Abstract
Survivin is an intracellular tumor-associated antigen that is broadly expressed in a large variety of tumors and also in tumor associated endothelial cells but mostly absent in differentiated tissues. Naked DNA vaccines targeting survivin have been shown to induce T cell as well as humoral immune responses in mice. However, the lack of epitope-specific CD8+ T cell detection and modest tumor protection observed highlight the need for further improvements to develop effective survivin DNA vaccination approaches. Here, the efficacy of a human survivin DNA vaccine delivered by intradermal electroporation (EP) was tested. The CD8+ T cell epitope surv20–28 restricted to H-2 Db was identified based on in-silico epitope prediction algorithms and binding to MHC class I molecules. Intradermal DNA EP of mice with a human survivin encoding plasmid generated CD8+ cytotoxic T lymphocyte (CTL) responses cross-reactive with the mouse epitope surv20–28, as determined by intracellular IFN-γ staining, suggesting that self-tolerance has been broken. Survivin-specific CTLs displayed an activated effector phenotype as determined by CD44 and CD107 up-regulation. Vaccinated mice displayed specific cytotoxic activity against B16 and peptide-pulsed RMA-S cells in vitro as well as against surv20–28 peptide-pulsed target cells in vivo. Importantly, intradermal EP with a survivin DNA vaccine suppressed angiogenesis in vivo and elicited protection against highly aggressive syngeneic B16 melanoma tumor challenge. We conclude that intradermal EP is an attractive method for delivering a survivin DNA vaccine that should be explored also in clinical studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Survivin, a member of the inhibitor of apoptosis (IAP) family, regulates important pathways implicated in cell cycle progression, cell proliferation and cell death [1]. This intracellular protein is broadly expressed during embryonic development [2], mostly absent in differentiated cells, but then strongly up-regulated in human cancer cells of different origins [3, 4]. Survivin expression is associated with enhanced tumor cell viability and resistance to cancer therapies [5]. Accordingly, knock-down of survivin expression in tumor cells has highly detrimental effects on cancer cell viability and tumor progression [6]. Survivin is also up-regulated in endothelial cells during tumor associated angiogenesis [7]. Widespread expression in several types of human cancers and tumor stroma, general absence in normal adult tissues and a requirement for tumor cell survival essentially identify survivin as an almost ideal “universal” tumor associated antigen (TAA).
Spontaneous cellular, as well as humoral immune responses against survivin have been detected in patients with several kinds of cancer [8, 9], further validating survivin as a TAA that can be exploited for therapeutic purposes. Importantly, CD8+ and CD4+ T cell responses restricted to different human and mouse MHC molecules have been characterized and several relevant survivin epitopes were identified. All together, these features make survivin a highly attractive target for T cell based immune strategies against cancer. Dendritic cell- and gene-based vaccines targeting survivin have been tested in preclinical settings where induction of T cell responses and tumor protection were observed [10, 11].
DNA vaccination is an attractive immunotherapeutic strategy that appears to mimic natural immunity and is able to induce long-lasting T cell- and antibody-mediated tumor protection [12]. Naked DNA vaccines have several advantages over other vaccination methods including that they are easy to generate, the desired response can be readily modified, they are cost-effective to produce and purify at large-scale, the same production platform can be employed for any antigen, native protein antigen is generated in vivo with no need for expensive purification schemes, the antigen is processed and presented by host cells independent of haplotype restriction, plasmid DNA contains “in-built” adjuvant danger signals that activate innate immunity, they are highly stable allowing long-term storage and finally, they are considered safe, since they are non-infectious and virtually no genomic integration has been observed. Despite high efficacy in preclinical models, naked DNA vaccines need to be improved in order to overcome the low efficacy which so far has been achieved in clinical trials. Recently, in vivo DNA electroporation (EP) has emerged as an efficient delivery method that allows efficient DNA uptake, leads to long-term and high levels of antigen expression as well as the induction of several cytokines and chemokines, thereby increasing the potency of DNA vaccines [13]. Immunization through the skin is an attractive approach for clinical applications, as skin is readily accessible and a high number of antigen presenting cells are present, e.g. Langerhans cells [14]. A novel intradermal (i.d.) DNA EP method has been developed for DNA vaccine delivery that ensures high levels of antigen expression as well as improved and protective anti-tumor cellular immunity [15, 16].
Naked DNA vaccines targeting survivin have been shown to induce T cell as well as humoral immune responses in animal models [17–19]. However, the induction of epitope-specific responses has not been conclusively demonstrated. Indeed, even the lack of detectable CD8+ cytotoxic T cell (CTL) responses restricted to previously published mouse epitopes has been reported [19]. Moreover, in cases where significant reduction of tumor growth was shown as a result of survivin DNA vaccination, modest or no long-term protection was observed. Therefore, further optimization and improvement of survivin DNA vaccine strategies are required that will lead to the induction of measurable survivin-specific T cell responses and efficient tumor protection. The present study demonstrates in a preclinical model the efficacy of a novel i.d. DNA EP approach in inducing anti-survivin CTL responses and long-term tumor protection in prophylactic as well as therapeutic settings.
Materials and methods
Mice and cell lines
C57BL/6 mice were bred and maintained at the animal facilities of the Microbiology and Tumor Biology Center at the Karolinska Institute. All animal studies have been reviewed and approved by the Swedish National Board for Laboratory Animals. B16 is a spontaneously raised murine melanoma and RMA-S is a transporter associated protein-deficient murine T cell lymphoma [20]. Cell lines were cultured in IMDM and RPMI media, respectively, supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 IU/ml penicillin and 100 μg/ml streptomycin (complete medium) (GibcoBRL, Life Technologies, Grand Island, NY, USA) in a humidified incubator at 37°C with 5% CO2. Mouse lymphocytes were cultured in RPMI complete medium supplemented with 1% non-essential amino acids and 1% sodium pyruvate (GibcoBRL).
