Abstract
Previously, we reported that the oral administration of high molecular mass poly-γ-glutamate (γ-PGA) induced antitumor immunity but the mechanism underlying this antitumor activity was not understood. In the present study, we found that application of high molecular mass γ-PGA induced secretion of tumor necrosis factor (TNF)-α from the bone-marrow-derived macrophages of wild type (C57BL/6 and C3H/HeN) and Toll-like receptor 2 knockout (TLR2−/−) mice, but not those of myeloid differentiation factor 88 knockout (MyD88−/−) and TLR4-defective mice (C3H/HeJ). Production of interferon (IFN)-γ-inducible protein 10 (IP-10) in response to treatment with γ-PGA was almost abolished in C3H/HeJ mice. In contrast to LPS, γ-PGA induced productions of TNF-α and IP-10 could not be blocked by polymyxin B. Furthermore, γ-PGA-induced interleukin-12 production was also impaired in immature dendritic cells (iDCs) from MyD88−/− and C3H/HeJ mice. Downregulation of MyD88 and TLR4 expression using small interfering RNA (siRNA) significantly inhibited γ-PGA-induced TNF-α secretion from the RAW264.7 cells. γ-PGA-mediated intracellular signaling was markedly inhibited in C3H/HeJ cells. The antitumor effect of γ-PGA was completely abrogated in C3H/HeJ mice compared with control mice (C3H/HeN) but significant antitumor effect was generated by the intratumoral administration of C3H/HeN mice-derived iDCs followed by 2,000 kDa γ-PGA in C3H/HeJ. These findings strongly suggest that the antitumor activity of γ-PGA is mediated by TLR4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poly-γ-glutamate (γ-PGA) naturally secreted from various strains of Bacillus is a safe and edible polymer in which the α-amino and γ-carboxy groups of d- or l-glutamic acid are linked by isopeptide bonds [29]. The treatment of γ-PGA (5 mg/ml) did not induce the cytotoxicity against various tumor cells even at 5 days post-treatment [21] and the toxicity was not observed in mice following the injection of 1 mg γ-PGA [32]. Recently, we reported that oral administration of high molecular mass (2,000 kDa) γ-PGA isolated from Bacillus subtilis sp. Chungkookjang, Korean traditional food, confers marked antitumor effects to a higher degree than that induced by lower molecular mass (10 kDa) γ-PGA in C57BL/6 mice [21]. However, the mechanism underlying the antitumor effect of high molecular mass (2,000 kDa) γ-PGA has not been previously investigated.
Because, many microbial products induce innate immune responses via mammalian Toll-like receptors (TLRs), and γ-PGA is produced by Bacillus [8], we hypothesized that this signaling may be mediated by TLRs. TLRs are abundantly expressed on professional antigen presenting cells (APCs), such as macrophages and dendritic cells (DCs), and the TLR-mediated activation of the innate immune response in APCs has been reported to promote antitumor immune responses via activation of natural killer (NK) and Th1 cells [7]. Among the immunity-related cells, DCs are potent APCs that play important roles in initiating innate and adaptive immune responses [10] by presenting antigens to naive T cells, leading to their activation. Efficient T cell activation requires the maturation of immature DCs (iDCs), which involves the induction of costimulatory surface molecules and production of cytokines, such as interleukin-12 (IL-12) and IL-18 [6]. IL-12 potently induces Th1 responses by augmenting cytolytic activity and inducing IFN-γ production and proliferation in NK and T cells, thereby bridging the gap between innate resistance and adaptive immunity [12, 36]. IL-12 is a heterodimer formed by a 35-kDa light chain and a 40-kDa heavy chain. Both MyD88-dependent and -independent signals are required for secretion of bioactive IL-12p70 following TLR4-mediated DC activation [12, 14]. TLR ligands can enhance the surface expression of costimulatory molecules such as CD40, CD80 and CD86 [9, 22]. This phenotypic modulation is a typical feature of DC maturation. Notably, while studies showed that LPS-induced maturation was completely abolished in TLR4-defective DCs, LPS-induced surface expression of costimulatory molecules was significantly upregulated in MyD88−/− DCs [17, 19], suggesting that LPS can induce phenotypic DC maturation via MyD88-independent signaling.
The TLR-mediated signaling cascades are generally separated into two groups: the MyD88-dependent pathways and the MyD88-independent pathways used by TLR3 and TLR4 [35]. In APCs, MyD88 recruits IL-1 receptor-associated kinase and TNF receptor-associated factor 6 (TRAF6), which then activates downstream mediators such as mitogen-activated protein kinases (MAPKs) and NF-κB [24, 25], triggering the subsequent activation of various inflammatory mediators and the production of cytokines, including TNF-α and IL-12p40 [3, 5, 7]. In MyD88-independent signaling, LPS or poly(I:C) can activate Toll/IL-1R domain-containing adaptor inducing IFN-β (TRIF), an adaptor molecule that functions independently of MyD88 [20]. TLR3 primarily activates this TRIF-mediated pathway, whereas TLR4 activates both MyD88- and TRIF-dependent pathways.
In the present study, we examined γ-PGA-induced innate immune responses in macrophages and DCs from control, MyD88−/− or TLR4-defective mice, in order to test the possible role of these molecules in activation of innate immune responses by γ-PGA. The antitumor activity of γ-PGA was checked in vivo by oral administration in control or TLR4-defective mice and the adaptive transfer of the antitumor activity by intratumoral administration of γ-PGA stimulated iDC from control mice was also studied in TLR4-defective mice.
Materials and methods
Reagents
γ-PGA molecules derived from B. subtilis (Chungkookjang), prepared as described previously [21], were kindly provided by BioLeaders Corporation (Daejeon, Korea) and dissolved in PBS. The number and weight-average molecular masses (Mn and Mw, respectively) along with the polydispersity (Mw/Mn) of the γ-PGA molecules were measured by gel permeation chromatography using a GMPWXL column (Viscotek, Houston, TX, USA) and a LR125 Laser Refractometer (Viscotek). Polydispersity of 2,000 and 10 kDa γ-PGA was measured as 4.3 and 1.5, respectively. The compositional D/L ratio of γ-PGA (2,000 and 10 kDa) was 60:40. D/L ratios were determined by HPLC system after FDAA modification for differentiation of d-glu and l-glu in γ-PGA hydrolysate. Polyacrylamide standards (American Polymer Standard, Mentor, OH, USA) were used to construct a calibration curve. The content of γ-PGA (10 or 2,000 kDa) was increased to > 99%, and polydispersity was decreased after anion-exchange chromatography. To get thoroughly solubilized gamma-PGA solution, we adjusted pH to 6.8 ~ 7.0 by adding 5 N NaOH solution to acid form PGA. LPS (Escherichia coli, O55:B5) and polymyxin B (PMB) sulfate were purchased from Sigma-Aldrich (St Louis, MO, USA) and Calbiochem (San Diego, CA, USA), respectively. The LPS concentration in γ-PGA was measured using an LPS-specific Limulus amoebocyte lysate (LAL) test with an endotoxin QCL-1000 kit (Bio Whittaker Inc., Walkersville, MD, USA).
