Abstract
Cationic antimicrobial peptides (CAPs) exhibit promising anticancer activities. In the present study, we have examined the in vivo antitumoral effects of a 9-mer peptide, LTX-302, which is derived from the CAP bovine lactoferricin (LfcinB). A20 B cell lymphomas of BALB/c origin were established by subcutaneous inoculation in syngeneic mice. Intratumoral LTX-302 injection resulted in tumor necrosis and infiltration of inflammatory cells followed by complete regression of the tumors in the majority of the animals. This effect was T cell dependent, since the intervention was inefficient in nude mice. Successfully treated mice were protected against rechallenge with A20 cells, but not against Meth A sarcoma cells. Tumor resistance could be adoptively transferred with spleen cells from LTX-302-treated mice. Resistance was abrogated by depletion of T lymphocytes, or either the CD4+ or CD8+ T cell subsets. Taken together, these data suggest that LTX-302 treatment induced long-term, specific cellular immunity against the A20 lymphoma and that both CD4+ and CD8+ T cells were required. Thus, intratumoral administration of lytic peptide might, in addition to providing local tumor control, confer a novel strategy for therapeutic vaccination against cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cationic antimicrobial peptides (CAPs) are natural-source agents that have been shown to exert anticancer activities [1–3]. Their positive charge and amphipathic properties enable CAPs to disrupt negatively charged membranes by electrostatic interactions. The plasma membrane of many cancer cells overexpress negatively charged phosphatidylserine and O-glycosylated mucins, thereby carrying a slightly higher net negative charge compared to normal eukaryotic cells [4–8]. This may partly explain why CAPs have a higher specificity for certain types of neoplastic cells compared to traditional chemotherapeutic drugs [9–12]. Moreover, CAPs kill chemoresistant cancer cells by membrane lysis, independently of proliferative status and resistance phenotype [13–15].
Bovine lactoferricin (LfcinB) has been reported to induce both apoptosis and necrosis in tumor cells in vitro, although a direct destabilization of the cytoplasmic membrane and collapse of mitochondria seem to be the main events responsible for the cytotoxic effect of LfcinB [16]. In vivo studies of LfcinB treatment have shown that systemic or intratumoral administration of the peptide inhibits tumor growth and metastasis in several experimental mouse models [17, 18]. By the use of extensive structure–activity studies and the optimization of structural parameters critical for antitumor activity [19–22], a 9-mer peptide LTX-302 has been constructed from its parental peptide LfcinB. It consists of an idealized α-helical secondary structure, containing five cationic Lys residues, three Trp residues, the bulky non-coded residue β-diphenylalanine (Dip) and an amidated C-terminal. In the current study, we investigated the effects of LTX-302 on the murine A20 B cell lymphoma in vitro and in vivo. The A20 lymphoma was selected on the basis of pilot experiments, and both wild-type and athymic nude mice of BALB/c origin were used to elucidate the mechanism of anticancer activity of the peptide.
The intratumoral administration of LTX-302 conferred complete and permanent tumor regression in a substantial portion of wild-type mice, but only transient inhibition of tumor growth in nude mice. Further studies revealed a T cell-dependent and long-term protective effect against A20 tumor cells in the cured animals. Hence, local treatment of A20 tumors by LTX-302 induces necrosis and local inflammation at the tumor site, and apparently elicits immunization against the tumor.
Materials and methods
Peptide synthesis
LTX-302 (WKKWDipKKWK-NH2, M = 1,439 g/mol) and LTX-328 (KAQDipQKQAW-NH2, M = 1,209 g/mol) were synthesized by solid-phase methods using standard Fmoc chemistry on a Pioneer Peptide synthesizer (Applied Biosystem, Foster City, CA, USA). The peptide was purified to >95% homogeneity by reverse-phase high-pressure liquid chromatography (Waters Corporation, Milford, MA, USA), and the peptide integrity was confirmed by mass spectrometry (VG Instruments Inc., Altringham, UK) [23].
Cell lines
A20, a naturally occurring murine B cell lymphoma of BALB/c mice origin [24], the human embryonic fibroblast cell line MRC-5 and the human umbilical vein endothelial cell line HUVEC were obtained from American Type Culture Collection (CRL-2873, ATCC, Rockville, MD, USA). Meth A is a chemically induced BALB/c fibrosarcoma [25]. A20 was grown in RPMI 1,640 medium containing 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES, 1.0 mM sodium pyruvate and 50 μM 2-mercaptoethanol. Meth A and MRC-5 were grown in RPMI 1640 and MEM medium, respectively. Media for A20, Meth A and MRC-5 were without antibiotics, but supplemented with 10% FBS and 1% l-glutamine. HUVEC was grown in an EGM-2 medium (Medprobe, Lonza). The cell cultures were maintained in a humidified atmosphere of 5% CO2 at 37°C. All cell lines were regularly tested for the presence of Mycoplasma (ATCC Mycoplasma Detection Kit) and A20 were additionally tested for viruses (RapidMAP™-27, Taconic, Europa).