Plasmids
Full-length human survivin cDNA was cloned into pcDNA3.1 as previously described [18]. The cloned fragment containing survivin cDNA was excised and then inserted into the pVAX vector (Invitrogen, Carlsbad, CA, USA) by using the compatible restriction sites HindIII and XhoI. E. coli (TOP10, Invitrogen) carrying the pVAX constructs were cultured in Luria-Bertani medium containing 50 mg/l Kanamycin and endotoxin-free plasmids used for immunizations were purified using the GigaPrep Endofree Kit (Qiagen GMBH, Hilden, Germany).
Intradermal DNA electroporation
C57BL/6 mice were anesthetized with isoflurane and injected intradermally with 40 μg (40 μl of PBS) of plasmid DNA at two sites (20 μg each) near the base of the tail using a 29-gauge insulin-grade syringe (Micro-Fine U-100, BD Consumer Healthcare, Franklin Lakes, NJ, USA). Immediately, a parallel needle array electrode (two rows of four 2-mm pins (1.5 × 4 mm gaps) was placed over the injection blebs, and electric pulses (two 1,125 V/cm pulses followed by eight 275 V/cm pulses) were applied using the Derma Vax™ DNA vaccine Skin Delivery System (provided by Cyto Pulse Sciences Inc.). Mice were immunized two times either at days −21 and −7 (early setting) or at days +10 and +17 (late setting) with respect to tumor challenge, referred as day 0.
B16 melanoma tumor challenge
Mice were challenged with a lethal dose of syngeneic B16 melanoma cells. B16 cells in the logarithmic growth phase (≤75% confluent) were harvested and a single cell suspension was prepared in PBS. Mice were injected subcutaneously in the left flank with 100 μl containing 105 B16 cells. Tumor growth was evaluated twice a week by measuring perpendicular tumor diameters with calipers, and the mean diameter was recorded for 100 days. Mice were sacrificed when they became moribund or when the mean tumor diameter reached 10 mm, the limit permitted according to the approved ethical protocol.
Epitope prediction and peptide synthesis
Full-length mouse and human survivin amino acid sequences were screened to identify potential H-2 Db-and Kb-restricted 9-mer epitopes using the following web-based prediction sites: The BioInformatics & Molecular Analysis Section (BIMAS) [21]; the Promiscuous MHC Class-I Binding Peptide Prediction Server (ProPred) [22]; and the SYFPEITHI database for MHC ligands and peptide motifs [23]. The score and ranking obtained using these three different web-sites were taken into account. The mouse-derived peptides; ATFKNWPFL (surv20–28), ACTPERMAE (surv32–40), FIHCPTENEP (surv43–52), CPTENEPDL (surv46–54), AFLTVKKQM (surv85–93), LDRQRAKNKI (surv104–113), AKETNNKQKE (surv114–123) as well as human survivin peptide STFKNWPFL (surv20–28), control TRP2 peptide SVYDFFVWL (trp2180–188) and mouse VEGFR2 peptide FSNSTNDILI (flk1615–624) were all obtained from UFPeptides (Ferrara, Italy) at >90% purity.
Antibodies and flow cytometry
Monoclonal antibodies anti-mouse CD107a (clone 1D4B), anti-mouse CD44 (clone IM7), anti-mouse CD8α (clone 53-6.7), anti-mouse H-2 Kb (clone AF6-88.5), anti-mouse H-2 Db (clone KH95), anti-mouse IFN-γ (clone XMG1.29) conjugated to FITC, PE, PerCP, or APC fluorochromes were used for flow cytometry analysis (Becton Dickinson Biosciences). Non-specific binding was blocked by adding unconjugated rat anti-mouse CD16/CD32 antibody (mouse BD Fc block, clone 2.4G2) prior to the surface staining procedure. The samples were analyzed with a FACSCalibur cytometer and the data obtained were processed using CellQuest Pro software (Becton Dickinson Biosciences).
MHC class I stabilization assay
RMA-S cells in logarithmic growth phase were harvested, kept at room temperature during 1.5 h and then seeded (2 × 105 cell/well) in 96-well plates (U-bottom) in complete RPMI medium containing different concentrations of H-2 Db- and Kb-restricted peptides. Cells were cultured during 16 h at 37°C, 5% CO2. Then, cells were stained for the detection of surface levels of H-2 Db and Kb MHC class I molecules and analyzed by flow cytometry.
Intracellular cytokine staining
Lymphocytes from immunized mice were isolated from peripheral blood. Mice were bled from the tail vein and blood samples collected in tubes containing heparin. Red blood cells (RBCs) were lysed using hypotonic buffer PharmLyse (Becton Dickinson Biosciences, Mountain View, CA, USA). Lymphocytes were seeded in U-bottom 96 wells plates and stimulated during 8 h with MHC class I-restricted peptides (10 μg/ml each). Anti-mouse CD107a antibody was added at this point and degranulation of cytotoxic granules was evaluated. CD8+ T cells producing IFN-γ were detected by intracellular cytokine staining following addition of GolgiPlug (Becton Dickinson Biosciences) during the last 6 h of stimulation. Staining was performed using Cytofix/Cytoperm™ Fixation/Permeabilization Solution set (Becton Dickinson Biosciences) according to the manufacturer’s instructions.
In vitro cytotoxicity assay
Single cell suspensions from spleen and lymph nodes were isolated from immunized mice and RBCs were lysed using hypotonic buffer PharmLyse. Lymphocytes were cultured for 5 days in the presence of 2 mM of surv20–28 peptide and 50 U/ml of recombinant human IL-2. After 5 days of stimulation, viable lymphocytes were counted by trypan blue exclusion and seeded in V-bottom 96-well plates. B16 and peptide-pulsed RMA-S target cells were labeled with 51Cr (Na2CrO4, Perkin Elmer) 1 h at 37°C, washed and 5,000 of target cells were cocultured with mouse lymphocytes at different effector: target ratios (100:1, 50:1, 25:1) in quadruplicates during 6 h at 37°C, 5% CO2. Release of 51Cr in culture supernatants was measured by a γ-counter (Wallac Sverige AB, Stockholm, Sweden) and the percentage of specific lysis was determined using the equation: 100 × [(sample release − spontaneous release)/(maximum release − spontaneous release)].
