Abstract
Heparanase is expressed in almost all advanced tumors, and therefore it may serve as a potential target for tumor therapy. Our previous study has shown that heparanase can serve as a potential universal tumor-associated antigen (TAA) for the immunotherapy of advanced tumors. Further study demonstrated that the HLA-A*0201-restricted Cytotoxic T lymphocytes (CTL) epitopes Hpa525 (PAFSYSFFV), Hpa277 (KMLKSFLKA) and Hpa405 (WLSLLFKKL) from human heparanase could induce a potent anti-tumor immune response in vitro. The present study was designed to investigate whether the above peptides could induce immune responses in mice. Our results demonstrated that the effectors from heparanase peptide-immunized mice could effectively lyse various tumor cells that were heparanase positive and HLA-A*0201 matched. We also found that these peptide-specific CTLs did not lyse autologous lymphocytes that had low heparanase activity. Further study revealed that Hpa525, Hpa277, and Hpa405 peptides increased the frequency of IFN-γ-producing T cells as compared to a negative peptide. These results suggest that Hpa525, Hpa277, and Hpa405 peptides are novel HLA-A*0201-restricted CTL epitopes capable of inducing heparanase-specific CTLs in mice. Because heparanase is expressed in most advanced malignant tumors, Hpa525, Hpa277, and Hpa405 peptide-based vaccines may be useful for the immunotherapy of patients with advanced tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overexpression of heparanase in tumor cells confers an invasive phenotype in experimental animals [1]. The enzyme also releases angiogenic factors from the ECM and thereby induces an angiogenic response in vivo [2]. Heparanase up-regulation correlates with increased tumor vascularity and poor prognosis of patients with cancers [3]. These results suggest that the heparanase enzyme is a promising target for anti-cancer drug development.
Cytotoxic T lymphocytes (CTLs) specific for various tumor antigens play a potent role in the antitumor immune response [4–6]. CTLs recognize antigens on both their target and on APCs as epitope fragments, composed of 8–12 amino acids that are complexed with MHC molecules [7–9]. Tumor cells expressing epitopes derived from tumor associated antigen (TAA) can be recognized and lysed by CTLs. Several TAAs have been identified, and the specific immune responses that can be generated by targeting these antigens are being studied in both human and mouse models of malignant disease [10, 11]. Moreover, immunization with epitopes derived from TAA-pulsed dendritic cells (DC) vaccines, as a modality of specific immunotherapy, has been applied to patients with malignancies and proven to have some clinical effectiveness [12].
Our previous study indicated that DC loading of full-length heparanase cDNA could induce heparanase-specific CTLs, which showed potent lysis of human gastric cancer cells in a MHC-restricted manner [13]. Recently, Sommerfeldt [14] predicted three epitopes derived from the human heparanase protein that could elicit heparanase-specific CTLs which lysed breast cancer cells in vitro. We also predicted and identified two H-2Kb-restricted epitopes from murine heparanase. Effectors induced by peptides of mouse heparanase at these residue positions 398–405 (LSLLFKKL, mHpa398) and 519–526 (FSYGFFVI, mHpa519) lysed three kinds of carcinoma cells expressing both heparanase and H-2Kb (B16 melanoma cell line, EL-4 leukoma cell line and Lewis lung cancer cell line). In vivo experiments indicated that mHpa398 and mHpa519 peptides offered the possibility not only to immunize against tumors but also to treat tumor-bearing hosts successfully [15]. Moreover, we successfully identified another three HLA-A*0201-restricted heparanase epitopes: Hpa525 (PAFSYSFFV), Hpa277 (KMLKSFLKA), and Hpa405 (WLSLLFKKL). Our results demonstrated that these three heparanase epitopes can induce heparanase-specific CTLs that lyse various tumor cells in an HLA-A*0201-restricted manner in vitro [16].