Hemolytic activity
Freshly isolated human red blood cells (RBC), prepared as described previously [17], were incubated for 1 h at 37°C with LTX-302 dissolved in PBS at concentrations ranging from 1 to 1,000 μg/ml. The samples were centrifuged at 4,000 rpm for 5 min before the absorbance of the supernatant was measured at 405 nm by a spectrophotometric microtiter plate reader (Thermomax Molecular Devises, NJ, USA). The 0 and 100% hemolysis were determined in samples exposed to PBS and 1% Triton X-100, respectively.
In vitro cytotoxicity
LTX-302 was diluted in a serum-free cell culture medium and incubated with A20, Meth A or MRC-5 cells for 4 h. Cell survival after peptide treatment was measured using the MTT viability assay [17, 26]. Results shown represent the mean of triplicate wells from three parallel experiments.
Scanning electron microscopy (SEM)
A20 cells were incubated with 34 μM LTX-302 for 30 min, which is the concentration that kills 80% of the cells within 30 min (IC80). Immediate fixation was performed by adding 1 ml of 4% formalin solution directly in the wells and specimen preparation was according to standard procedures [27]. After 30 min, the fixed cells were transferred to vials and centrifuged. The supernatant was removed and the cells fixed overnight in a 4% formalin solution. The cells were transferred to coverslips, washed with phosphate buffer before counter-fixation in 1% osmium tetroxide for 20 min, dehydrated in ethanol and dried with hexamethyl disilazane. Cells on coverslips were coated with gold and analyzed by JEOL JSM-6300 scanning electron microscopy.
Transmission electron microscopy (TEM)
A20 cells incubated with 34 μM LTX-302 for 30 min were harvested, aspirated and fixed in Karnovsky’s fixative overnight, followed by post-fixation, dehydration and embedding in Epon-araldite according to standard procedures [28]. Ultrathin sections were prepared and examined on a JEOL JEM-1010 transmission electron microscope (Tokyo, Japan).
Histological examination
Tumors were excised and immersed in 4% formalin in PBS at 4°C for 24 h and thereafter embedded in paraffin and sectioned at 5 μm thickness. The sections were stained with hematoxylin and eosin (H&E), and examined by a Zeiss axiophot light microscope (CarlZeiss, Oberkochen, Germany).
Release of high mobility group box-1 (HMGB1) from A20 cells
Murine A20 cells (6 × 105 cells/well) were seeded in 96-well plates and treated with LTX-302 at different concentrations (0, 10, 50, 100, 500 μg/ml) and incubated at 37°C and with 5% CO2 for different time points (5, 10, 30, 60 or 120 min).
Supernatants (S) were collected after centrifugation at 1400g for 5 min. To make cell lysates (L), the cells were washed with PBS before being lysed using cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA). Supernatants and cell lysates were boiled in reducing NuPAGE LDS sample buffer, resolved on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Norway). Filters were hybridized with HMGB1 antibody (rabbit polyclonal to HMGB1, Abcam), followed by horseradish peroxidase-conjugated secondary reagent (goat anti-rabbit IgG, Abcam) and developed by WB Luminol Reagent (Santa Cruz Biotechnology, Heidelberg, Germany) according to the manufacturer’s instructions.
In vivo tumor treatment protocol
Mice
Wild-type and athymic nude female BALB/c mice, 6–8 weeks of age, were purchased from Charles River, Germany. All mice were housed in cages in a specific pathogen-free animal facility according to the local and European Ethical Committee guidelines.
Tumor treatment
A20 tumor cells were harvested, washed in RPMI-1640 and injected s.c. into the left side of the abdomen in wild-type or nude BALB/c mice (5 × 106 cells per mouse/50 μl RPMI-1640). When the tumor size reached 20 mm2, single doses of LTX-302 dissolved in saline (0.5 mg LTX-302/50 μl saline) were administered intratumorally once a day for 3 consecutive days. Vehicle control was saline only. Additionally, LTX-328 (0.5 mg LTX-328/50 μl saline) was used as a nonlytic control peptide in wild-type mice.