In vivo cytotoxicity assay
Target cell populations were prepared from naive C57BL/6 mice. RBC-lysed splenocytes (108 cells/ml PBS 0.5% FBS) were stained with 0.2 or 2 μM carboxyfluorescein succinimidyl ester (CFSE) for 5 min. Ten volumes of PBS 10% FBS were subsequently added to stop the labeling. Target cells were pulsed (60 min at 37°C) with antigen-specific or irrelevant peptide as an internal control. Cells (6 × 106) from each population were then mixed together in equal proportions and injected intravenously into empty or survivin DNA vaccinated C57BL/6 mice. Spleens and lymph nodes were removed 20 h later and single-cell suspensions were generated before analysis on a FACSCalibur cytomoter (Becton Dickinson Biosciences). CFSE+ donor target splenocytes were distinguished from host cells and the percentage of target cell killing was determined as follows: 100 − [(percentage of surv20–28 peptide-pulsed targets in pSURV vaccinated recipients/percentage of control targets in pSURV vaccinated recipients)/(percentage of surv20–28 peptide-pulsed targets in pEMPTY vaccinated recipients/control targets in pEMPTY vaccinated recipients) × 100].
In vivo matrigel plug angiogenesis assay
Ten days after the last immunization, mice were injected subcutaneously in the midventral abdominal region with 250 μl of matrigel (Becton Dickinson Biosciences) containing 200 ng of human bFGF (PeproTech, Rocky Hill, NJ). Seven days later matrigel plugs were removed, fixed overnight in 10% formalin-PBS and stored in 30% sucrose-PBS. Then, plugs were washed, embedded in OCT compound (Tissue-Tek, Histolab, Sweden) and stored frozen at −20°C until further analysis. The glass slides were dried at room temperature for 1 h, then an ice cold mixture of methanol and acetone (1:1) was applied to fix samples and remove residual OCT. Subsequently, samples were permeabilized in 0.3% Triton X-100. After blocking with 5% BSA, samples were then stained with anti-mouse CD31 (MEC 13.3 Becton Dickinson Pharmingen) antibody over night at 4°C and then with the Texas Red-conjugated secondary anti rat immunoglobulin antibody (712-075-153, Jackson ImmunoResearch Laboratories Inc., Newmarket, Suffolk, UK) during 2 h at room temperature. Slides were mounted with Flourescein-FragEL Mounting media (Calbiochem, Nottingham, UK) and visualized using a Zeiss axioplan 2 microscope (Carl Zeiss GmbH, Oberkochen, Germany). Photos were taken with an Axiocam HRm camera and processed using Zeiss Axiovision 4.5 software. The presence of vessels was evaluated in ten random visual fields per sample at 20× magnification. Vascular density was expressed as the number of vessels per field.
Statistical analysis
Statistically significant differences between different groups of data were evaluated using a two tailed t test. Statistical significance for comparison of survival curve was evaluated with the log rank test. Differences were considered significant when a P value less than 0.05 was obtained (*P < 0.05).
Results
Predicted epitope surv20–28 binds to H-2b molecules
The primary aim of our study was to investigate whether i.d. EP with a human survivin encoding plasmid could induce efficient survivin-specific CD8+ T cell and mediate tumor protection. To this end, we first sought to identify a mouse survivin epitope which T cells would recognize following immunization with human full-length survivin plasmid enabling us to measure CD8+ T cell responses. Candidate epitopes restricted to H-2 Db and Kb molecules were identified in the amino acid sequence of mouse survivin antigen using three different web-based algorithms; BIMAS [21]; ProPred [22]; and SYFPEITHI [23]. The search focused on the identification of 9-mer peptides restricted to H-2 Db and Kb MHC class I molecules with conserved sequences between mouse and human survivin proteins. Epitopes with a single mouse/human amino acid difference, not expected to interfere with T cell Receptor (TCR) recognition, were also considered.
High scoring peptides have better probabilities of binding to and being presented by MHC class I molecules. The peptide surv20–28 was identified as a candidate epitope with high scores in each of the three prediction algorithms, ranking in first and fifth positions for H-2 Db and Kb, respectively (Table 1). Since mouse and human surv20–28 peptides only differ in the first amino acid (alanine in mouse, serine in human), which is located outside the TCR recognition area, the likelihood that immunization with the gene encoding for human survivin would lead to the induction of CTLs that recognize the mouse epitope was considered to be high. Both mouse and human surv20–28 peptides yielded exactly the same scores in the epitope prediction analysis. We tested the ability of this peptide and other mouse survivin peptides previously published [24] to bind to H-2 Db and Kb molecules in the MHC class I stabilization assay [25] using RMA-S cells [20]. Interestingly, surv20–28 was the only survivin peptide that could efficiently stabilize H-2 Db molecules at the surface of RMA-S cells (Fig. 1a), although to a lesser extent compared to a high affinity H-2 Db-restricted peptide derived from the mouse VEGFR2 protein (flk1) [26]. Importantly, the mouse and human surv20–28 peptides bound MHC class I molecules with similar efficiency as measured by the RMA-S stabilization assay (Fig. 1b). Taken together, these data indicated that the predicted surv20–28 peptide fulfilled the desired features of a potential CTL epitope.