However, the presentation of antigen in vivo is a more complicated process, and many in vitro experiments cannot be repeated in vivo. Transgenic mice expressing unmodified HLA class I molecules have been used in many laboratories as a suitable animal model for the study of HLA class I-restricted CTL responses [17]. HLA-A*0201 transgenic mice represent a powerful model for the induction and examination of HLA-A*0201-restricted CTLs responses in vivo [18]. In order to investigate the immune response elicited by naturally processing of the heparanase-specific CTL epitope in vivo and to provide evidence for the clinical use of heparanase epitopes to treat patients with advanced tumors, DCs derived from HLA-A*0201 transgenic C57BL/6 mice were pulsed with the above three epitope peptides and then used to immunize HLA-A*0201 syngenic mice by three subcutaneous injections. The splenic lymphocytes were used as effectors to investigate the specific lysis of various tumor cells from different tissues. The results showed that the peptides Hpa525, Hpa277, and Hpa405 can be presented naturally in vivo and elicit the heparanase-specific lysis of various tumor cells expressing both heparanase and HLA-A*0201. Our results suggest that the Hpa525, Hpa277, and Hpa405 peptides are new HLA-A*0201-restricted CTL epitopes capable of inducing heparanase-specific CTLs in mice. Because heparanase is expressed in most advanced malignant tumors, Hpa525, Hpa277, and Hpa405 peptide-based vaccines may be useful for the immunotherapy of patients with advanced tumors.
Materials and methods
Mice and cell lines
C57BL/6 transgenic mice, which express a chimeric heavy chain of the MHC-I molecule (HLA-A*0201 α1 and α2 and H-2Kb, transmembrane and intracytoplasmic domains), were kindly provided by Prof. Ni B from Institute of Immunology of PLA, Third Military Medical University, Chongqing, P. R. China.
The human colon carcinoma cell line SW480 (Hpa+, HLA-A*0201+), human gastric carcinoma cell line KATO-III (Hpa+, HLA-A*0201+), human osteogenic sarcoma cell line U-2 OS (Hpa+, HLA-A*0201+), human breast cancer cell line MCF-7 (Hpa−, HLA-A*0201+), and hepatoma carcinoma cell line HepG2 (Hpa+, HLA-A*0201−) were obtained from our laboratory. In addition, HepG2/HLA-A2 cells (Hpa+, HLA-A*0201+), which were transfected with a plasmid vector encoding the chimeric heavy chain of the MHC class I molecule (HLA-A*0201) and MCF-7/Hpa cells (Hpa+, HLA-A*0201+), which were transfected with a plasmid containing heparanase, were constructed and stored in our laboratory. U-2 OS cells were cultured in Dulbecco’s Modified Eagle Media (DMEM, HyClone) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine. KATO-III, HepG2, MCF-7, and SW480 cells were all cultured in RPMI-1640 medium containing 10% FCS, penicillin (100 U/ml), and streptomycin (100 μg/ml). All cells mentioned above were kept at 37°C in a humidified atmosphere containing 5% CO2.
Synthetic peptides
Three nonapeptides derived from the human heparanase (Hpa) amino acid sequence [16] and one nonapeptide from HIV virus sequence [HIVpol(476–484) (ILLEPVHGV)], which served as a negative control in inducing heparanase-specific CTL reactions, were synthesized by the Beijing Scilight Biotechnology Ltd. Co. (Beijing, China) with a purity >90% as determined by HPLC (Delta 600 Wasters Company). The molecular weight of the peptides was validated by mass spectrometry (API2000, PE). Lyophilized peptides were dissolved in DMSO (Sigma) and stored at −20°C.
DC generation from mouse bone marrow
The DCs from mouse bone marrow (mDCs) were generated as described previously [15, 16, 19]. In brief, bone marrow was flushed from the tibia and femur of C57BL/6-Tg mice and depleted of erythrocytes with commercial lysis buffer (Sigma, St. Louis, MO, USA). The cells were washed twice in serum-free RPMI-1640 medium and cultured in a six-well plate at 5 × 105 cells/well with RPMI-1640 medium containing 500 U/ml recombinant murine GM-CSF (mGM-CSF, R&D System, Inc., USA) and 1000 U/ml recombinant murine IL-4 (mIL-4, R&D System, Inc., USA). On days 3, 5, and 7, half of the media was refreshed without discarding any cells and fresh cytokine-containing (mGM-CSF and mIL-4) media was added. On day 8 of culture, mTNF-α (R&D System, Inc., USA) was added to the media. On day 9, non-adherent cells obtained from these cultures were considered mature bone marrow-derived DC.
Flow cytometric analysis of cell populations
The DCs were collected and resuspended in cold FACS buffer (phosphate-buffered saline with 0.2% BSA and 0.09% sodium azide). Cells were immunostained with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse CD11c, H-2Kb, MHC-II and CD86 antibodies (eBioscience, USA). Corresponding FITC immunoglobulin G (IgG) isotype control antibody (eBioscience, USA) was used. A total of 1 × 106 cells were incubated overnight at 4°C with antibodies. The cells were then washed once with FACS buffer, resuspended, and tested on a FACScan (Becton–Dickinson, USA).