Mice and cell lines
Female C3H/HeN and C3H/HeJ mice were purchased from Japan SLC (Hamamatsu, Japan). C57BL/6 mice were purchased from KOATECH (Pyeongtaek, Korea). MyD88−/− and TLR2−/− mice were kindly provided by S. Akira (Osaka University, Osaka, Japan) [2]. Mice were housed in the specific pathogen-free animal facility at the Korea Research Institute of Bioscience and Biotechnology. The RAW264.7 (TIB-71) mouse macrophage cell line and the K1735 mouse melanoma cell line were obtained from the American Type Culture Collection and the Korea Cell Line Bank (Seoul, Korea), respectively. The cell lines were cultured as described previously [13, 30].
Isolation of macrophages and dendritic cells
Thioglycollate-elicited peritoneal macrophages were collected 4 days after i.p. injection of 2 ml 4% thioglycollate broth (Difco Laboratories, Detroit, MI, USA). Bone marrow cells were isolated and cultured as described by Lutz et al. [23], with minor modifications. Briefly, femora and tibiae from two female mice, 8–12 weeks, were removed and the marrow was flushed with PBS using a 27-gauge needle. The resulting cell suspension was centrifuged for 10 min at 300 × g and washed once in PBS. To enrich for macrophages, the bone marrow cells were plated in 10% FBS-RPMI 1640 supplemented with 10 ng/ml murine M-CSF (PeproTech, London, UK), as described previously [34]. To enrich for DCs, cells were plated at 1 × 106 cells/ml in 12-well plates in 10% FBS-RPMI 1640 supplemented with 20 ng/ml GM-CSF and 10 ng/ml IL-4 (PeproTech). The purity of the obtained DCs populations was determined by the expression of DC phenotypic markers by flow cytometric staining using fluorochrome-conjugated mAbs to CD11c. The purity was consistently about 95–97% CD11c-positive cells. On day 7, the cultured cells were used to evaluate effects of γ-PGA on expression of costimulatory molecules and cytokine release. Intestinal DCs were isolated from entire small intestine. Luminal contents were flushed with PBS, and the tissues were cut open longitudinally, the cut into <5-mm pieces. The pieces were incubated two times for 20 min at 37°C with continuous agitation in Ca2+Mg2+-free HBSS (Gibco, Karlsruhe, Germany) supplemented with 5 mM EDTA and 1 mM DTT to remove epithelial cells and digested in PBS supplemented with 0.5 mg/ml collagenase D (Roche, Mannheim, Germany), 3 mg/ml dispase II (Roche), 0.5 mg/ml DNase I (Roche) for 20 min at 37°C with continuous agitation. The resulting cell suspension was passed through a 40 μm pore size cell strainer (BD bioscience) and incubated with CD11c microbeads (Miltenyi Biotec) according to the manufacturer’s instructions. CD11c-positive cell purification was conducted over LS + columns on an OctoMACS separator (Miltenyi Biotec).
Cytokine analysis
Supernatants were collected after stimulation and kept at −20°C until assayed. The concentrations of TNF-α and IL-12p70 in the culture supernatants were determined with an OptEIA™ kit (BD Biosciences, San Diego, CA, USA), and IP-10 levels were determined with a DuoSet ELISA kit (R&D Systems, Minneapolis, MN, USA).
RNA interference
For MyD88 and TLR4 knockdown studies, 1 × 106 RAW264.7 cells were transiently transfected with 100 nM of small interfering RNA (siRNA) against MyD88 (siMyD88), TLR4 (siTLR4), or control siRNA. siRNA targeting the coding regions of MyD88 and TLR4, and the siCONTROL® were obtained from Dharmacon (Lafayette, IN, USA). Cells were electroporated using a MicroPorator electroporation system (Digital Bio Technology, Seoul, Korea), according to the manufacturer’s protocol. The transfected cells were cultured in complete medium for 24 h, and then used as indicated in each experiment.
RT-PCR analysis
Total RNA was prepared using an RNeasy Mini kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions and reverse transcribed by using M-MLV reverse transcriptase (Promega, Madison, WI, USA). cDNAs were amplified with specific primers for targets. Primer sequences were as follows: for MyD88, sense, 5′ATCCGAGAGCTGGAAACG, and antisense, 5′GCAAGGGTTGGTATAGTC; for TLR4, sense, 5′GCATGGCTTACACCACCTCT, and antisense, 5′GTGCTGAAA ATCCAGGTGCT, for β-actin, sense, 5′GCTACAGCTTCACCACCACA, and antisense, 5′CATCGTACTCCTGCTTGCTG.
Flow cytometry and immunofluorescence microscopy
For analysis of cell surface markers, cells were fixed and stained with anti-CD40-FITC, anti-CD80-PE, or anti-CD86-PE (all mouse specific; BD Bioscience) at 4°C for 40 min and then washed twice. The stained cells were analyzed by using a BD FACSCalibur flow cytometer (Becton Dickinson, San Joes, CA, USA), and the data were processed using the CELLQuest Pro software (Becton Dickinson). For checking the expression level of siRNA target molecules, the transfected cells were washed, fixed, and permeabilized with 0.1% (v/v) Triton X-100 in PBS for 10 min. The cells were then incubated with anti-MyD88 or anti-TLR4 antibodies (Santa Cruz Biotechnology, CA, USA) for 2 h at 4°C, washed twice and then incubated with an FITC-conjugated secondary antibody (dilution of 1:200; Sigma-Aldrich) or tetramethylrhodamine isothiocyanate (TRITC)-conjugated secondary antibody (dilution of 1:200; Sigma-Aldrich) for 1 h at 4°C. The samples were analyzed by using a BD FACSCalibur flow cytometer or the mounted slides were examined on a fluorescence microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY, USA) and photographed with an Axiocam HRC (Carl Zeiss MicroImaging Inc.).
Western blot analysis
Peritoneal macrophages and bone-marrow-derived dendritic cells (BMDCs) from C57BL/6, MyD88−/−, C3H/HeN, or C3H/HeJ mice were treated with LPS (100 ng/ml) and γ-PGA (1 mg/ml) for 30 min and then lysed in a buffer containing 1% NP-40, 0.5% sodium deoxycholate, 1% SDS, 1 mM EDTA, 1 mM EGTA, 1 mM sodium orthovanadate, 1 mM leupeptin, and 1 mM PMSF in PBS. Equivalent amounts of protein (30 μg) were size-fractionated by 12% SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% skim milk in TBS-T (TBS containing 0.05% Tween-20) and blotted with a polyclonal anti-phospho JNK, p38, or ERK1/2 antibody (Cell Signaling Technology, Beverly, MA, USA). The blots were developed using a horseradish peroxidase-conjugated secondary antibody and visualized by chemiluminescence using an ECL kit (GE Healthcare, Uppsala, Sweden). After stripping, the membranes were reprobed with a polyclonal anti-JNK, p38, or ERK1/2 antibody (Cell Signaling) as loading controls.
