow-up of IL-2-treated patients
All 30 patients who received chemotherapy for metastatic melanoma after intratumoral IL-2 treatment were considered in the response evaluation. A complete response was observed in 5 patients (16.7%) and a partial response in 6 patients (20%), resulting in an overall response rate of 36.7%. Furthermore, 5 patients (16.7%) showed stable disease. The other 14 patients (46.7%) had already progressed at the first tumor assessment after starting chemotherapy. Response to chemotherapy was observed in all M-substages, including 5 of 17 (29.4%) patients with M1c, 4 of 8 (50%) patients with M1b and 2 of 5 (40%) patients with M1a disease, respectively (Tables 2, 3). With regard to prior intratumoral IL-2 treatment, the total dose of IL-2 was positively correlated with response to subsequent chemotherapy. Ten of 18 (55.6%) patients who had received at least 60 MIU IL-2 showed a response upon subsequent chemotherapy, while this was true only for 1 of 12 (8.3%) patients who had received less IL-2. Stage at initiation of IL-2 was not associated with outcome. While 8 of 16 (50%) patients who received the first systemic cytotoxic treatment within one year of starting IL-2 manifested a response, this was true only for 3 of 14 (21.4%) patients with a longer time interval between the two therapies. A further correlation was found between response rate and different cytotoxic agent(s). While 11 of 23 patients (48%) treated with a dacarbazine/temozolomide-based regimen showed an objective response, all 7 patients treated by polychemotherapy or other cytotoxic drugs had progressive disease. Treatment characteristics and status of responding patients are shown in Table 3.
Discussion
The present study analyzed the long-term outcome of melanoma patients after intratumoral IL-2 treatment. Overall survival rates exceeded those described by Balch et al. for patients whose disease was limited to cutaneous metastases in stage III (stage IIIB, N2c: 5-year survival rate 86.6% vs. ~70% [10]) and to soft-tissue metastases in stage IV (stage IV M1a: 2-year survival rate 66.7% vs. ~43% [10]), while the long-term outcome of patients with additional locoregional lymph node involvement (IIIC) and visceral disease (stage IV, M1b,c) does not seem to be influenced by intratumoral IL-2. The observed long survival of stage IIIB and stage IV M1a patients unlikely represents a positive selection bias considering that these patients presented with a high number of metastases (median 10 metastases) and were extensively pretreated.
Eighteen of 72 patients have had no recurrence thus far. Of the other 54 patients, 30 required systemic chemotherapy due to progression in the later course of disease after intratumoral IL-2. We observed a response rate of 36.7% to this subsequent chemotherapy. In the subgroup treated with dacarbazine- or temozolomide-based regimens, 11 of 23 patients (48%) manifested a response. In contrast, overall response rates ranging between 7.5% for dacarbazine [11] and 13.4% for temozolomide [12] were reported in controlled trials. It is unlikely that the association is observed by chance because of the additional correlations between response and (1) a high total IL-2 dose applied before and (2) a short time interval between intratumoral IL-2 and chemotherapy.
The phenomenon of high response rates and favorable outcome upon chemotherapy after prior immunotherapy was already described in several clinical trials [13–19]. Wheeler et al. reported improved survival after chemotherapy in glioblastoma patients who had received prior dendritic cell vaccination compared with the same chemotherapy without vaccination [16]. In another trial, 10 of 11 cancer patients who had developed specific immune responses on vaccination responded to salvage chemotherapy in contrast to only 1 of 6 patients without measurable immunization [17]. Antonia et al. described a high response rate and an unexpectedly favorable long-term outcome upon paclitaxel or carboplatin chemotherapy treatment in non-small-cell lung cancer patients who had been previously treated with a p53-based dendritic cell vaccine [15, 18]. Finally, Arlen et al. reported a long progression-free survival on docetaxel treatment of patients with prostate cancer, if vaccination was applied before chemotherapy [13].
The favorable long-term outcome observed here may suggest a beneficial systemic effect induced by the local treatment, despite the fact that no responses of metastases that had not been directly injected have been observed thus far. Systemic responses after intratumoral IL-2 have, however, been described in various animal models. Maas et al. showed regression of distant, non-injected lesions after intratumoral treatment in lymphoma-bearing mice. The same IL-2 doses given systemically were far less effective [20]. Van Es et al. [21] described in a transplanted rabbit carcinoma model that peritumoral IL-2 induced complete regressions of untreated contralateral tumors. A second challenge of the cured animals resulted in tumor rejection, suggesting the generation of specific immunity.