Generation of CTLs in transgenic mice with synthetic Hpa peptides
We generated Hpa-specific CTLs according to procedures previously described [19, 20]. Briefly, transgenic mice, 8–12-week-old, were immunized by subcutaneous injection once a week in the back with 2 × 106 syngenic mature dendritic cells pulsed with the above epitope peptides. One week after the final immunization, the mice were killed, and the splenocytes were collected and re-stimulated with the vaccination peptides. On day 1 after re-stimulation, IL-2 (R&D System, USA) 50 U/ml was added. After 5 days the splenocytes were harvested as effectors.
Cytotoxicity assay
Target cells, SW480, KATO-III, U-2 OS, HepG2, HepG2/HLA-A2, MCF-7/Hpa, were labeled with Na51CrO4 (100 μCi Na51CrO per 1 × 106 cells) in 1 ml of RPMI-1640 containing 10% FCS for 2 h at 37°C in 5% CO2. The labeled target cells were washed three times by RPMI-1640 medium without serum. Then the target cells were plated in triplicate at a final concentration of 1 × 104 cells/well in 96-well V-bottom microtiter plates. The 51Cr-labeled target cells (100 μl) were then mixed with effector cells at effector-to-target ratios (E:T) of 10:1, 20:1, 40:1, and 80:1. After incubation for 4 h at 37°C in 5% CO2, the release of the 51Cr label was measured by collecting 100 μl of the supernatant followed by quantitation in an automated gamma counter (Beijing Nuclear Instrument Factory, China). The percentage of specific lysis was calculated as the percentage of specific 51Cr release using the following formula:
IFN-γ ELISPOT assay
The generated CTLs mentioned above were assayed in 24-h ELISPOT cultures (96-well coated microtiter plates, Dakewe Biotech Company Limited, China) for IFN-γ production. Briefly, effector cells were plated in triplicate at a final concentration of 2 × 105, 4 × 105, and 8 × 105 cells/well in 96-well nitrocellulose plates. Effector cells were stimulated by candidate peptides at the final concentration of 30 μM. The plate was incubated in 37°C, 5% CO2 for 24 h. The plate was processed using biotin labeled anti-mouse IFN-γ, enzyme labeling marker, and anti-marker. Then, freshly prepared developer was added and incubated in the dark at 37°C for 8 min (Quick Spot Mouse IFN-γ Precoated ELISPOT kit, DAKEWE, Shenzheng, China). Spots were quantified using the ELISPOT reader (BioReader 4000 Pro-X, BIOSYS, Germany).
Statistics
All experiments were run in triplicate, and the results are given as the mean ± SD of triplicate determinations. Statistical analysis was performed using Student’s t test. Differences were considered statistically significant when the P value was less than 0.05. All statistical analyses were carried out with SPSS 11.5 software.
Results
Identification of mDC from murine bone marrow
For phenotypic analyses, mDCs were incubated with the labeled antibodies for 45 min at 4°C before flow cytometry analysis. The results demonstrated a strong upregulation of the expression of H-2Kb (98.5%), CD86 (94%), CD11c (97%), and MHC-II (92%) in the dendritic cells on day 9. These data indicated that these dendritic cells have phenotypically and functionally maturated.
Generation of CTLs specific to heparanase by synthetic peptides
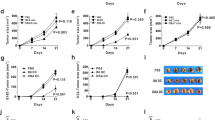
To analyze CD8+ T cell responses against heparanase, HLA-A*0201/H-2Kb transgenic mice were immunized three times at 1-week intervals with synthetic peptides. One week after the last immunization, splenocytes were harvested and restimulated with peptides (30 μmol/L). Five days later, they were analyzed for their ability to lyse human target cells. The results demonstrated that peptide Hpa277, Hpa405, and Hpa525 were able to elicit heparanase-specific CTLs, which could lyse SW480, KATO III, and U-2 OS cells expressing both heparanase and HLA-A*0201 [16] (Fig. 1). In contrast, the effectors could not lyse HepG2 (Hpa+, HLA-A*0201−) or MCF-7 cells (HLA-A*0201+, Hpa−) [16], even at the highest E:T ratios. However, when we transfected HepG2 cells with an HLA-A*0201 cDNA plasmid or MCF-7 cells with heparanase cDNA, the specific lysis rate rose to 74% and 52%, respectively (Fig. 2). These results are consistent to our previous study in vitro [16].