NF-κB and IRF-3 luciferase assay
Peritoneal macrophages from C57BL/6, MyD88−/−, C3H/HeN, or C3H/HeJ mice were transiently transfected with 1 μg of pNF-κB-luc (Stratagene, La Jolla, CA, USA) or interferon regulatory factor-3 (IRF-3)-luc (a kind gift from Dr Kate Fizerald) [38] and a Renilla luciferase control vector (Promega), using a MicroPorator. The transfected cells were cultured in complete medium for 1 h with LPS or for 2 h with PBS or γ-PGA, and luciferase activity was determined with a luciferase reporter gene assay kit (Promega). Briefly, cell lysates were mixed with the provided luciferase assay substrate, and firefly luminescence was measured using a VICTOR3 1420 multilabel counter (Wallac, Perkin-Elmer, Boston, MA, USA). Relative luciferase units (RLU) were calculated as the ratio of firefly luciferase activity versus Renilla luciferase activity.
Tumor challenge assay
For checking the protective antitumor effect, six mice per group (C3H/HeN or C3H/HeJ) were orally treated with PBS, 10 kDa γ-PGA or 2,000 kDa γ-PGA at the previously reported dose (400 μg/day, 5 days/week). At 7 days after the initial treatment, syngeneic K1735 melanoma cells (2.5 × 106/mouse) were injected s.c. into the left inguinal region. For the treatment effect of γ-PGA, 3 days after the inoculation of K1735 melanoma cells (2.5 × 106/mouse), mice were treated with daily oral treatment of 400 μg γ-PGA (10 or 2,000 kDa) or PBS. Tumor size was assessed as the tumor area (mm2), which was determined by monitoring two perpendicular diameters of the tumors twice a week using a caliper.
Intratumoral administration of γ-PGA and DCs
Syngeneic K1735 cells (2.5 × 106/mouse) were injected s.c. into the left inguinal region of C3H/HeJ mice. Approximately, 7 days after the inoculation when tumors were about 50 mm2 in size, the mice were randomly divided into six groups consisting of five mice with equal mean tumor sizes. The mice were administered with iDCs (1 × 106), derived from C3H/HeN or C3H/HeJ mice, intratumorally on days 7, 14, 21, and 28 after tumor inoculation and followed by PBS or 150 μg of 10 kDa γ-PGA or 2,000 kDa γ-PGA intratumorally on days 8, 15, 22, and 29.
Cytotoxicity assay of natural killer cell
Natural killer cells were purified from spleens of C3H/HeN mice by MACS-negative selection beads (Miltenyi Biotec). The purity of the NK cell preparations was confirmed by flow cytometry and a purity of more than 95% was achieved routinely. BMDCs isolated from wild-type (C3H/HeN) and TLR4-defective (C3H/HeJ) mice were stimulated with 1 mg/ml γ-PGA for 24 h and washed, and NK cells were added to the cultures at a DC/NK cell ration of 1:2. After 24 h, NK cells were incubated with YAC-1 cells for 6 h at the indicated effector-to-target cell ration (E/T ratio).
YAC-1 cells were sub-cultured in RPMI-1640 complete cell culture medium supplemented with 10% FBS. The cytotoxicity activity of NK cell was assessed by a lactate dehydrogenase (LDH) release cytotoxicity assay (Promega). NK cell was harvested as described earlier, and was incubated with 1 × 104 YAC-1 cells in increasing effector-to-target cell ratios (30:1, 15:1, 7.5:1) for 6 h at 37°C. LDH was assayed in the supernatant by optical density (OD) measurement at 490 nm. Target cell lysis was calculated as: (OD of sample −OD with spontaneous release of LDH from target cells −OD with spontaneous release of LDH from effector cells) × 100/(OD with maximal release of LDH from target cell − OD with spontaneous release of LDH from target cell).
Cytolytic activity of cytotoxic T lymphocytes against K1735 tumor cells
Mice were orally administered with 400 μg of 10 kDa γ-PGA, 2,000 kDa γ-PGA, or PBS (5 days/week) for 3 weeks. 7 days after the initial treatment, the mice were subcutaneously challenged with 2.5 × 106 K1735 melanoma cells. Twenty-eight days after tumor cell injection, the splenocytes were cultured in a 96-well round bottom plates and stimulated with lysate of K1735 melanoma cell lines at a final concentration of 100 μg/ml. After 24 h, splenocytes were incubated with K1735 cells for 6 h at the indicated effector-to-target cell ration (100:1, 50:1, 25:1). The cytotoxic activity of cytotoxic T lymphocytes (CTL) was evaluated using LDH release assays described as in NK assay above.
Statistical analysis
Statistical analyses were performed using Student’s t-tests for data. A P-value of <0.05 was considered a statistically significant difference.
Results
γ-PGA-induced activation of macrophages is mediated by MyD88-dependent and -independent pathways
MyD88 is a general adaptor protein that plays an import role in TLR family signaling. To determine whether MyD88 signaling might be involved in γ-PGA-induced immune cell activation, we examined cytokine production following γ-PGA (1 mg/ml) treatment of bone-marrow-derived macrophages (BMDMs) from wild-type (C57BL/6) and MyD88−/− mice. Treatment with LPS or 2,000 kDa γ-PGA induced the production of TNF-α (LPS: 1663.1 ± 298.6 pg/ml, 2,000 kDa γ-PGA: 1730.8 ± 228.6 pg/ml) in macrophages from wild-type mice and the ability of 2,000 kDa γ-PGA to induce TNF-α production was dose-dependent (Supplementary Figure 1a), whereas TNF-α was not produced in PBS- or 10 kDa γ-PGA-treated macrophages from wild-type or MyD88−/− mice (Fig. 1a). Notably, TNF-α production was impaired in 2,000 kDa γ-PGA-treated macrophages prepared from MyD88−/− mice (Fig. 1a).