Surface stabilization of H-2 Db molecules by predicted epitope surv20–28. RMA-S cell were incubated with 100 μM of different MHC class I-restricted peptides. Then surface levels of H-2 Db molecules were detected by flow cytometry analysis and compared with those observed after incubation without peptide (control, filled gray histograms) or with the flk1615–624 peptide (flk1, fragmented line histograms) employed as negative and positive controls, respectively. a Histograms representing H-2 Db levels after incubation with individual mouse survivin-derived peptides are shown, including the predicted epitope surv20–28 (thick line) as well as surv32–40, surv43–52, surv46–54, surv85–93, surv104–113, surv114–123 (dotted lines) that could not stabilize H-2 Db molecules under these conditions. b Histograms representing H-2 Db levels after incubation with mouse (thick line) and human (thin line) surv20–28 peptides are shown
Intradermal electroporation with a survivin DNA vaccine induces surv20–28-specific CD8+ T cell responses
The efficacy of i.d. DNA EP in inducing survivin-specific CD8+ T cell responses was evaluated by intracellular IFN-γ staining. Peripheral lymphocytes from vaccinated mice were stimulated ex vivo with trp2180–188 peptide as negative control, a pool of six H-2b-restricted survivin-derived peptides, and mouse or human surv20–28 peptides. The percentage of CD8+ T cells producing IFN-γ was determined by flow cytometry (Fig. 2a). Approximately, 1% of all CD8+ T cells were shown to specifically produce IFN-γ when stimulation was performed with surv20–28 peptide in mice immunized with survivin encoding plasmid, while only low background levels were detected in mice vaccinated with empty plasmid or when T cells were stimulated with the trp2 control peptide instead. Almost identical responses were observed stimulating with either mouse or human surv20–28 peptides in accordance with the induction of cross-reactive CD8+ T cells able to recognize both peptides. Importantly, the observed induction of CD8+ T cell responses against the mouse epitope indicates that i.d. EP with the human survivin plasmid is able to break self-tolerance to the mouse survivin TAA. No detectable CD8+ T responses were obtained when T cells were stimulated with a pool of previously described H-2b-restricted survivin peptides [24], suggesting that the predicted surv20–28 epitope is immunodominant among the peptides tested. The cytotoxic potential of surv20–28-specific CD8+ T cells was evaluated by extracellular staining of the late endosome/lysosome marker CD107a (Lysosome-Associated Membrane Protein 1, LAMP-1) and the activation marker CD44. A higher mean fluorescence intensity (MFI) for CD107 staining was observed in surv20–28-specific CD8+ T cells (IFN-γ(+)) compared to the overall non-specific CD8+ T cell population (IFN-γ(−)) (Fig. 2b). A higher MFI is indicative of CD107 exposure at the cell surface and represents an indirect measure of cytotoxic vesicle degranulation. Additionally, surv20–28-specific CD8+ T cells displayed high levels of the activation marker CD44, a key regulator of intratumoral CTL migration that determines the ability of T cells to kill cancer cells [27]. High levels of CD44 (CD44high phenotype) were observed in surv20–28-specific CD8+ T cells (IFN-γ(+)) when compared to the broad distribution (CD44−/low/high) observed on the overall non-specific CD8+ T cell population (IFN-γ(−)) (Fig. 2c). Taken together, these results demonstrate that i.d. EP with survivin DNA efficiently induced surv20–28-specific CTL responses.
Surv20–28-specific IFN-γ production, CD44 and CD107 up-regulation in peripheral CD8+ T cells induced by intradermal survivin DNA electroporation. a Lymphocytes from mice vaccinated with empty (pEMPTY, n = 3) or survivin (pSURV, n = 6) encoding plasmids were isolated from peripheral blood and stimulated during 8 h with MHC class I-restricted peptides, including: trp2180–188 (trp2); a pool of surv32–40, surv43–52, surv46–54, surv85–93, surv104–113, surv114–123 (survivin pool); mouse surv20–28 (m surv20); and human surv20–28 (h surv20). CD8+ T cells producing IFN-γ were detected by intracellular staining and flow cytometry analysis. The percentages of IFN-γ positive cells in CD8+ population are shown (mean + SEM). b Peripheral lymphocytes from mice vaccinated with pSURV (n = 9) were stimulated with mouse surv20–28 peptide and CD107 levels were analyzed. Mean fluorescence intensity (MFI) in IFN-γ negative (−) and positive (+) CD8+ T cell populations are shown (mean + SEM). c Peripheral lymphocytes from mice vaccinated with pSURV were stimulated with mouse surv20–28 peptide and CD44 expression was analyzed. Representative histograms displaying CD44 levels in IFN-γ negative (−) (filled histogram) and positive (+) (empty histogram) CD8+ T cell populations are shown
Intradermal DNA electroporation induces survivin-specific CTLs with killing activity in vitro and in vivo
To evaluate whether surv20–28 specific CTLs induced by survivin DNA EP displayed functional killing activity, standard chromium release assays were performed. Cytotoxic activity of lymphocytes cultured in vitro for 5 days in the presence of surv20–28 peptide was determined. As shown in Fig. 3a, specific cytotoxic activity was observed with effector cells from mice vaccinated with pSURV against surv20–28-pulsed but not against control-pulsed RMA-S targets. No detectable cytotoxic activity was observed in control vaccinated mice. These results show the potential of surv20–28 specific CTLs to efficiently kill tumor cells presenting surv20–28 epitope at the cell surface. The ability of surv20–28 specific CTLs to recognize endogenously processed epitope and kill B16 cells in vitro was also evaluated (Fig. 3b). Data from individual mice vaccinated with pSURV show that two out of five mice displayed detectable cytotoxic activity against B16 cells (Fig. 3b, right panel). No cytotoxic activity was detected in mice vaccinated with control pEMPTY plasmid. These results indicate that the surv20–28 epitope is naturally processed and presented at the surface of B16 cells and potentially accessible to CTLs.