Tumor size was measured three times a week using an electronic caliper and expressed as the area of an ellipse [(maximum dimension/2) × (minimum dimension/2) × 3.14]. Animals were killed when the tumor exceeded 100 mm2, and if metastasis or tumor ulceration were evident.
Secondary tumor challenge
Wild-type mice with complete regression of A20 tumors were given a second s.c. A20 tumor inoculation (5 × 106 cells) on the right side of the abdomen (contralateral to the first tumor site) 4 weeks after they were cured by LTX-302. Another group of A20-cured mice were given Meth A sarcoma (5 × 106 cells/50 μl RPMI-1640) s.c. in the right side of the abdomen. Two control groups of naïve mice were inoculated with 5 × 106 A20 or Meth A cells, respectively. All mice were monitored for tumor size and survival.
Irradiation of mice before secondary tumor challenge
Wild-type mice were inoculated with A20 cells and treated with LTX-302 as previously described. When treated mice had been tumor free for 4 weeks, they were irradiated with 500 cGy with a 137Cs irradiator (JL: Shephard, San Fernando, CA, USA) 1 day before the rechallenge of A20 (5 × 106 cells) and thereafter monitored for tumor growth and survival.
Preparation of splenocytes for adoptive transfer
Spleens were disaggregated in warm RPMI medium containing 2% FBS, fragments were allowed to settle out, and the supernatant was centrifuged at 1,100 rpm for 10 min. Red blood cells were removed by hypotonic lysis and an extensive washing with RPMI 1640 containing 2% FBS. Splenocytes were then resuspended in RPMI 1640, the medium for the transfer of splenocytes.
Adoptive transfer of splenocyte
Wild-type mice were inoculated with A20 cells and treated with LTX-302 as described above. Spleens were harvested from treated mice 4 weeks after macroscopically visible tumors disappeared. Recipient mice were irradiated with 500 cGy 1 day before the transfer of splenocytes, and 100 μl heparin (10 IU) was administered intraperitoneally a few minutes before splenocytes were injected i.v. into the tail vein (2 × 107 splenocytes/100 μl RPMI 1640). The next day these mice were s.c. challenged with A20 cells (5 × 106 cells) and monitored for tumor growth and survival. Control mice received splenocytes from naïve mice.
T cell depletion experiments
Spleen single cell suspensions from mice cured of A20 were depleted of T cells by magnetic cell sorting (Miltenyi Biotec, Bergish Gladbach, Germany) using antibodies specific for CD90 (Thy1.2), CD8a (Ly-2) or CD4 (L3T4) coupled to microbeads. The remaining lymphocytes were infused i.v. into naïve irradiated mice (2 × 107 cells), which received an s.c. A20 challenge (5 × 106 cells) the next day and monitored for tumor growth and survival. Control mice received unfractionated splenocytes from either treated tumor-free or naïve mice. The depletion conditions were validated by flow cytometry analysis using anti-CD90 (Thy1.2), anti-CD4 (L3T4) and anti-CD8a (Ly-2) monoclonal antibodies (Miltenyi Biotec, Bergish Gladbach, Germany). Under these conditions, >98% of the relevant T cells were depleted.
Statistical analysis
Survival curves were compared by the log-rank test. Groups were compared using one-way ANOVA test and Tukey’s multiple comparison test. Differences were considered significant when P < 0.05.
Results
Tumor cells were more sensitive to LTX-302 than normal cells
The LTX-302 peptide exhibited an eight- to tenfold higher activity against the A20 and Meth A cancer cell lines than against the normal fibroblasts MRC-5 and HUVEC, and no hemolytic activity was obtained against RBC over the concentration range tested (Table 1). No cytotoxic activity against the murine tumor cell lines or the normal human cells were observed using the control peptide LTX-328 (data not shown).
LTX-302-induced necrosis in A20 tumor cells
To investigate the mode of action underlying the cytotoxic activity of LTX-302, A20 cells were incubated with the peptide for 30 min and prepared for electron microscopy studies. Representative micrographs of both untreated and LTX-302-treated A20 cells studied by SEM (Fig. 1a) revealed a smooth cell membrane on untreated A20 cells, while pore formation and cell swelling were evident on A20 plasma membranes after treatment with LTX-302. TEM studies revealed intact organelles and nuclei in untreated A20 cells, while LTX-302-treated A20 cells showed more condensed nuclei and cell swelling with disrupted plasma membranes and subsequent leakage of intracellular content (Fig. 1b). These results demonstrate that LTX-302 kills the tumor cells via a lytic mode of action.