γ-PGA induced the activation of macrophage by MyD88-dependent and -independent pathways. BMDMs (1 × 106 cells/ml) isolated from wild-type or MyD88−/− mice were stimulated with 100 ng/ml LPS or 1 mg/ml γ-PGA in the presence or absence of 10 μg/ml polymyxin B (PMB) for 24 h. Concentration of TNF-α (a) and IP-10 (b) in the culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA). RAW264.7 cells, murine macrophage cell line, were transiently transfected with siRNA targeting the coding regions of MyD88 (siMyD88) and the control siRNA. The mRNA and protein expression levels of MyD88 of siRNA transfected cells were determined by RT-PCR, fluorescence microscopy (×200) and flow cytometry analysis (c). The RAW264.7 cells transfected with siRNA were treated with 100 ng/ml LPS and 1 mg/ml γ-PGA. The concentrations of TNF-α in the supernatants of siMyD88- or the control siRNA-transfected cells were determined by ELISA (d). Asterisks represent not detected. The results are expressed as mean ± SE of three independent experiments
Although a LAL assay showed that the level of endotoxin in our γ-PGA stock solution was <0.01 EU, we further examined whether LPS was involved in the production of TNF-α and IP-10 in 2,000 kDa γ-PGA-treated macrophages by administering 10 μg/ml PMB and testing its effect on γ-PGA-treated macrophages from wild-type or MyD88−/− mice. No difference in the effectiveness of γ-PGA treatment was observed in macrophages from wild-type mice or MyD88−/− mice treated with or without PMB (Fig. 1a). By contrast, PMB completely blocked TNF-α induction by LPS in wild-type mice. These results confirm that the effect of γ-PGA on macrophages was not due to LPS contamination. We also investigated the production of TNF-α in macrophages treated with low molecular mass γ-PGA (< 50 kDa) obtained by digestion of 2,000 kDa γ-PGA with γ-PGA-degrading enzymes (γ-glutamyltransferase and glutamyl amidohydrolase) secreted by B. subtilis [1]. As expected, the ability of 2,000 kDa γ-PGA to induce TNF-α production was completely impaired in γ-PGA-treated macrophages from wild-type mice (data not shown).
Because IP-10 production normally occurs independently of MyD88 [7, 16, 18], we next investigated the production of IP-10 in γ-PGA-treated macrophages. Similar amounts of IP-10 were produced in macrophages from both wild-type and MyD88−/− mice treated with γ-PGA (2,000 kDa) and LPS (Fig. 1b), and the ability of 2,000 kDa γ-PGA to induce IP-10 production was also dose-dependent (Supplementary Figure 1b). As expected, induction of IP-10 by LPS, but not γ-PGA, was blocked by PMB.
To confirm the role of MyD88 in γ-PGA-induced innate immunity, we developed MyD88-targeting siRNA (siMyD88), and checked the effect of MyD88 knockdown on the production of TNF-α in γ-PGA-stimulated RAW264.7 cells (a murine macrophage cell line). RT-PCR analysis showed reduced mRNA expression of MyD88, but not β-actin, in RAW264.7 cells transfected with siMyD88, compared to control siRNA-transfected cells (Fig. 1c). Similarly, the protein expression levels of MyD88, as checked by flow cytometry and immunofluorescence microscopy, were significantly attenuated in the siMyD88-transfected cells (Fig. 1c), confirming successful siRNA knockdown of the target molecules. In control siRNA-transfected RAW264.7 cells, TNF-α production in response to LPS and γ-PGA (2,000 kDa) treatment was 3045 ± 81 pg/ml and 2582 ± 117 pg/ml, respectively. In contrast, RAW264.7 cells transfected with siMyD88 produced significantly less TNF-α upon stimulation of LPS or 2,000 kDa γ-PGA (LPS: 1809 ± 284 pg/ml, 2,000 kDa γ-PGA: 1205 ± 279 pg/ml; P < 0.05; Fig. 1d). These results indicate that γ-PGA activates macrophages via both MyD88-dependent and -independent pathways.
γ-PGA-induced macrophage activation is mediated by TLR4
Because γ-PGA treatment was found to induce both MyD88-dependent and -independent pathways in macrophages, we next examined whether this innate immune response is mediated by TLR4 using BMDMs isolated from TLR4-defective mice (C3H/HeJ) and TLR2−/− mice. C3H/HeJ mice harbor a proline to histidine point mutation at residue 712 of TLR4, making them unresponsive to LPS [18, 31]. γ-PGA treatment dose-dependently induced production of TNF-α (984.3 ± 152.5 pg/ml) and IP-10 (865.4 ± 106.9 pg/ml) in macrophages from C3H/HeN wild-type mice. In contrast, the productions of TNF-α (LPS: 109.9 ± 72.8 pg/ml, 2,000 kDa γ-PGA: 52.2 ± 49.4 pg/ml) and IP-10 (LPS: 104.2 ± 22.1 pg/ml, 2,000 kDa γ-PGA: 124.9 ± 25.2 pg/ml) were strongly suppressed in 2,000 kDa γ-PGA- or LPS-treated macrophages from C3H/HeJ mice (Fig. 2a, b). Also, macrophages from wild-type (C57BL/6) and TLR2−/− mice produced similar amounts of TNF-α and IP-10 in response to the 2,000 kDa γ-PGA, which indicated that the response to γ-PGA was not mediated by TLR2. Taken together, these findings display that TLR4 is essential receptor for the γ-PGA-induced cytokine production of macrophages.
γ-PGA induced activation of macrophage was mediated by TLR4. BMDMs (1 × 106 cells/ml) isolated from TLR4-defective (C3H/HeJ), TLR2-deficient mice, or wild-type (C3H/HeN, C57BL/6) mice were stimulated with 100 ng/ml LPS or 1 mg/ml γ-PGA for 24 h. Concentration of TNF-α (a) and IP-10 (b) in the culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA). RAW264.7 cells, murine macrophage cell line, were transiently transfected with siRNA targeting the coding regions of TLR4 (siTLR4) and the control siRNA. The mRNA and protein expression levels of TLR4 of siRNA transfected cells were determined by RT-PCR, fluorescence microscopy (×200) and flow cytometry analysis (c). The RAW264.7 cells transfected with siRNA were treated with 100 ng/ml LPS and 1 mg/ml γ-PGA. The supernatants were collected 24 h later and concentrations of TNF-α of siTLR4- or the control siRNA-transfected cells were determined by ELISA (d). Asterisks represent not detected. The results are expressed as mean ± SE of three independent experiments
To confirm the role of TLR4 in γ-PGA-induced innate immunity, we also checked the effect of TLR4 downregulation on the production of TNF-α in γ-PGA-stimulated RAW264.7 cells. RT-PCR analysis showed reduced mRNA expression of TLR4, but not β-actin, in RAW264.7 cells transfected with TLR4-specific siRNA (siTLR4), compared to control siRNA-transfected cells (Fig. 2c). Similarly, the protein expression levels of TLR4, as checked by flow cytometry and immunofluorescence microscopy, were significantly attenuated in the siTLR4-transfected cells (Fig. 2c), confirming successful siRNA knockdown of the target molecules. LPS- and 2,000 kDa γ-PGA-stimulated TNF-α production was also markedly inhibited in siTLR4-transfected RAW264.7 cells compared with control cells (P < 0.05; Fig. 2d). These observations, which are consistent with our finding of decreased LPS- and 2,000 kDa γ-PGA-stimulated TNF-α production from TLR4-defective (C3H/HeJ) mice macrophages (Fig. 2a), further support the notion that TLR4 plays a major role in the innate immunity of γ-PGA.