Three observations support the hypothesis that at least the local rejection of intratumorally treated metastases and, moreover, potentially a favorable long-term outcome, is mediated by a specific anti-melanoma immune response: (1) the onset of vitiligo and (2) an increase in anti-melanoma T-cells in PBMC of single patients [7]. (3) The predominance of CD8+ and CD4+ T-cells, the most important mediators of specific cellular immune responses, in the intra- and peritumoral lymphocytic infiltrate, which arises 1–2 weeks after the start of treatment and precedes regression of the lesion [8]. This systemic vaccination effect after local treatments was described as in situ vaccination [22].
Intravenous high-dose IL-2 as a monotherapy, or after adoptive T-cell transfer, is efficient in melanoma and can cause long-term regressions in a small subset of patients [23, 24]. The higher local efficacy if applied intratumorally compared with the systemic route may be explained by the high peak concentration of IL-2 at the tumor site after intratumoral injection, which cannot be achieved by systemic therapy even at dose-limiting concentrations applied intravenously [25]. This high peak concentration might reverse the tolerogenic state in the tumor tissue, which is maintained by serum factors (e.g. TGF-β) or by immunosuppressive T-cell subsets like regulatory T-cells (Tregs). Tregs are characterized by expression of CD25—the high-affinity receptor for IL-2—and are therefore candidates to play a thus-far uncharacterized role in the context of intratumoral IL-2 treatment.
The limitation of a potential effect on survival to IL-2 treated patients without lymph node involvement (stage IIIB) or visceral metastases (stage IV M1a) as well as the lack of response of distant, not directly injected, metastases may be explained by the consideration that homing and trafficking of induced T-cells is substantially influenced by the site of activation. Targeting of effector and memory T-cells to tissue is instructed during priming and mediated by adhesion receptors such as integrins expressed on the cell surface [26]. The direct treatment with intratumoral IL-2, which was limited to cutaneous or subcutaneous metastases, might lead to a preferential trafficking of T-cells to the soft-tissue compared with lymphatic or visceral tissue. As a consequence, a benefit on survival would be limited to those patients in whom the cutaneous or subcutaneous metastases are decisive for prognosis (stage IIIB due to intransit metastases and a subset of stage IV M1a patients). All of the stage IIIB as well as the majority of our stage IV M1a patients had cutaneous or subcutaneous metastases only, without additional lymph node involvement.
Limitations of this study are indeed the lack of control groups and the short follow-up of the patients of the second trial, which impede forming definite conclusions with regard to survival from our observations described here. However, our data can at least serve as a basis for planning a randomized controlled trial analyzing a survival endpoint with the patients receiving intratumoral IL-2 in the experimental arm.
In conclusion, we observed a favorable long-term outcome in stage III patients with cutaneous metastases only and in stage IV patients with injectable soft-tissue metastases only, whereas stage III patients with additional lymph node metastases and stage IV patients with additional visceral metastases did obviously not benefit in terms of overall survival. Furthermore, a high response rate on first subsequent chemotherapy in stage IV was observed after the pretreatment with intratumoral IL-2. A possible explanation for these observations may be a beneficial systemic effect induced by the local treatment; however, the underlying mechanisms are not yet understood. Our data support further pursuit of this therapeutic approach in a randomized trial including a control group of patients with standard care.