Surv20–28-specific CTL killing induced by intradermal survivin DNA electroporation. Mice were immunized two times at a 2-week interval and 12 days after the last immunization in vitro as well as in vivo cytotoxicity assays were performed. Effector cells were obtained from mice vaccinated with empty (pEMPTY, squares, n = 5) or survivin (pSURV, triangles, n = 5) plasmids and stimulated for 5 days with surv20–28 peptide. a Lymphocytes were co-cultured with 51Cr-labeled RMA-S cells pulsed with trp2180–188 (white symbols) or surv20–28 (gray symbols) peptides at different effector: target ratios. Release of 51Cr was measured in culture supernatants and the percentage of specific lysis was determined (mean + SEM). b The same experiment as described above but using B16 cells as targets (black symbols). Data from individual mice are shown (mean + SEM). c Target splenocytes from naive mice were stained with 0.2 or 2 μM CFSE and pulsed with irrelevant trp2 (control, CFSElow) or mouse surv20–28 (surv20, CFSEhigh) peptides, respectively. Cells from each population were then mixed together in equal proportions and injected i.v. into empty (pEMPTY, n = 4) or survivin (pSURV, n = 4) DNA vaccinated mice. Spleens were removed 20 h later and single-cell suspensions were analyzed by flow cytometry. Representative histograms (gated on CFSE positive cells) displaying CFSElow and CFSEhigh populations detected in vaccinated recipient mice are shown. d The percentage of surv20–28-specific killing detected in vaccinated mice is shown (mean + SEM)
To further evaluate whether surv20–28 specific CTL killing occurred in vivo, spleen cells from naïve mice were simultaneously stained with either a high or a low concentration of CFSE and pulsed with surv20–28 or control peptide, respectively. Then both populations were mixed in equal proportions and adoptively transferred to immunized mice by i.v. injection. Specific killing of the surv20–28-pulsed population (CFSEhigh) relative to the internal control population (CFSElow) was analyzed by flow cytometry. Spleens and lymph nodes from DNA vaccinated recipient mice were analyzed 20 h after target cell inoculation. Essentially the same number of surv20–28-pulsed (CFSEhigh) and control (CFSElow) target cells were detected in mice vaccinated with empty plasmid (Fig. 3c, left panel), while a selective decrease of surv20–28-pulsed (CFSEhigh) population with respect to control (CFSElow) target cells (Fig. 3c, right panel) was observed in mice vaccinated with survivin plasmid. An overall surv20–28-specific in vivo killing of 17% was observed when the spleens of these mice (pSURV) were analyzed (Fig. 3d). Interestingly, in vivo cytotoxicity was even higher (25%) when pooled inguinal lymph nodes were analyzed instead of individual spleens (data not shown). These results demonstrated that surv20–28 specific CTLs were able to mediate specific in vivo killing.
Survivin DNA electroporation suppresses angiogenesis in vivo
The efficacy of i.d. DNA EP in targeting survivin-expressing endothelial cells forming new blood vessels (angiogenesis) was evaluated by the in vivo matrigel assay. Mice were vaccinated twice at a 2-week interval with either empty (pEMPTY) or survivin (pSURV) encoding plasmids. Ten days after the last immunization, mice were s.c. inoculated with matrigel containing bFGF, an angiogenic factor that strongly up-regulates survivin expression in endothelial cells [7]. Matrigel plugs were removed 1 week later, fixed and immunofluorescence analysis was performed (Fig. 4a). Representative images of matrigel sections showing nuclei (top panels) and endothelial cells (middle panels) from each group vaccinated with either empty (pEMPTY, left panels) or survivin (pSURV, right panels) encoding plasmids. Merged fluorescent images are also shown (lower panels). Vascular density, here expressed as the number of blood vessels per visual field, was quantified and shown to be decreased by nearly 50% in mice vaccinated with survivin encoding plasmid (pSURV) when compared with empty plasmid (pEMPTY) vaccinated mice (Fig. 4b). These results indicate that the formation of new blood vessels, a key process during tumor progression, can be targeted by i.d. EP with a survivin DNA vaccine.
Suppression of in vivo angiogenesis induced by intradermal survivin DNA electroporation. Mice were immunized two times with empty (pEMPTY, n = 4) or survivin (pSURV, n = 4) plasmids and 10 days after last immunization matrigel was s.c. inoculated. One week later, matrigel plugs were removed, fixed and immunofluorescence analysis was performed. a Representative pictures for each group are shown. Cell nuclei in the matrigel are shown in blue (top panels). The presence of endothelial cells was detected by CD31 staining (red, middle panels). Merged fluorescent images are also shown (lower panels). b Blood vessel density was quantified as described in the material and methods section. Data plotted from both groups are shown (mean + SEM)
Survivin DNA electroporation confers tumor protection
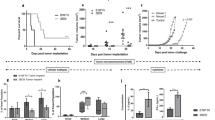
The anti-tumor efficacy of i.d. survivin DNA EP was tested by evaluating the protection against the aggressive B16 melanoma tumor model. Mice were immunized twice at a 2-week interval with either empty (pEMPTY) or survivin (pSURV) encoding plasmids and 1 week after the last immunization mice received a s.c. tumor challenge with a lethal dose of B16 cells (early setting). In this early vaccination setting, nearly 40% of mice vaccinated with pSURV were protected from tumor development and remained tumor-free during the entire period of the experiment (Fig. 5a), in contrast to mice vaccinated with pEMPTY which all succumb to the tumor. Additionally, a very similar proportion of mice (40%) were protected when the vaccine was administered in a “late setting” twice at a 1-week interval starting 10 days after the mice had received the tumor challenge (Fig. 5b). However, tumor growth data from individual mice showed that none of the survivin immunized mice bearing a palpable tumor underwent tumor regression (Fig. 5c), why we cannot conclude that this late vaccination setting can be considered as therapeutic. We therefore surmise that i.d. EP with a survivin DNA vaccine can confer tumor protection to mice when delivered either before or after challenge with B16 melanoma cells.