γ-PGA induced intracellular signaling is TLR4-dependent
To study the direct impact of γ-PGA on TLR4-mediated signaling events, we examined TLR4-mediated intracellular signaling in peritoneal macrophages from wild-type (C57BL/6, C3H/HeN), MyD88−/−, and C3H/HeJ mice. Because ligation of TLR4 triggers the activation of MAP kinase and NF-κB, leading to production of proinflammatory cytokines through the MyD88-dependent pathway, we investigated the activation of MAP kinase such as JNK, p38, and ERK1/2 in macrophages treated with LPS or 2,000 kDa γ-PGA. As shown in Fig. 3a, b, treatment with LPS or 2,000 kDa γ-PGA increased the phosphorylations of JNK and p38 kinase in macrophages from wild-type mice. However, the γ-PGA-induced phosphorylation of JNK and p38 was markedly decreased in peritoneal macrophages from MyD88−/− and C3H/HeJ mice. Interestingly, as shown in Fig. 3c, LPS induced phosphorylation of ERK1/2 was decreased in macrophage from MyD88−/− and C3H/HeJ mice but the activation level of ERK1/2 induced by the 2,000 kDa γ-PGA treatment was not significantly different in macrophages from wild-type, MyD88−/−, and C3H/HeJ mice.
Intracellular signaling induced by γ-PGA was MyD88 and TLR4-dependent. Peritoneal macrophages from C57BL/6, MyD88−/−, C3H/HeN, or C3H/HeJ mice were treated with 100 ng/ml LPS and 1 mg/ml γ-PGA for 30 min. The expression level of phosphorylated JNK (pJNK) (a), p38 kinase (pp38) (b), and ERK1/2 (pERK1/2) (c) were determined by Western blot analysis. Peritoneal macrophages from C57BL/6, MyD88−/−, C3H/HeN, or C3H/HeJ mice were transiently transfected with pNF-κB-Luc and IRF-3-Luc reporter gene plasmids and then cultured in complete medium for 24 h. At 24 h after transfection, cells were treated with 100 ng/ml LPS for 1 h and 1 mg/ml γ-PGA for 2 h and then NF-κB activity (d), IRF-3 activity (e) were determined by luciferase reporter assay. The data presented in this figure are representative of triplicate experiments. All data are representative of at least three experiments
We then examined whether γ-PGA treatment induces NF-κB activation in peritoneal macrophages, using an NF-κB luciferase reporter assay. As expected, treatment with LPS (100 ng/ml) or 2,000 kDa γ-PGA (1 mg/ml) induced significant NF-κB activation in macrophages from wild-type mice, but NF-κB activation in macrophages from MyD88−/− and C3H/HeJ mice was greatly reduced, to a level similar to that seen in 10 kDa γ-PGA-treated macrophages (Fig. 3d). Since treatment with 2,000 kDa γ-PGA induced MyD88-independent production of IP-10, we further examined the activation of IRF-3, a signaling molecule upstream of IP-10, in peritoneal macrophages, using an IRF-3 luciferase reporter gene. Treatment with LPS or 2,000 kDa γ-PGA induced IRF-3 activation in macrophages from MyD88−/− and wild-type mice, but this effect was significantly decreased in macrophages from C3H/HeJ mice (Fig. 3e). In addition, we examined intracellular signaling in BMDCs from wild-type, MyD88−/−, and C3H/HeJ mice. Similar to the results of peritoneal macrophage (Fig. 3), 2,000 kDa γ-PGA induced activations of JNK, p38 kinase, NF-κB, and IRF-3 were mediated by TLR4 in BMDCs (Supplementary Figure 2). Taken together, these results collectively indicate that γ-PGA-induced intracellular signaling is mediated by TLR4.
DC maturation by γ-PGA is TLR4 dependent
To determine whether DC maturation by γ-PGA require TLR4, we then examined γ-PGA-induced cytokine production and upregulation of maturation markers (CD40, CD80, and CD86) in BMDCs from wild-type, MyD88−/− and TLR4 defective mice (Fig. 4). Significant increases in the expressions of CD40, CD80 and CD86 were observed in γ-PGA-treated BMDCs from wild-type and MyD88−/− mice similarly to LPS (Fig. 4a). However, while production of IL-12p70 (1870.07 ± 68.7 pg/ml) was observed in γ-PGA-treated BMDCs from wild-type mice, this response was completely absent in iBMDCs from MyD88−/− mice (Fig. 4b). Hence, MyD88−/− cells lose the ability to produce cytokines but retain the ability to upregulate DC maturation markers in response to γ-PGA. This change was also observed in LPS-treated BMDCs, which is consistent with a reported finding [19].
γ-PGA induced maturation of DCs was TLR4-dependent. BMDCs isolated from wild-type (C57BL/6) and MyD88−/− mice were stimulated with 100 ng/ml LPS or 1 mg/ml γ-PGA for 12 h, and then the expression of surface molecules, CD40, CD80, and CD86 (a) was analyzed by flow cytometry and concentration of IL-12p70 (b) was measured by ELISA. The results are expressed as mean ± SE of three independent experiments. BMDCs from wild-type (C3H/HeN) or TLR4-defective (C3H/HeJ) mice were stimulated with LPS or γ-PGA, and then expression of surface molecules (c) was analyzed by flow cytometry and concentration of IL-12p70 (d) in culture supernatants was measured by ELSIA. Filled histogram and dotted line indicated γ-PGA treated BMDCs and untreated control (media only), respectively. Asterisks represent not detected. All data are representative of at least three experiments
We further compared the responses of BMDCs isolated from C3H/HeN and C3H/HeJ mice to determine whether TLR4 signaling might be involved in γ-PGA-induced cytokine production and BMDC maturation. Treatment with high molecular mass γ-PGA or LPS induced significant up-regulation of CD40, CD80, and CD86 expression in wild-type DCs. In contrast, DCs from TLR4-defective mice did not show enhanced expressions of CD40, CD80 and CD86 in response to 2,000 kDa γ-PGA treatment (Fig. 4c). Furthermore, the induction of IL-12p70 observed in wild-type BMDCs in response to 2,000 kDa γ-PGA treatment was abrogated to the control level (PBS or 10 kDa γ-PGA treatment) in DCs derived from TLR4-defective mice (Fig. 4d). Taken together, these findings indicate that TLR4 is essential for the γ-PGA-induced innate immune responses of DCs as well as macrophage and the γ-PGA-induced activation of APCs is mediated by both MyD88-dependent and -independent pathways.