LTX-302 induces damage on the cell membrane of A20 cells in vitro. A20 cells were treated with 34 μM LTX-302 for 30 min (IC80), fixed and prepared for electron microscopic studies. SEM micrographs showing untreated A20 cells with a normal smooth surface and A20 cells treated with LTX-302, showing disruption and pore formation of the cell membrane (a). TEM micrographs showing intact untreated A20 cells and A20 cells treated with LTX-302, having completely destroyed cell membrane and organelles (b). TEM micrographs showing overview pictures of untreated and treated A20 (c)
Treatment of established A20 tumors with LTX-302 resulted in complete regression in a proportion of immunocompetent mice
A20 cells was injected subcutaneously in both wild-type and nude (T cell deficient) BALB/c mice to compare the antitumoral effects for the treatment of tumors by LTX-302 in mice with different immunological status. After 6 days, the tumors reached an average size of 20 mm2 and were injected intratumorally with LTX-302 (or LTX-328 or vehicle only) once a day for 3 consecutive days. As presented in Fig. 2a, LTX-302 induced a significant inhibition of tumor growth in all treated animals. Moreover, complete tumor regression in five out of eight wild-type mice was achieved (Fig. 2b). No recurrence was observed in these tumor-free mice during 90 days of follow-up (data not shown). In nude mice, the inhibition of tumor growth was only transient and all mice succumbed to tumor regrowth (Fig. 2c). Hence, complete tumor regression induced by LTX-302 peptide was achieved only in wild-type mice.
Intratumoral effects of LTX-302 in established A20 tumors in BALB/c wild-type mice and nude mice. Tumor-bearing mice were treated intratumorally with vehicle (saline 50 μl), control peptide LTX-328 (0.5 mg/50 μl saline) or LTX-302 (0.5 mg/50 μl saline) for 3 consecutive days (marked by arrows in the graphs) when tumor sizes were approximately 20 mm2. Wild-type mice injected with saline, LTX-328 or LTX-302 (a).*P < 0.05 at day 13 and 22, LTX-302 versus other treatment. Survival curves after peptide treatment showing the number of survivors of wild-type mice (b). P < 0.005 for LTX-302 treated mice. Nude mice injected with saline or LTX-302 (c). Experiments were done several times with similar results
Selective and long-term protection against the A20 lymphoma was induced by LTX-302 in immunocompetent mice
The absence of complete regression after peptide treatment in T cell-deficient (nude) mice indicated that the tumoricidal effects involved specific immunity. Accordingly, upon rechallenge with the same tumor the animals might mount a secondary immune response. In support of this contention, A20 tumor cells grew only transiently when inoculated into immunocompetent mice previously rendered tumor free by peptide treatment (Fig. 3a). Thus, whereas some tumor growth was observed initially, all the reinoculated mice became tumor free within 15 days without receiving any treatment. In contrast, a different syngeneic tumor, the Meth A sarcoma rapidly established large and lethal tumors when inoculated in mice previously cured of the A20 lymphoma (Fig. 3b). This is consistent with specific immunity.
Selective and long-term protection against A20 lymphoma was induced by LTX-302 treatment in wild-type mice. Mice cured of A20 tumor were rechallenged with A20 or Meth A cells (5 × 106) 4 weeks after they became tumor free by LTX-302 treatment, and were monitored for tumor growth. Rechallenge of A20 cells in cured mice (filled triangles) or naïve mice (control) (filled squares) (a). Challenge of syngeneic and antigenic irrelevant Meth A tumor cells in mice cured of A20 (open triangles) or naïve mice (open squares) (b). The numbers of surviving mice with complete tumor regression are indicated for each group. Experiments were done several times with similar results
A20 tumors were characterized by infiltration of inflammatory cells and necrosis after LTX-302 treatment and after tumor rechallenge of cured mice
Histological sections from wild-type mice with established A20 tumors treated with LTX-302 and tumor-free mice rechallenged with the same tumor were examined. Figure 4a shows the histology of the viable A20 tumor parenchyma of a control tumor-bearing animal (injected with vehicle only). Figure 4b represents a section taken from an LTX-302 treated tumor, demonstrating extensive infiltration of inflammatory cells, necrotic areas, hemorrhage and a loss of viable tumor parenchyma. The arrow points to a small part of the section demonstrating viable tumor tissue. Figure 4c shows an A20 tumor 4 days after reinoculation in a mouse previously cured of A20 by LTX-302 injection. A massive infiltration of lymphocytes having small, dark nuclei was evident and almost no viable tumor parenchyma was left, demonstrating a regressing tumor.