Survival of mice immunized by intradermal survivin DNA electroporation following challenge with B16 melanoma cells. a Mice were immunized two times with empty (pEMPTY, n = 20) or survivin (pSURV, n = 16) plasmids at days −21 and −7 and then challenged with a lethal dose of syngeneic B16 melanoma cells at day 0 (early setting). Data are shown as the percentage of survival after tumor challenge from two independent experiments. b Mice were s.c. challenged with a lethal dose of syngeneic B16 melanoma cells at day 0 and then immunized two times with empty (pEMPTY, n = 10) or survivin (pSURV, n = 12) encoding plasmids at days +10 and +17 (late setting). Data are shown as the percentage of survival after tumor challenge from two independent experiments. c Tumor growth in mice treated with the late vaccination setting (described above) was registered as the mean tumor diameter. Data from individual mice immunized with empty (pEMPTY, n = 10, left panel) or survivin (pSURV, n = 12, right panel) encoding plasmids are shown
Discussion
We have shown that a naked DNA vaccine encoding the human survivin antigen delivered by i.d. EP could efficiently induce CTL responses against a newly described mouse survivin self-epitope. Also, in vivo anti-angiogenic effects as well as anti-tumor protection against the highly aggressive B16 melanoma tumor challenge were generated. To the best of our knowledge, this is the first report showing that a naked DNA vaccine targeting the survivin antigen elicits CTL responses against a defined self-epitope, efficiently suppresses angiogenesis and, most importantly, yields long-lasting tumor protection.
Previously, we and others have shown in various mouse experimental models that naked survivin DNA vaccination is able to induce cellular as well as humoral responses against survivin [17–19]. In these studies, however, no epitope specific CTL responses were reported. We have here extended our studies by testing a more efficient DNA delivery approach and also by improving the detection of survivin-specific CTL responses, which is of considerable importance when targeting an intracellular antigen, such as survivin. T cell epitopes for survivin have been described in preclinical studies using mouse models, but it was not until after the initiation of our studies that epitopes restricted to H-2b (C57BL/6 background) were described [24, 28]. For this reason, we began our study by searching for potential epitopes in the mouse survivin sequence and then evaluated whether a CTL response against the relevant mouse self-antigen could be induced. Since a plasmid containing the full-length human survivin cDNA was used for the immunization, the search for potential mouse epitope candidates was restricted to peptides that were either absolutely conserved between mouse and human sequences or that contained a single mouse/human amino acid difference not expected to interfere with TCR recognition. The peptide surv20–28 showed the highest scores and ranked top in the analysis for candidate epitopes restricted to H-2 Db and also yielded relatively good scores for the H-2 Kb allele. We hypothesized that DNA vaccination using the human survivin antigen could generate a CTL response against the predicted epitope surv20–28 because the difference between mouse (alanine) and human (serine) peptides is in first amino acid and was not expected to affect the interaction between MHC/peptide complex and the TCR. Moreover, both peptides showed exactly the same scores in all the epitope prediction algorithms employed (Table 1) and bound to H-2 Db molecules with the same efficiency (Fig. 1b), therefore we anticipated the induction of cross-reactive CTL responses. We further compared the ability to bind H-2 Db and Kb of our newly identified candidate epitope as well as others survivin-derived epitopes previously described in the literature [24]. Rather surprisingly, the surv20–28 peptide was the only one able to bind and stabilize H-2 Db (Fig. 1a) and, to a lesser extent, Kb (data not shown) molecules on RMA-S cells, indicating that it has a higher probability of being presented than the other peptides.
Recently, i.d. EP has been described as an efficient delivery method for DNA vaccines that is able to induce enhanced cellular responses against human TAAs in mice [16, 29]. Here we showed that i.d. survivin DNA EP efficiently induced CTL responses against the mouse as well as human epitope surv20–28 measured by intracellular IFN-γ staining. This indicates that self-tolerance to this antigen has been overcome. We also observed that individual mice responded to the same extent against both peptides (data not shown), supporting the notion that a cross-reactive CTL response has been induced. No detectable responses were observed when lymphocytes were stimulated with a pool of previously described survivin epitopes (Fig. 2a). These results suggest that the surv20–28 is an immunodominant epitope among the peptides tested. Surv20–28-specific CTLs were found to be functionally activated after peptide stimulation as measured by IFN-γ production (Fig. 2a) and up-regulation of the cytotoxic vesicle degranulation marker CD107 (Fig. 2b). Interestingly, surv20–28-specific CTLs also showed high expression levels of the activation marker CD44 (Fig. 2c), recently described as a key regulator of intratumoral CTL infiltration that determines the ability of T cells to kill cancer cells and ultimately efficiently reject tumor [27]. Importantly, surv20–28 CTLs displayed specific functional cytotoxic activity against tumor cells as well as peptide-pulsed targets in vitro and in vivo (Fig. 3). Moreover, surv20–28 CTLs could recognize and kill B16 cells that endogenously process and present survivin-derived epitopes in 40% of the tested mice (Fig. 3b). Although, this is the first study to report surv20–28 as a relevant TAA epitope in mice, the human peptide was previously described as a CTL epitope restricted to HLA-A24 [30], a very frequent allele expressed especially in the Asian population. The presence of surv20–28 CTLs in cancer patients supports the notion that this peptide is processed and presented by cancer cells. Therefore, surv20–28 is a relevant survivin-derived epitope that has been demonstrated to be immunogenic in both preclinical models and cancer patients.
One of the outstanding advantages of survivin as a TAA is that it is also expressed in endothelial cells forming blood vessels during tumor associated angiogenesis and molecular targeting of survivin results in suppression of angiogenesis [31]. Therefore, i.d. DNA EP-induced survivin CTLs were expected to target tumor as well as endothelial cells. The later cells represent an attractive target population because they are genetically more stable than tumor cells and angiogenesis is essential for tumor growth. Here we showed efficient suppression of angiogenesis induced by i.d. EP with the survivin encoding vaccine (Fig. 4) reinforcing the therapeutic potential of this vaccination approach.