The antitumor effect of γ-PGA is TLR4-depedent and generated by adaptive transfer of C3H/HeN iDC followed by γ-PGA in C3H/HeJ mice
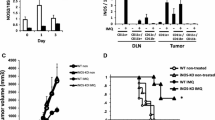
Because the γ-PGA-induced activation of APCs is TLR4-dependent, we next investigated whether TLR4 also plays an important role in γ-PGA-induced antitumor effects in C3H/HeN and C3H/HeJ mice. Mice were orally administered 400 μg of 10 kDa γ-PGA, 2,000 kDa γ-PGA, or PBS (5 days/week). 7 days after the initial treatment, the mice were subcutaneously challenged with 2.5 × 106 K1735 melanoma cells. Twenty-eight days after tumor cell injection, the average tumor size in the 2,000 kDa γ-PGA-treated group (50.0 ± 18.2 mm2) was significantly smaller (P < 0.005) than that in the PBS control group (547.8 ± 82.3 mm2) or the 10 kDa γ-PGA-treated group (309.5 ± 95.1 mm2; Fig. 5a). However, there was no significant tumor size difference in C3H/HeJ mice treated with 2,000 kDa γ-PGA (360.4 ± 90.8 mm2), 10 kDa γ-PGA (316.6 ± 80.5 mm2), or PBS (389.4 ± 87.6 mm2; Fig. 5b). 100% of C3H/HeN mice receiving PBS or 10 kDa γ-PGA, as well as all experimental C3H/HeJ mice (i.e., regardless of PBS or γ-PGA treatment) developed tumors within 16 days after the tumor challenge. In contrast, 33% of C3H/HeN mice treated with 2,000 kDa γ-PGA remained tumor free until 30 days after the K1735 tumor challenge (Fig. 5c). Consistently, administration of PBS or 10 kDa γ-PGA failed to protect against K1735 challenge. All C3H/HeJ and C3H/HeN mice, with the notable exception of C3H/HeN mice treated with 2,000 kDa γ-PGA, succumbed to tumor within 60 days after tumor inoculation (Fig. 5d). In contrast, C3H/HeN mice treated with 2,000 kDa γ-PGA exhibited 100% survival 75 days after tumor inoculation. Importantly, the treatment of 2,000 kDa γ-PGA did not yield antitumor protection in TLR4-defective C3H/HeJ mice. To check the therapeutic antitumor effect of γ-PGA, the mice were subcutaneously challenged with 2.5 × 106 K1735 melanoma cells 3 days prior to its treatment. The average tumor size (185.0 ± 102.2 mm2) in the 2,000 kDa γ-PGA-treated C3H/HeN mice was significantly smaller (P < 0.05) than that in the PBS control group (620.1 ± 83.9 mm2) or the 10 kDa γ-PGA-treated group (520.3 ± 74.3 mm2; Fig. 6a). No significant tumor size difference was observed in C3H/HeJ mice treated with 2,000 kDa γ-PGA, 10 kDa γ-PGA or PBS (Fig. 6b). As expected, C3H/HeN mice treated with 2,000 kDa γ-PGA exhibited enhanced tumor free mice and survival rates after tumor inoculation (data not shown). Collectively, these results indicate that TLR4 is essential for γ-PGA-induced antitumor effects in vivo.
The protective antitumor effect of γ-PGA is mediated by TLR4-depedent. C3H/HeN and C3H/HeJ mice (six mice per group) were treated with daily oral treatment of 400 μg γ-PGA (10 or 2,000 kDa), and were then injected s.c. with 2.5 × 106 K1735 tumor cells at 7 days after the initial treatment. The group of PBS-treated mice was used as a negative control. Tumor size of C3H/HeN (a) or C3H/HeJ (b) mice was measured twice a week and tumor free mice (c) were counted. Survival rate of K1735-bearing mice (d) was observed by 75 days after tumor cell injection. The data presented in this figure are representative of duplicate experiments
The therapeutic antitumor effect of γ-PGA is mediated by TLR4-dependent and DCs play an important role in antitumor immunity of γ-PGA. C3H/HeN and C3H/HeJ mice were inoculated s.c. with 2.5 × 106 K1735 tumor cells into the left inguinal region. Three days after the inoculation, mice were randomly divided and treated with daily oral treatment of 400 μg γ-PGA (10 or 2,000 kDa) or PBS. Tumor size of C3H/HeN (a) or C3H/HeJ (b) mice was measured twice a week. The data presented in this figure are representative of duplicate experiments. 2.5 × 106 K1735 cells were injected s.c. into the left inguinal region of C3H/HeJ mice. Approximately 7 days after the inoculation and when the size of tumors was about 50 mm2, the mice were randomly divided into six groups consisting of five mice with almost equal mean tumor sizes. iDCs (1 × 106), derived from C3H/HeN (c) or C3H/HeJ (d) mice, were intratumorally injected on days 7, 14, 21, and 28 after tumor inoculation and PBS, 150 μg of 10 kDa γ-PGA, or 150 μg of 2,000 kDa γ-PGA were intratumorally injected on days 8, 11, 15, 18, 22, 25, 29, and 32
In order to check the role of γ-PGA-activated DCs in in vivo antitumor effect, we investigated the antitumor effect after intratumoral administration of wild-type iDCs (C3H/HeN-derived BMDCs) or TLR4-defective iDCs (C3H/HeJ-derived BMDCs) followed by γ-PGA into the tumor-bearing TLR4-defective mice (C3H/HeJ). After tumor administration of C3H/HeN-derived iDCs, the size of tumor was significantly decreased (190.0 ± 82.5 mm2; P < 0.005) in 2,000 kDa γ-PGA-treated mice than that in the PBS-treated mice (397.9 ± 54.1 mm2) or the 10 kDa γ-PGA-treated mice (457.4 ± 65.9 mm2; Fig. 6c). In contrast, the mice administered with TLR4-defective iDCs, the antitumor effect was not observed by the administration of 2,000 kDa γ-PGA (520.1 ± 80.4 mm2), PBS (499.3 ± 81.3 mm2), or 10 kDa γ-PGA (449.3 ± 53.9 mm2; Fig. 6d). Intratumoral administration of γ-PGA alone did not induce the antitumor effect in TLR4-defective (C3H/HeJ) mice. In wild-type (C3H/HeN) mice, the slight inhibition of tumor growth was examined after intratumoral administration of 2,000 kDa γ-PGA, which may be induced by the activation of TLR4 expressing APCs near tumor (data not shown). Taken together, these results reveal that γ-PGA-stimulated DCs may play a major role in the TLR4-dependent antitumor effect of γ-PGA.
γ-PGA induced TLR4-dependent activation of NK cells and CTLs
Previously, we reported that oral administration of high molecular mass gamma-PGA generated NK cell-mediated antitumor activity. In order to determine whether oral administration of 2,000 kDa γ-PGA induce activation of DCs in vivo, we checked the expression of maturation marker (CD40) in intestinal DCs by FACS analysis. Significant increase of CD40 expression was observed only in intestinal DCs of 2,000 kDa γ-PGA orally administered mice but not in those of PBS or 10 kDa γ-PGA-administered mice (Supplementary Figure 3). To evaluate the relationship between γ-PGA matured DCs via TLR4 signaling and NK cell mediated antitumor activity, we performed in vitro NK cell activity assay. Cytolytic activity of NK cells incubated with 2,000 kDa γ-PGA-treated wild-type DCs (C3H/HeN) (69.5%) was significantly higher than that with 2,000 kDa γ-PGA-treated TLR4-defective DCs (C3H/HeJ) (35.8%) which was similar with that with 10 kDa or PBS treated DCs (Fig. 7b). Thus, these findings indicate that the TLR4-dependent activation of NK cells is induced by matured DCs in response to γ-PGA stimulation.