LTX-302 induces inflammation and necrosis of A20 tumors in vivo and rechallenged A20 tumors are strongly infiltrated by lymphocytes. Histological examinations of A20 tumors in wild-type mice were performed by H&E staining at ×200. Primary tumors were excised 1 day after the last injection with saline (a) or LTX-302 (b). Secondary tumor challenges in tumor-free mice were excised 4 days after the inoculation of A20 cells (c). The arrows point to the small part of viable tissue in b and the rest of viable tumor cells in c. HMGB1 was assessed at different time points by Western blotting in the cell lysate (L) or in the supernatant (S) of A20 cells treated with LTX-302 (50 μg/ml) or control untreated cells (d)
The danger signal HMGB1 is shown to be released when cells are damaged by necrosis [29]. In our study, immunoblotting (Fig. 4d) demonstrated that LTX-302 treatment of A20 cells induced release of HMGB1 in the supernatant after 30 min. After 120 min of peptide treatment, large amount of HMGB1 was detected in the supernatant, while almost no protein was detected in the cell lysate. In the untreated controls, HMGB1 was detected only in the cell lysates and not in the supernatants.
Protection against tumor growth was T cell dependent
To study lymphocyte involvement in the protection against the A20 lymphoma, cured mice were subjected to lymphocyte-depleting whole body irradiation prior to tumor rechallenge. This substantially reduced the capacity to reject a secondary A20 inoculum, as complete tumor regression occurred only in half of the irradiated animals (Fig. 5a). Additionally, regression of the tumors took 5–10 days more than in non-irradiated controls as measured by tumor growth (data not shown). The attenuation of tumor rejection conferred by irradiation further support the assumption that cellular immunity is involved in the protection against A20 lymphoma.
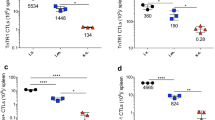
The active principle underlying tumor protection against the A20 tumor. Protection against the A20 tumor in cured mice can be turned off by irradiation (a). To induce non-myeloablative lymphodepletion in cured mice, the animals were whole body irradiated (500 cGy) 1 day before the rechallenge of A20 cells (5 × 106). Survival curves of irradiated cured mice (inverted filled triangles), non-irradiated cured mice (filled triangles) and naïve mice (filled squares) are shown. P < 0.05 irradiated and non-irradiated cured mice compared to the control mice. Prevention against A20 tumor growth is T cell dependent and can be adoptively transferred (b). Splenocytes (2 × 107) from cured mice were infused into irradiated (500 cGy) naïve mice. One day later, the mice were challenged s.c. with A20 tumor cells (5 × 106) and monitored for tumor growth and survival. T cells from cured donors were depleted from the splenocyte suspension by using CD90 (Thy1.2) microbead magnetic cell sorting before being transferred into recipient mice. Survival curves of mice receiving splenocytes from cured mice (filled triangles), naive mice (filled squares) or all T cell-depleted cured mice (filled circles) are shown. P < 0.0005 for the cured mice compared to naïve or all T cell-depleted cured mice. Both CD4+ and CD8+ T cell subsets are required for long-lasting protection (c). CD4+ or CD8+ T cells in splenocytes from cured donors were depleted by using CD4 (L3T4) or CD8 (Ly-2) microbead magnetic cell sorting. After magnetic selection, whole splenocytes (filled triangles), CD4+ T cell-depleted splenocytes (filled diamonds), and CD8+ T cell-depleted splenocytes (filled circles) were infused i.v. into irradiated naïve mice. Mice treated with splenocytes from naïve mice (filled squares) served as a control group. One day later, these mice were s.c. challenged with a lethal number (5 × 106) of A20 cells and monitored for tumor size and survival. P < 0.05 for the cured mice compared to naïve or depleted mice, while there was no significance between CD4 and CD8 depleted mice. The numbers of survivors from the total number of animals in each group is indicated in parentheses. All survivors remained tumor free. Mice were killed when the tumors exceeded 100 mm2 or if metastasis occurred
If the protection against an A20 tumor depends on cellular immunity, we hypothesized that the immunity could be transferred between animals by the adoptive transfer of splenocytes. Splenocytes from cured mice were therefore transferred into naïve mice followed by the challenge of A20 cells 1 day later. Mice receiving splenocytes from cured mice showed that protection was transferred between animals since seven out of nine mice survived tumor challenge, and the survivors remained tumor free during 90 days of follow-up (Fig. 5b). When the splenocytes from cured mice were depleted of all T cells before transference, it conferred no inhibition of tumor growth and there were no surviving animals after tumor challenge (Fig. 5b), thereby proving that T cells were needed for protection against A20.