Specific lysis of effectors induced by Hpa525 (a), Hpa277 (b), and Hpa405 (c) peptides against different cancer cell lines expressing both Hpa and HLA-A*0201. Effector-to-target ratios are presented on the X-axis, while the Y-axis represents the percent of specific lysis. Cytotoxic T lymphocytes generated from HIV virus [HIVpol(476–484)] served as a negative peptide (NP). Results are given as the mean ± SD. *Statistically significant values at P < 0.05 using a paired Student’s t test compared with the corresponding controls
Study of the specificity and restriction of CTLs generated from Hpa525, Hpa277, and Hpa405 epitopes. Effector-to-target ratios are presented on the X-axis, while the Y-axis represents the percent of specific lysis. Cytotoxic T lymphocytes generated from HIV virus [HIVpol(476–484)] served as a negative control (NP). a, b Study of heparanase specificity of CTLs generated from different heparanase epitopes. MCF-7 breast cancer cells were Hpa negative but HLA-A*0201 positive. The effectors derived from Hpa525, Hpa277, and Hpa405 did not lyse the cells (a), but when we transduced MCF-7 cells with full-length cDNA of heparanase by the lipofection method, cytotoxic activity of CTLs induced by Hpa525, Hpa277, and Hpa405 was detected at various E/T ratios (b). c, d Study of the HLA-A*0201 restriction of CTLs generated from Hpa525, Hpa277, and Hpa405 epitopes. HepG2 liver cancer cells were HLA-A*0201-negative but Hpa positive. The CTLs induced by Hpa525, Hpa277, and Hpa405 did not lyse HepG2 cells (c). However, when we transduced the cells with a plasmid containing full-length of cDNA of HLA-A*0201 by the DOTAP method, cytotoxic activity of CTLs induced by Hpa525, Hpa277, and Hpa405 was detected at various E/T ratios (d). Results are given as the mean ± SD. *Statistically significant values at P < 0.05 using a paired Student’s t test compared with the corresponding controls
Effect of heparanase-specific CTLs on autologous lymphocytes
Heparanase can also be detected in some normal tissues and cells at a lower degree. Previous studies have shown that heparanase can be expressed in immunologically competent cells, NK cells, and inflammatory cells such as neutrophils, granulocytes, activated T cells, and B cells [16]. In order to investigate the capacity of effectors to lyse autologous lymphocytes and mDCs, the 51Cr release test was employed to detect the specific killing rate again. The results indicate that heparanase vaccination has no detectable lysis effect on these cells (Fig. 3).
Study of the side effect of Hpa525, Hpa277, and Hpa405 epitopes. Cytotoxic T lymphocytes generated from HIV virus [HIVpol(476–484)] served as a negative control (NP). a Specific lysis of CTLs induced by heparanase epitopes on autologous lymphocytes. b Specific lysis of CTLs induced by heparanase epitopes on autologous mDCs. Results are given as the mean ± SD
Enzyme-linked immunospot (ELISPOT) assay for IFN-γ
Since CTLs are known to produce the Th1 cytokine IFN-γ, peptide-specific T cells were enumerated by measuring IFN-γ-producing cells by ELISPOT assay. As shown in Fig. 4, Hpa525, Hpa277, and Hpa405 peptides were found to generate a strong peptide-specific T cell response by virtue of their ability to induce increased frequencies of IFN-γ-producing T cells, as compared to the negative peptide control (P < 0.01). These results suggest that heparanase peptide vaccines can increase IFN-γ secretion by effectors and enhance the Th1 immune response.
IFN-γ-producing cells were enumerated by the ELISPOT method. Phosphate buffered solution (PBS) served as a negative control. Phytohemagglutinin (PHA) served as a positive control. Columns indicate mean, bars, and SEM. **Statistically significant values at P<0.01 by a paired Student’s t test compared with the negative peptide group
Discussion
The identification of tumor-associated antigens (TAAs) opened new opportunities for the treatment of patients with cancer [21, 22]. By targeting these TAAs, the treatment is tumor specific, less toxic, and can have a long-lasting effect. Great progress has been made in the discovery of CTLs recognizing tumor-associated antigens [23, 24].
Although the results of immunotherapeutic cancer treatments have been promising in experimental models, as yet, the overall success in human trials has been modest [25]. Therefore, the identification of more tumor-associated antigens is very important to expanding the repertoire of antitumor immunotherapies and providing more powerful clinical effectiveness. We previously identified immunogenic CTL epitopes restricted by HLA-A*0201 from heparanase. A potential disadvantage to targeting heparanase-derived antigens for immunotherapy is that they may be subject to self-tolerance. Nevertheless, in this study we observed that the epitopes we identified were able to generate heparanase peptide-specific CTL responses in HLA-A*0201 transgenic mice, proving that the T cell repertoire for heparanase exists in vivo. By establishing CD8+ CTL lines specific for these peptides, our result show that the three epitopes Hpa525, Hpa277, and Hpa405 can be recognized on various tumor cells, suggesting that they are naturally processed and expressed.