Activations of NK cells and splenic cytolytic T cells were TLR4-dependent. DCs prepared from C3H/HeN (a) and C3H/HeJ (b) mice were incubated with γ-PGA for 24 h, and NK cells were added to the culture at an DCs/NK ratio of 1:2. After 24 h, NK cells were incubated with YAC-1 cells for 6 h at the E:T ratios (30:1, 15:1, 7.5:1). C3H/HeN (c) and C3H/HeJ (d) mice (five mice per group) were treated with daily oral treatment of 400 μg γ-PGA (10 or 2,000 kDa), and were then injected s.c. with 2.5 × 106 K1735 tumor cells at 7 days after the initial treatment. The group of PBS-treated mice was used as a negative control. Splenocytes were isolated from mice orally administered (10 and 2,000 kDa) and stimulated with lysate of K1735 cell for 24 h. These splenocytes were used as effector cells and K1735 tumor cells served as target cells. The K1735 cells were mixed with splenocytes at various E:T ratios (100:1, 50:1, 25:1). Specific lysis was determined by quantitative measurements of LDH
In addition, we tested whether splenic T cells from mice orally administered 2,000 kDa γ-PGA showed cytolytic activity against K1735 melanoma cells. Splenocytes were isolated from mice orally administered (10 and 2,000 kDa) γ-PGA for 3 weeks and stimulated in vitro with K1735 lysates. Stimulated splenocytes were then tested for recognition and lysis of K1735 cells. CTL response in the 2,000 kDa γ-PGA-treated C3H/HeN mice (64.0 ± 5.0%) was significantly higher than that in the PBS control group (17.6 ± 2.6%) or the 10 kDa γ-PGA-treated group (22.0 ± 2.1%; Fig. 7c). After oral administration of 2,000 kDa γ-PGA, the TLR4-defective C3H/HeJ mice did not show any increase of cytolytic activity (Fig. 7d). These results display that splenic T cells from mice orally administered 2,000 kDa γ-PGA show tumor-specific cytolytic activity via TLR4 stimulation.
Discussion
Bacillus subtilis sp. Chungkookjang, isolated from chungkookjang, a traditional Korea fermented soybean food, was reported to produce a higher molecular mass (>1,000 kDa) γ-PGA than the forms produced by B. subtilis (natto) [8]. Previously, we showed that oral administration of high molecular mass γ-PGA (average M.W. 2,000 kDa) elicited NK cell-mediated antitumor immunity in mice [21]. Here, we further showed that treatment with 2,000 kDa, but not 10 kDa γ-PGA, induced the activation of macrophages and the maturation of iDCs via TLR4 in a mouse system. Recent reports have proposed that TLR4 ligands such as OK-432, a Streptococcus-derived agent, the cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin (BCG-CWS), and a 55 kDa protein from Aeginetia indica, all induce antitumor activity by activating innate and acquired immunity through NK and T cell activation [4, 26–28]. These findings indicate that TLR4-mediated activation of both innate and adaptive immunity is important for some antitumor effects, further suggesting that TLR4 agonists may prove useful for tumor therapy. Here, we demonstrated that treatment with 2,000 kDa γ-PGA induced antitumor effects in C3H/HeN but not in TLR4-defective C3H/HeJ mice. These results strongly suggest that the antitumor activity of high molecular mass γ-PGA may be induced by TLR4-dependent activation of innate immune signals, such as the production of TNF-α and IP-10 in macrophages, and the increased expression of costimulatory molecules and IL-12 in DCs. Even though the treatment of 10 kDa γ-PGA did not induce the significant production of cytokines (TNF-α, IP-10) and upregulation of CD40, CD80 or CD86, its treatment enhanced the significant NF-κB activation compared with PBS treatment (Fig. 3), which may induce the slight tumor growth suppression (Fig. 5). The depletion of NK, CD8 T, and CD4 T cells using anti-asialo GM1, -CD4, and -CD8 antibodies reduced the antitumor effects of 2,000 kDa γ-PGA in C3H/HeN mice challenged with K1735 tumor cells (unpublished result). Thus, our findings strongly suggest that the antitumor effects of higher molecular mass γ-PGA (2,000 kDa) are mediated by TLR4-dependent activation of APCs, NK cells, and T cells.
To our knowledge, γ-PGA is a first polymer ligand of TLR4 reported, which is distinctive from the known TLR4 ligands. Different from other TLR4 ligands to induce the antitumor activity by parenteral administration, the marked antitumor immunity was generated by oral uptake of high molecular mass γ-PGA. Because high molecular mass γ-PGA was naturally produced by B. subtilis sp. Chungkookjang, isolated from Korea fermented soybean food, it was thought to be safe for oral application. The viabilities of γ-PGA (1 mg/ml) treated cells (RAW264.7, BMDM, BMDC, and peritoneal macrophages) were more than 95% monitored by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. The toxicity study of 2,000 mg/kg in 13-week oral repeated dose did not induce any adverse effect in SD rats (unpublished result).
Oral administration of higher molecular mass γ-PGA (2,000 kDa) induced TLR4-dependent antitumor immunity, whereas treatment with lower molecular mass γ-PGA (10 kDa) did not. Only higher molecular mass γ-PGA induced TLR4 dependent innate immunity such as the activation of macrophages and the maturation of iDCs. Furthermore, the enhanced antitumor effects after γ-PGA subcutaneously administered mice was observed in wild type (C3H/HeN) but not in TLR4-defective (C3H/HeJ) mice (unpublished data). We demonstrated that the activation of intestinal DCs (Supplementary Figure 3) and the increase of CTL activity (Fig. 7c) were induced by oral administration of γ-PGA and only high molecular mass γ-PGA treated wild type DCs (C3H/HeN) enhanced the cytolytic activity of NK cells (Fig. 7a). These results might indicate that treatment of γ-PGA induce the activation of DCs via TLR4, which migrate into the regional lymph nodes, where they activate NK cells and T cells.
Our experimental results consistently indicated that higher molecular mass γ-PGA is a more potent inducer of TLR4-mediated innate immunity. A recent report showed that TLR binds with diverse exogenous and endogenous ligands, and the hydrophobicity of TLR ligands may be important for initiating TLR-mediated innate immunity [33]. Because γ-PGA is a polymer consisting of the hydrophilic amino acid, glutamate, it was not understood why high molecular mass γ-PGA is a more potent agonist of TLR4 compared to low molecular mass γ-PGA. The larger size, the polyanionic nature, and γ-isopeptide linkage of high molecular mass γ-PGA may contribute to the initiation of innate immunity. The further studies are required to understand why high molecular mass γ-PGA is better agonist for TLR4.