To study the role of the main T cell subsets, splenocytes from cured animals were depleted of CD4+ or CD8+ T cells, respectively, before transfer to naïve mice (Fig. 5c). Depletion of CD8+ T cells virtually abrogated the capacity of spleen cells to confer tumor protection, as no animals survived. On the other hand, in three out of five mice receiving CD4+ T cell-depleted splenocytes, tumor regression commenced 6–8 days after tumor inoculation (data not shown). However, regrowth of all these tumors was evident during 90 days of follow-up. Thus, both CD4+ and CD8+ T cells are necessary for the long-lasting protection against A20.
Discussion
A number of previous studies have reported anticancer activities of CAPs. Almost all of the in vivo studies have been performed in xenograft models by inoculating human tumor cells in nude mice. Some CAPs have been administered systemically, as propeptides [11], peptides conjugated to targeting domains [30] or diastereomeric peptides [31], whereas other CAPs have been administered locally by intratumoral injection [32, 33]. Relatively few studies on the anticancer activity of CAPs have been performed in syngeneic models with immunocompetent mice [34, 35]. Our group has previously reported that the CAP LfcinB inhibits growth of a solid murine tumor after intratumoral administration [17].
In the present study, A20 B cell lymphomas of BALB/c origin were established by subcutaneous inoculation in syngeneic mice. Intratumoral administration of LTX-302, a synthetic 9-mer peptide having optimized structural properties critical for membrane interaction and lysis, resulted in complete regression of the tumors in the majority of animals. This involved a T cell-dependent mechanism, since the treatment was inefficient in nude mice.
In vitro studies revealed that LTX-302 was highly active against A20 lymphoma cells. The efficacy was selective since the peptide displayed an eight- to tenfold lower activity against normal fibroblasts. This differential susceptibility most likely reflects differences in the cell membrane composition of neoplastic and normal cells, i.e., many cancer cell membranes are abnormally anionic due to a high expression of phosohatidylserine, lipoproteins, O-glycocylated mucines and sialic acid [4–6].
Histological studies of treated tumors show extensive hemorrhagic necrosis of the tumor parenchyma. Electron microscopy demonstrated that in vitro treated A20 cells were killed by LTX-302-induced lysis. Taken together, our observations in vitro and in vivo indicate that intratumoral LTX-302 peptide injection leads to extensive tumor necrosis initiated by a direct disruptive effect of the peptide on the plasma membrane of tumor cells.
The anticancer effect of LTX-302 was examined in immunodeficient mice by local treatment of A20 tumors in nude mice, revealing a significant tumor growth inhibition although no long-lasting complete regression was evident. This demonstrates that an intact immune system is crucial for inducing the complete regression of A20 lymphomas. The contention that the specific immune system plays a role was supported by histological studies showing massive infiltration of lymphocytes at the tumor site after peptide treatment of wild-type mice. In contrast to our studies, Mai et al. [36] reported that a proapoptotic peptide induced tumor regression in immunocompetent mice after intratumoral administration, but this treatment, however, led to minimal lymphocytic infiltration and did not prevent tumor relapse. The discrepancy in the treatment effects of these peptides may be due to their different mode of killing, since the proapoptotic peptide induces apoptosis rather than necrosis. The lytic mode of action of LTX-302 therefore seems to be an important precondition in achieving infiltration of lymphocytes into the tumor site and the following complete regression.
Interestingly, A20 lymphoma-bearing mice cured by LTX-302 were protected against the A20 tumor upon rechallenge. This effect was systemic since the A20 cells were reinoculated at a different site from the primary tumor. Protection was transferable with spleen cells from cured, but not naïve mice. Additionally, the protection was T cell dependent, sensitive to depletion of the major T-lymphocyte subsets and did not affect growth of a different syngeneic tumor. Taken together, these observations strongly suggest that protection against tumor regrowth reflects a specific, cell-mediated secondary immune response and that both CD4+ and CD8+ T cells are crucially involved.