In our previous investigation, the heparanase-specific CTL lines used for tumor recognition were generated from healthy donors in vitro [16]. In the present study, heparanase-specific CTLs from transgenic mice were shown to have the ability to kill tumor cell lines. All three peptides were able to induce specific CTLs in vivo, indicating that the HLA-A*0201 transgenic mice possessed an adequate T-cell repertoire that allowed for the recognition of the heparanase-derived epitopes. Here, we found a general correlation of CTL responses with peptide affinity for the HLA-A*0201 molecule, supporting our previous investigation. Interestingly, mouse peptide-specific CD8+ T cells can recognize endogenous peptides on human tumor cells, indicating that T cells educated in these mice are able to recognize peptide bound to the native HLA-A*0201 molecule. Furthermore, we showed that all the candidate peptides are processed and elicit effective immune response in vivo.
Heparanase expression is mainly restricted to tumor cells. Rare normal cells, including progenitor and activated lymphocytes, as well as dendritic cells, are known to express heparanase. Consequently, any heparanase-based vaccine therapy may result in autoimmunity and destruction of normal cells that express this antigen. In the present study, the heparanase-specific CD8+ CTLs generated from transgenic mice were not reactive against autologous lympholeukocytes and mDCs after peptide pulsing. This result is similar to J. Vieweg’s [26] study and supports our previous data that heparanase-specific CTLs failed to lyse autologous lymphocytes and DCs [16].
In conclusion, our results suggest that Hpa525, Hpa277, and Hpa405 peptides derived from heparanase can induce HLA-A*0201-restricted CD8+ CTLs in vivo, which can lyse various tumor cells expressing heparanase and HLA-A*0201. These novel CTL epitopes may therefore serve as an attractive component of peptide-based vaccines to treat cancer patients. We are going to evaluate the Hpa525, Hpa277, and Hpa405 peptide-pulsed dendritic cell vaccine in Hpa+/HLA-A*0201+ patients with cancer.
Abbreviations
- Hpa:
-
Heparanase
- TAA:
-
Tumor-associated antigen
- DC:
-
Dendritic cell
- CTLs:
-
Cytotoxic T lymphocytes
- E/T:
-
Effector-to-target
References
Joyce JA, Freeman C, Meyer-Morse N, Parish CR, Hanahan D (2005) A functional heparan sulfate mimetic implicates both heparanase and heparan sulfate in tumor angiogenesis and invasion in a mouse model of multistage cancer. Oncogene 24(25):4037–4051
Vlodavsky I, Goldshmidt O, Zcharia E, Atzmon R, Rangini-Guatta Z, Elkin M, Peretz T, Friedmann Y (2002) Mammalian heparanase: involvement in cancer metastasis, angiogenesis and normal development. Semin Cancer Biol 12(2):121–129
Beckhove P, Helmke BM, Ziouta Y, Bucur M, Dörner W, Mogler C, Dyckhoff G, Herold-Mende C (2005) Heparanase expression at the invasion front of human head and neck cancers and correlation with poor prognosis. Clin Cancer Res 11(8):2899–2906
Bercovici N, Haicheur N, Massicard S, Vernel-Pauillac F (2008) Analysis and characterization of antitumor T-cell response after administration of dendritic cells loaded with allogeneic tumor lysate to metastatic melanoma patients. J Immunother 31(1):101–112
Zhu B, Chen Z, Cheng X, Lin Z, Guo J, Jia Z, Zou L, Wang Z, Hu Y, Wang D, Wu Y (2003) Identification of HLA-A*0201-restricted cytotoxic T lymphocyte epitope from TRAG-3 antigen. Clin Cancer Res 9(5):1850–1857
Harada M, Li YF, El-Gamil M, Rosenberg SA, Robbins PF (2001) Use of an in vitro immunoselected tumor line to identify shared melanoma antigens recognized by HLA-A*0201-restricted T cells. Cancer Res 61(3):1089–1094
Zinkernagel RM, Doherty PC (1997) The discovery of MHC restriction. Immunol Today 18(1):14–17
York IA, Rock KL (1996) Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol 14(1):369–396