References
Young SE, Martinez SR, Essner R (2006) The role of surgery in treatment of stage IV melanoma. J Surg Oncol 94:344–351
Grunhagen DJ, Brunstein F, Graveland WJ, van Geel AN, de Wilt JH, Eggermont AM (2004) One hundred consecutive isolated limb perfusions with TNF-alpha and melphalan in melanoma patients with multiple in-transit metastases. Ann Surg 240:939–947
Tsao H, Atkins MB, Sober AJ (2004) Management of cutaneous melanoma. N Engl J Med 351:998–1012
Eggermont AM, Kirkwood JM (2004) Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer 40:1825–1836
Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA (1999) High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 17:2105–2116
Gutwald JG, Groth W, Mahrle G (1994) Peritumoral injections of interleukin 2 induce tumour regression in metastatic malignant melanoma. Br J Dermatol 130:541–542
Weide B, Derhovanessian E, Pflugfelder A, Eigentler TK, Radny P, Zelba H, Pfohler C, Pawelec G, Garbe C (2010) High response rate after intratumoral treatment with interleukin-2: results from a phase 2 study in 51 patients with metastasized melanoma. Cancer 116:4139–4146
Radny P, Caroli UM, Bauer J, Paul T, Schlegel C, Eigentler TK, Weide B, Schwarz M, Garbe C (2003) Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br J Cancer 89:1620–1626
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC Jr, Morton DL, Ross MI, Sober AJ, Sondak VK (2009) Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27:6199–6206
Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, Pavlick AC, DeConti R, Hersh EM, Hersey P, Kirkwood JM, Haluska FG (2006) Bcl-2 antisense (Oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen melanoma study group. J Clin Oncol 24:4738–4745
Kaufmann R, Spieth K, Leiter U, Mauch C, von den Driesch P, Vogt T, Linse R, Tilgen W, Schadendorf D, Becker JC, Sebastian G, Krengel S, Kretschmer L, Garbe C, Dummer R (2005) Temozolomide in combination with interferon-alfa versus temozolomide alone in patients with advanced metastatic melanoma: a randomized, phase III, multicenter study from the dermatologic cooperative oncology group. J Clin Oncol 23:9001–9007
Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosenbach DW, Feldman J, Steinberg SM, Jones E, Chen C, Marte J, Schlom J, Dahut W (2006) A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res 12:1260–1269
Davies AM, Evans WK, Mackay JA, Shepherd FA (2004) Treatment of recurrent small cell lung cancer. Hematol Oncol Clin North Am 18:387–416
Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, Bepler G, Simon G, Janssen W, Lee JH, Menander K, Chada S, Gabrilovich DI (2006) Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res 12:878–887
Wheeler CJ, Das A, Liu G, Yu JS, Black KL (2004) Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res 10:5316–5326
Gribben JG, Ryan DP, Boyajian R, Urban RG, Hedley ML, Beach K, Nealon P, Matulonis U, Campos S, Gilligan TD, Richardson PG, Marshall B, Neuberg D, Nadler LM (2005) Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res 11:4430–4436
Chiappori AA, Soliman H, Janssen WE, Antonia SJ, Gabrilovich DI (2010) INGN-225: a dendritic cell-based p53 vaccine (Ad.p53-DC) in small cell lung cancer: observed association between immune response and enhanced chemotherapy effect. Expert Opin Biol Ther 10:983–991
Gabrilovich DI (2007) Combination of chemotherapy and immunotherapy for cancer: a paradigm revisited. Lancet Oncol 8:2–3
Maas RA, Van Weering DH, Dullens HF, Den Otter W (1991) Intratumoral low-dose interleukin-2 induces rejection of distant solid tumour. Cancer Immunol Immunother 33:389–394
Van Es RJ, Baselmans AH, Koten JW, Van Dijk JE, Koole R, Den Otter W (2000) Perilesional IL-2 treatment of a VX2 head-and-neck cancer model can induce a systemic anti-tumour activity. Anticancer Res 20:4163–4170
Neville ME, Robb RJ, Popescu MC (2001) In situ vaccination against a non-immunogenic tumour using intratumoural injections of liposomal interleukin 2. Cytokine 16:239–250
Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE (1994) Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst 86:1159–1166
Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE (1994) Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 271:907–913
Phan GQ, Attia P, Steinberg SM, White DE, Rosenberg SA (2001) Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol 19:3477–3482
Denucci CC, Mitchell JS, Shimizu Y (2009) Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there. Crit Rev Immunol 29:87–109
Acknowledgments
Parts of this work were funded by Deutsche Forschungsgemeinschaft grant SFB685, a research grant of Novartis GmbH, Nürnberg, Germany, and a research grant of Chiron Therapeutics, Ratingen, Germany.
Conflict of interest
There are no financial disclosures of any of the authors to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
A comment to this article is available at http://dx.doi.org/10.1007/s00262-014-1583-2.
Rights and permissions
About this article
Cite this article
Weide, B., Eigentler, T.K., Pflugfelder, A. et al. Survival after intratumoral interleukin-2 treatment of 72 melanoma patients and response upon the first chemotherapy during follow-up. Cancer Immunol Immunother 60, 487–493 (2011). https://doi.org/10.1007/s00262-010-0957-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-010-0957-3