Previous efforts targeting tumors using naked survivin DNA vaccines delivered by classical intramuscular injection have shown anti-tumor effects in vivo [17, 19]. In these studies, however, modest or no long-lasting tumor protection was reported, motivating us to use a more efficient vaccination approach. Here we show, in accordance with the observed efficient induction of CTL responses and suppression of angiogenesis, that i.d. survivin DNA EP protected mice from challenges with highly aggressive B16 melanoma cells, a tumor model that is hard to target with DNA vaccine approaches [32] and that has not been previously targeted using survivin-based vaccines. Our vaccination approach prolonged survival and completely protected 40% of the mice immunized either before (early setting, Fig. 5a) or after (late setting, Fig. 5b) tumor challenge, when small tumors were developing in some but not all of the mice. Tumor protection observed upon the late immunization setting cannot be considered strictly therapeutic because no tumor regression was registered in mice immunized with the survivin encoding plasmid (Fig. 5c, right panel), and probably reflects the protection of mice that had not developed tumors. Accordingly, whereas some of the mice had palpable tumors after 10 days, others did not develop palpable tumors until 20 days or more. Moreover, once tumors become palpable a highly aggressive growth was observed in all mice regardless the treatment received, reaching the size limit (mean tumor diameter, 10 mm) within 2 weeks. Considering the aggressive nature of the B16 tumor model, the therapeutic window using this model is very small for testing our vaccination approach. Importantly, it should be noted that mice which were protected from tumor development following survivin vaccination remained tumor-free during the entire one hundred day experimentation period. Intriguingly, the proportion of mice protected against B16 tumor challenge in vivo coincides with the proportion of mice that showed detectable cytotoxicity against B16 cells in vitro (Fig. 3b). This observation indicates that while the majority of the surv20–28-specific CTLs are able to recognize peptide-pulsed cells (Fig. 3a), much fewer can recognize naturally processed peptides on B16 tumors, as commonly is the case for CTLs specific for tumor-derived peptide epitopes known to be naturally processed [33].
To what extent potential direct anti-tumor or antiangiogenic effects contribute to the observed tumor protection and whether CTLs against surv20–28 or other survivin-derived epitopes mediate the anti-tumor responses remains to be established. The anti-angiogenic effects observed upon id EP with pSURV could, at least in part, account for the anti-tumor effects of the vaccination but also could potentially lead to the generation of autoimmune side effects. In theory, targeting angiogenesis could interfere with physiological processes that require the formation of new blood vessels. Although all vaccinated mice appeared healthy, further studies are needed to carefully evaluate this possibility. Survivin gene-based vaccination has, however, previously been shown to induce appropriate responses in the absence of apparent signs of angiogenesis-related autoimmunity [10]. In the aforementioned report, survivin vaccination suppressed angiogenesis in vivo and elicited CTL-mediated killing of murine endothelial cells in vitro without impairing either wound healing or fertility.
Therapeutic as well as prophylactic tumor protection using survivin gene-based vaccine have previously been reported [10]. In these studies, a far more complex vaccine, consisting of an attenuated S. typhimurium carrying a plasmid encoding a mutant ubiquitin-survivin fusion in combination with the chemokine CCL21, was used. Both anti-angiogenic and anti-tumor effects in prophylactic as well as therapeutic settings were demonstrated using a pulmonary metastasis model of non-small cell lung carcinoma. However, specificity of CTL responses against survivin-derived epitopes was not conclusively shown. Furthermore, although anti-tumor efficacy was evident, protection was not evaluated for longer than 4 weeks after i.v. challenge. Importantly, the delivery of both the survivin DNA vaccine and the encoded CCL21 chemokine adjuvant by attenuated S. typhimurium were required to achieve effective tumor-protective immunity and the oral vaccine encoding only the survivin antigen was markedly less efficient.
With respect the EP method, we have previously shown a potent induction of human prostate-specific antigen (PSA)- and human epidermal growth factor receptor 2 (HER2)-specific CTLs [16, 29]. However, PSA is an antigen that is not expressed in mice and thus this study did not evaluate the ability to overcome tolerance against a self-antigen. In the case of HER2, CTLs against shared HLA-A2-restricted epitopes between mouse and human sequences were induced although tumor protection was evaluated using cells transfected with human HER2, which is a foreign antigen for mouse immune system. In another study, i.d. EP with a DNA vaccine encoding for the immature laminin receptor protein (OFA-iLRP), an embryonic self-antigen expressed by a variety of tumors, failed to induce anti-tumor responses [15]. CTL-mediated tumor protection was achieved only when OFA-iLRP was fused to CCR6 ligands that targeted the antigen to antigen-presenting cells.
I.d. delivery of DNA vaccines has a great clinical potential, not only because skin is readily accessible and injections are better tolerated, but also because of the unique immunological properties of the skin [14]. EP seems to combine high levels of antigen expression and adequate activation of both innate and adaptive immunity along with safety, tolerability, reproducibility and clinically acceptable administration [13]. Thus, our studies extend upon former reports by showing that long-lasting tumor protection can be achieved by i.d. EP with a plasmid encoding for the human survivin antigen. We anticipate that the simplicity of our vaccination strategy will greatly facilitate the clinical application of survivin DNA vaccines with a considerable therapeutic potential.