Rammensee HG, Falk K, Rotzschke O (1993) Peptides naturally presented by MHC class I molecules. Annu Rev Immunol 11(1):213–244
Tuting T, Steitz J, Bruck J et al (1999) Dendritic cell-based genetic immunization in mice with a recombinant adenovirus encoding murine TRP2 induces effective anti-melanoma immunity. J Gene Med 1(1):400–406
Kong J, Diao Z, Deng X, Zhong H, Yao W, Hu X (2007) Anti-tumor effects of immunotherapeutic peptide on the treatment of hepatocellular carcinoma with HBc carrier. Oncol Rep 18(1):279–285
Holmes JP, Benavides LC, Gates JD, Carmichael MG, Hueman MT, Mittendorf EA, Murray JL, Amin A, Craig D, von Hofe E, Ponniah S, Peoples GE (2008) Results of the first phase I clinical trial of the novel II-key hybrid preventive HER-2/neu peptide (AE37) vaccine. J Clin Oncol 26(20):3426–3433
Cai YG, Fang DC, Chen L et al (2007) Dendritic cells reconstituted with a human heparanase gene induce potent cytotoxic T-cell responses against gastric tumor cells in vitro. Tumor Biol 28(4):238–246
Sommerfeldt N, Beckhove P, Ge Y et al (2006) Heparanase: a new metastasis-associated antigen recognized in breast cancer patients by spontaneously induced memory T lymphocytes. Cancer Res 66:7716–7723
Tang XD, Wan Y, Chen L, Chen T, Yu ST, Xiong Z, Fang DC, Liang GP, Yang SM (2008) H-2Kb-restricted CTL epitopes from mouse heparanase elicit an antitumor immune response in vivo. Cancer Res 68(5):1529–1537
Chen T, Tang XD, Wan Y, Chen L, Yu ST, Xiong Z, Fang DC, Liang GP, Yang SM (2008) HLA-A2-restricted cytotoxic T lymphocyte epitopes from human heparanase as novel targets for broad-spectrum tumor immunotherapy. Neoplasia 10(9):977–986
Arnold B, Hämmerling GJ (1991) MHC class-I trans-genic mice. Annu Rev Immunol 9(1):297–322
Vitiello AD, Marchesini J, Furze LA, Sherman RW (1991) Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in transgenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J Exp Med 173(4):1007–1015
Chen L, Tang XD, Yu ST, Ai ZH, Fang DC, Cai YG, Luo YH, Liang GP, Yang SM (2009) Induction of anti-tumour immunity by dendritic cells transduced with hTERT recombinant adenovirus in mice. J Pathol 217(5):685–692
Alexander J, Sidney J, Southwood S, Ruppert J, OseroV C, Maewal A (1994) Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity 1(1):751–761
Boon T, Old LJ (1997) Cancer tumor antigens. Curr Opin Immunol 9(5):681–683
Pardoll DM (1998) Cancer vaccines. Nat Med 4(5):525–531
Storkus WJ, Herrem C, Kawabe M, Cohen PA, Bukowski RM, Finke JH, Wesa AK (2007) Improving immunotherapy by conditionally enhancing MHC class I presentation of tumor antigen-derived peptide epitopes. Crit Rev Immunol 27(5):485–493
Morris LF, Ribas A (2007) Therapeutic cancer vaccines. Surg Oncol Clin N Am 16(4):819–831
Schmitz M, Bornhäuser M, Ockert D, Rieber EP (2002) Cancer immunotherapy: novel strategies and clinical experiences. Trends Immunol 23(9):428–429
Vieweg J, Jackson A (2004) Antigenic targets for renal cell carcinoma immunotherapy. Expert Opin Biol Ther 4(11):1791–1801
Acknowledgments
This study is supported by grants from the National Program on Key Basic Research Project of China (973 Program) (No.2010CB529406), the National Nature Science Foundation of China (No. 30800520), the Key Project of Science and Technology of Chongqing (CSTC, 2008AB5002) and the Chongqing Science Fund for Distinguished Young Scholars (CSTC, 2009BA5045).
Author information
Authors and Affiliations
Corresponding author
Additional information
X.-D. Tang, G. P. Liang and C. Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tang, XD., Liang, GP., Li, C. et al. Cytotoxic T lymphocyte epitopes from human heparanase can elicit a potent anti-tumor immune response in mice. Cancer Immunol Immunother 59, 1041–1047 (2010). https://doi.org/10.1007/s00262-010-0829-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-010-0829-x