Sensitization of T cells following intratumoral administration of LTX-302 migth reflect two not mutually exclusive mechanisms. LTX-302-mediated tumor cell lysis and subsequent release of membrane-bound and intracellular compounds to the extracellular compartment might augment the amount of tumor-associated antigen available for T cell priming by dendritic cells (DC) [37]. Alternatively, or in addition, tumor necrosis might provide appropriate danger signals for maturation and activation of DCs [38–40], thus enhancing their capacity to activate T cells. This was supported by the release of HMGB1, which is a danger molecule shown to stimulate DCs to mature and become immunostimulatory [41].
Intratumoral treatment with CAPs might be developed into a novel, dual action therapeutic strategy for cancer by mediating local tumor control via direct tumor cell lysis and subsequent protection against recurrence and metastasis by inducing specific immunity.
References
Hoskin DW, Ramamoorthy A (2008) Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta 1778:357–375
Mader JS, Hoskin DW (2006) Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin Investig Drugs 15:933–946
Papo N, Shai Y (2005) Host defense peptides as new weapons in cancer treatment. Cell Mol Life Sci 62:784–790
Iwasaki T, Ishibashi J, Tanaka H, Sato M, Asaoka A, Taylor D, Yamakawa M (2009) Selective cancer cell cytotoxicity of enantiomeric 9-mer peptides derived from beetle defensins depends on negatively charged phosphatidylserine on the cell surface. Peptides 30:660–668
Utsugi T, Schroit AJ, Connor J, Bucana CD, Fidler IJ (1991) Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res 51:3062–3066
Dobrzynska I, Szachowicz-Petelska B, Sulkowski S, Figaszewski Z (2005) Changes in electric charge and phospholipids composition in human colorectal cancer cells. Mol Cell Biochem 276:113–119
Burdick MD, Harris A, Reid CJ, Iwamura T, Hollingsworth MA (1997) Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumor cell lines. J Biol Chem 272:24198–24202
Yoon WH, Park HD, Lim K, Hwang BD (1996) Effect of O-glycosylated mucin on invasion and metastasis of HM7 human colon cancer cells. Biochem Biophys Res Commun 222:694–699
Mader JS, Salsman J, Conrad DM, Hoskin DW (2005) Bovine lactoferricin selectively induces apoptosis in human leukemia and carcinoma cell lines. Mol Cancer Ther 4:612–624
Chen Y, Xu X, Hong S, Chen J, Liu N, Underhill CB, Creswell K, Zhang L (2001) RGD-Tachyplesin inhibits tumor growth. Cancer Res 61:2434–2438
Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, Krajewski S, Lombardo CR, Rao R, Ruoslahti E, Bredesen DE, Pasqualini R (1999) Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med 5:1032–1038
Risso A, Braidot E, Sordano MC, Vianello A, Macri F, Skerlavaj B, Zanetti M, Gennaro R, Bernardi P (2002) BMAP-28, an antibiotic peptide of innate immunity, induces cell death through opening of the mitochondrial permeability transition pore. Mol Cell Biol 22:1926–1935
Kim S, Kim SS, Bang YJ, Kim SJ, Lee BJ (2003) In vitro activities of native and designed peptide antibiotics against drug sensitive and resistant tumor cell lines. Peptides 24:945–953
Sharom FJ, DiDiodato G, Yu X, Ashbourne KJ (1995) Interaction of the P-glycoprotein multidrug transporter with peptides and ionophores. J Biol Chem 270:10334–10341
Johnstone SA, Gelmon K, Mayer LD, Hancock RE, Bally MB (2000) In vitro characterization of the anticancer activity of membrane-active cationic peptides. I. Peptide-mediated cytotoxicity and peptide-enhanced cytotoxic activity of doxorubicin against wild-type and p-glycoprotein over-expressing tumor cell lines. Anticancer Drug Des 15:151–160
Eliassen LT, Berge G, Leknessund A, Wikman M, Lindin I, Lokke C, Ponthan F, Johnsen JI, Sveinbjornsson B, Kogner P, Flaegstad T, Rekdal O (2006) The antimicrobial peptide, lactoferricin B, is cytotoxic to neuroblastoma cells in vitro and inhibits xenograft growth in vivo. Int J Cancer 119:493–500
Eliassen LT, Berge G, Sveinbjornsson B, Svendsen JS, Vorland LH, Rekdal O (2002) Evidence for a direct antitumor mechanism of action of bovine lactoferricin. Anticancer Res 22:2703–2710
Yoo YC, Watanabe S, Watanabe R, Hata K, Shimazaki K, Azuma I (1997) Bovine lactoferrin and lactoferricin, a peptide derived from bovine lactoferrin, inhibit tumor metastasis in mice. Jpn J Cancer Res 88:184–190