References
Altieri DC (2008) Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 8:61–70
Adida C, Crotty PL, McGrath J et al (1998) Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am J Pathol 152:43–49
Ambrosini G, Adida C, Altieri DC (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3:917–921
Velculescu VE, Madden SL, Zhang L et al (1999) Analysis of human transcriptomes. Nat Genet 23:387–388
Yamamoto H, Ngan CY, Monden M (2008) Cancer cells survive with survivin. Cancer Sci 99:1709–1714
Altieri DC (2006) Targeted therapy by disabling crossroad signaling networks: the survivin paradigm. Mol Cancer Ther 5:478–482
O’Connor DS, Schechner JS, Adida C et al (2000) Control of apoptosis during angiogenesis by survivin expression in endothelial cells. Am J Pathol 156:393–398
Andersen MH, Pedersen LO, Capeller B et al (2001) Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res 61:5964–5968
Rohayem J, Diestelkoetter P, Weigle B et al (2000) Antibody response to the tumor-associated inhibitor of apoptosis protein survivin in cancer patients. Cancer Res 60:1815–1817
Xiang R, Mizutani N, Luo Y et al (2005) A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res 65:553–561
Zeis M, Siegel S, Wagner A et al (2003) Generation of cytotoxic responses in mice and human individuals against hematological malignancies using survivin-RNA-transfected dendritic cells. J Immunol 170:5391–5397
Gurunathan S, Klinman DM, Seder RA (2000) DNA vaccines: immunology, application, and optimization. Annu Rev Immunol 18:927–974
Luxembourg A, Evans CF, Hannaman D (2007) Electroporation-based DNA immunisation: translation to the clinic. Expert Opin Biol Ther 7:1647–1664
Nicolas JF, Guy B (2008) Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines 7:1201–1214
Biragyn A, Schiavo R, Olkhanud P et al (2007) Tumor-associated embryonic antigen-expressing vaccines that target CCR6 elicit potent CD8+ T cell-mediated protective and therapeutic antitumor immunity. J Immunol 179:1381–1388
Roos AK, Moreno S, Leder C et al (2006) Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol Ther 13:320–327
Decker WK, Qiu J, Farhangfar F et al (2006) A retrogen plasmid-based vaccine generates high titer antibody responses against the autologous cancer antigen survivin and demonstrates anti-tumor efficacy. Cancer Lett 237:45–55
Lladser A, Parraga M, Quevedo L et al (2006) Naked DNA immunization as an approach to target the generic tumor antigen survivin induces humoral and cellular immune responses in mice. Immunobiology 211:11–27
Zhu K, Qin H, Cha SC et al (2007) Survivin DNA vaccine generated specific antitumor effects in pancreatic carcinoma and lymphoma mouse models. Vaccine 25:7955–7961
Karre K, Ljunggren HG, Piontek G et al (1986) Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319:675–678
Parker KC, Bednarek MA, Coligan JE (1994) Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol 152:163–175
Singh H, Raghava GP (2003) ProPred1: prediction of promiscuous MHC Class-I binding sites. Bioinformatics 19:1009–1014
Rammensee H, Bachmann J, Emmerich NP et al (1999) SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50:213–219
Nagaraj S, Pisarev V, Kinarsky L et al (2007) Dendritic cell-based full-length survivin vaccine in treatment of experimental tumors. J Immunother 30:169–179
Townsend A, Ohlen C, Foster L et al (1989) A mutant cell in which association of class I heavy and light chains is induced by viral peptides. Cold Spring Harb Symp Quant Biol 54(Pt 1):299–308
Dong Y, Qian J, Ibrahim R et al (2006) Identification of H-2Db-specific CD8+ T-cell epitopes from mouse VEGFR2 that can inhibit angiogenesis and tumor growth. J Immunother 29:32–40
Mrass P, Kinjyo I, Ng LG et al (2008) CD44 mediates successful interstitial navigation by killer T cells and enables efficient antitumor immunity. Immunity 29:971–985
Ciesielski MJ, Kozbor D, Castanaro CA et al (2008) Therapeutic effect of a T helper cell supported CTL response induced by a survivin peptide vaccine against murine cerebral glioma. Cancer Immunol Immunother 57:1827–1835
Vertuani S, Triulzi C, Roos AK et al (2008) HER-2/neu mediated down-regulation of MHC class I antigen processing prevents CTL-mediated tumor recognition upon DNA vaccination in HLA-A2 transgenic mice. Cancer Immunol Immunother 58(5):653–664
Andersen MH, Soerensen RB, Becker JC et al (2006) HLA-A24 and survivin: possibilities in therapeutic vaccination against cancer. J Transl Med 4:38
Blanc-Brude OP, Mesri M, Wall NR et al (2003) Therapeutic targeting of the survivin pathway in cancer: initiation of mitochondrial apoptosis and suppression of tumor-associated angiogenesis. Clin Cancer Res 9:2683–2692
Tuting T, Gambotto A, DeLeo A et al (1999) Induction of tumor antigen-specific immunity using plasmid DNA immunization in mice. Cancer Gene Ther 6:73–80
Conrad H, Gebhard K, Kronig H et al (2008) CTLs directed against HER2 specifically cross-react with HER3 and HER4. J Immunol 180:8135–8145
Acknowledgments
Related results have been presented previously in a preliminary form at the AACR Special Conference in Cancer Research “Tumor Immunology: New Perspectives”. December 2–5, 2008, Miami, FL, USA. Research described here has been supported by grants from the Swedish Cancer Society, the Swedish Medical Research Council, the Cancer Society of Stockholm, the European Union (Grants “EUCAAD” and “DC-THERA”), the Karolinska Institutet, “ALF-Project” grants from the Stockholm City Council (to RK), as well as the ICGEB (International Center of Genetic Engineering and Biotechnology, Trieste, Italy) grant CRP/CH102-01, Wellcome Trust award WT06491I/Z/01/Z and FONDAP grant 15010006 (to AFGQ). AL has been supported by President of the Republic International Fellowship for Postgraduate Studies, CONICYT Ph.D. Fellowship award, MECESUP Fellowship awards UCH9903 and UCH0306. KL has been supported by a postdoctoral fellowship from the Swedish Society for Medical Research.
Conflict of interest statement
The authors declare that they have no conflict of interest. AKR is an employee at Cyto Pulse Sciences Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lladser, A., Ljungberg, K., Tufvesson, H. et al. Intradermal DNA electroporation induces survivin-specific CTLs, suppresses angiogenesis and confers protection against mouse melanoma. Cancer Immunol Immunother 59, 81–92 (2010). https://doi.org/10.1007/s00262-009-0725-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-009-0725-4