Eliassen LT, Haug BE, Berge G, Rekdal O (2003) Enhanced antitumour activity of 15-residue bovine lactoferricin derivatives containing bulky aromatic amino acids and lipophilic N-terminal modifications. J Pept Sci 9:510–517
Yang N, Lejon T, Rekdal O (2003) Antitumour activity and specificity as a function of substitutions in the lipophilic sector of helical lactoferrin-derived peptide. J Pept Sci 9:300–311
Yang N, Stensen W, Svendsen JS, Rekdal O (2002) Enhanced antitumor activity and selectivity of lactoferrin-derived peptides. J Pept Res 60:187–197
Yang N, Strom MB, Mekonnen SM, Svendsen JS, Rekdal O (2004) The effects of shortening lactoferrin derived peptides against tumour cells, bacteria and normal human cells. J Pept Sci 10:37–46
Strom MB, Rekdal O, Svendsen JS (2000) Antibacterial activity of 15-residue lactoferricin derivatives. J Pept Res 56:265–274
Kim KJ, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, Asofsky R (1979) Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol 122:549–554
Sveinbjornsson B, Olsen R, Seternes OM, Seljelid R (1996) Macrophage cytotoxicity against murine meth A sarcoma involves nitric oxide-mediated apoptosis. Biochem Biophys Res Commun 223:643–649
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Bozzola JJ, Russell LD (1992) Specimen preparation for scanning electron microscopy. In: Electron microscopy: principles and techniques for biologists. Jones and Bartlett Publishers, Boston, pp 48–69
Bozzola JJ, Russell LD. 1992. Specimen preparation for transmission electron microscopy. In: Electron microscopy: principles and techniques for biologists. Jones and Bartlett Publishers, Boston, pp 16–45
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Leuschner C, Enright FM, Gawronska B, Hansel W (2003) Membrane disrupting lytic peptide conjugates destroy hormone dependent and independent breast cancer cells in vitro and in vivo. Breast Cancer Res Treat 78:17–27
Papo N, Seger D, Makovitzki A, Kalchenko V, Eshhar Z, Degani H, Shai Y (2006) Inhibition of tumor growth and elimination of multiple metastases in human prostate and breast xenografts by systemic inoculation of a host defense-like lytic peptide. Cancer Res 66:5371–5378
Papo N, Braunstein A, Eshhar Z, Shai Y (2004) Suppression of human prostate tumor growth in mice by a cytolytic d-, l-amino acid peptide: membrane lysis, increased necrosis, and inhibition of prostate-specific antigen secretion. Cancer Res 64:5779–5786
Soballe PW, Maloy WL, Myrga ML, Jacob LS, Herlyn M (1995) Experimental local therapy of human melanoma with lytic magainin peptides. Int J Cancer 60:280–284
Baker MA, Maloy WL, Zasloff M, Jacob LS (1993) Anticancer efficacy of magainin2 and analogue peptides. Cancer Res 53:3052–3057
Rodrigues EG, Dobroff AS, Cavarsan CF, Paschoalin T, Nimrichter L, Mortara RA, Santos EL, Fazio MA, Miranda A, Daffre S, Travassos LR (2008) Effective topical treatment of subcutaneous murine B16F10-Nex2 melanoma by the antimicrobial peptide gomesin. Neoplasia 10:61–68
Mai JC, Mi Z, Kim SH, Ng B, Robbins PD (2001) A proapoptotic peptide for the treatment of solid tumors. Cancer Res 61:7709–7712
den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, Adema GJ (2004) In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res 64:4024–4029
Gallucci S, Lolkema M, Matzinger P (1999) Natural adjuvants: endogenous activators of dendritic cells. Nat Med 5:1249–1255
Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N (2000) Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 191:423–434
Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 12:1539–1546
Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Muller S, Iannacone M, Traversari C, Bianchi ME, Manfredi AA (2004) HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 5:825–830
Acknowledgments
This work was funded by a grant from the Norwegian Research Council and Lytix Biopharma. We thank Ragnhild Osnes for her technical assistance at the Animal Department. The staff at the Department of Electron Microscopy is acknowledged for their assistance. Kristel Berg and Inger Lindin at the Tunor Biology Research Group are acknowledged for their technical assistance during this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berge, G., Eliassen, L.T., Camilio, K.A. et al. Therapeutic vaccination against a murine lymphoma by intratumoral injection of a cationic anticancer peptide. Cancer Immunol Immunother 59, 1285–1294 (2010). https://doi.org/10.1007/s00262-010-0857-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-010-0857-6