TLR4-mediated intracellular signaling reportedly leads to the activation of NF-κB, MAPKs, and IRF-3 [39]. The activations of NF-κB and IRF-3 were observed in 2,000 kDa γ-PGA-treated macrophages (Fig. 3). However, treatment of 2,000 kDa γ-PGA did not significantly induce the activation of ERK but JNK and p38 were activated in 2,000 kDa γ-PGA treated peritoneal macrophages. Consistent with our data, it has been reported that treatment with nanoparticles (NPs) composed of γ-PGA and l-phenylalanine ethylester induced TNF-α production by activating DCs in an ERK-independent fashion, as shown by the lack of response to an ERK inhibitor [37]. LPS treatment has been reported to induce ERK, JNK, and p38 activation in macrophages [15]. Thus, it is possible that the intracellular signaling induced by 2,000 kDa γ-PGA may differ from LPS-induced signaling, even though TLR4 is involved in both responses. Even though it is clear that the innate immunity and antitumor activity of γ-PGA is TLR4 dependent, it is still possible that γ-PGA acts as a ligand for other TLR(s) or innate immune receptor(s). Further studies will be required for us to gain a clear understanding of the mechanism(s) underlying γ-PGA-mediated innate immunity.
In summary, our results demonstrated that treatment with high molecular mass γ-PGA (2,000 kDa) initiated innate immune responses, such as iDC maturation, via TLR4 signaling in mice. Furthermore, TLR4-dependent antitumor effects were induced by the oral administration of γ-PGA (2,000 kDa) in a mouse tumor model in vivo. Because γ-PGA is safe, edible, and inexpensive polymer [11], these findings strongly suggest that γ-PGA may be a new immunomodulator targeting TLR4, and may warrant consideration as a therapeutic agent for cancer and other diseases.
Abbreviations
- γ-PGA:
-
Poly-gamma-glutamate
- MyD88:
-
Myeloid differentiation factor 88
- TLR4:
-
Toll-like receptor 4
- JNK:
-
c-Jun N-terminal kinase
- IRF-3:
-
Interferon regulatory factor-3
References
Abe K, Ito Y, Ohmachi T, Asada Y (1997) Purification and properties of two isozymes of gamma-glutamyltranspeptidase from Bacillus subtilis TAM-4. Biosci Biotechnol Biochem 61:1621–1625
Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S (1998) Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150
Aderem A, Ulevitch RJ (2000) Toll-like receptors in the induction of the innate immune response. Nature 406:782–787
Ahmed SU, Okamoto M, Oshikawa T, Tano T, Sasai A, Kan S, Hiroshima T, Ohue H, Moriya Y, Ryoma Y, Saito M, Sato M (2004) Anti-tumor effect of an intratumoral administration of dendritic cells in combination with TS-1, an oral fluoropyrimidine anti-cancer drug, and OK-432, a streptococcal immunopotentiator: involvement of Toll-like receptor 4. J Immunother 27:432–441
Akira S, Hemmi H (2003) Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett 85:85–95
Akira S, Hoshino K (2003) Myeloid differentiation factor 88-dependent and -independent pathways in Toll-like receptor signaling. J Infect Dis 187(Suppl 2):S356–S363
Akira S, Takeda K, Kaisho T (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2:675–680
Ashiuchi M, Kamei T, Baek DH, Shin SY, Sung MH, Soda K, Yagi T, Misono H (2001) Isolation of Bacillus subtilis (chungkookjang), a poly-gamma-glutamate producer with high genetic competence. Appl Microbiol Biotechnol 57:764–769
Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K (2000) Immunobiology of dendritic cells. Annu Rev Immunol 18:767–811
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Buescher JM, Margaritis A (2007) Microbial biosynthesis of polyglutamic acid biopolymer and applications in the biopharmaceutical, biomedical and food industries. Crit Rev Biotechnol 27:1–19
Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, Trinchieri G, Caux C, Garrone P (2005) A type I interferon autocrine–paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med 201:1435–1446
Gee MS, Koch CJ, Evans SM, Jenkins WT, Pletcher CH Jr, Moore JS, Koblish HK, Lee J, Lord EM, Trinchieri G, Lee WM (1999) Hypoxia-mediated apoptosis from angiogenesis inhibition underlies tumor control by recombinant interleukin 12. Cancer Res 59:4882–4889
Goriely S, Molle C, Nguyen M, Albarani V, Haddou NO, Lin R, De Wit D, Flamand V, Willems F, Goldman M (2006) Interferon regulatory factor 3 is involved in Toll-like receptor 4 (TLR4)- and TLR3-induced IL-12p35 gene activation. Blood 107:1078–1084
Guha M, Mackman N (2001) LPS induction of gene expression in human monocytes. Cell Signal 13:85–94
Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B (2003) Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424:743–748
Hoshino K, Kaisho T, Iwabe T, Takeuchi O, Akira S (2002) Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. Int Immunol 14:1225–1231
Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S (1999) Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162:3749–3752
Kaisho T, Takeuchi O, Kawai T, Hoshino K, Akira S (2001) Endotoxin-induced maturation of MyD88-deficient dendritic cells. J Immunol 166:5688–5694
Kawai T, Adachi O, Ogawa T, Takeda K, Akira S (1999) Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11:115–122
Kim TW, Lee TY, Bae HC, Hahm JH, Kim YH, Park C, Kang TH, Kim CJ, Sung MH, Poo H (2007) Oral administration of high molecular mass poly-gamma-glutamate induces NK cell-mediated antitumor immunity. J Immunol 179:775–780
Klechevsky E, Kato H, Sponaas AM (2005) Dendritic cells star in Vancouver. J Exp Med 202:5–10
Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223:77–92
Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA Jr (1998) MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 2:253–258
Muzio M, Polentarutti N, Bosisio D, Manoj Kumar PP, Mantovani A (2000) Toll-like receptor family and signalling pathway. Biochem Soc Trans 28:563–566
Okamoto M, Furuichi S, Nishioka Y, Oshikawa T, Tano T, Ahmed SU, Takeda K, Akira S, Ryoma Y, Moriya Y, Saito M, Sone S, Sato M (2004) Expression of toll-like receptor 4 on dendritic cells is significant for anticancer effect of dendritic cell-based immunotherapy in combination with an active component of OK-432, a streptococcal preparation. Cancer Res 64:5461–5470
Okamoto M, Oh EG, Oshikawa T, Furuichi S, Tano T, Ahmed SU, Akashi S, Miyake K, Takeuchi O, Akira S, Himeno K, Sato M, Ohkubo S (2004) Toll-like receptor 4 mediates the antitumor host response induced by a 55-kilodalton protein isolated from Aeginetia indica L., a parasitic plant. Clin Diagn Lab Immunol 11:483–495
Okamoto M, Sato M (2003) Toll-like receptor signaling in anti-cancer immunity. J Med Invest 50:9–24
Oppermann-Sanio FB, Steinbuchel A (2002) Occurrence, functions and biosynthesis of polyamides in microorganisms and biotechnological production. Naturwissenschaften 89:11–22
Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, Vogel SN (2001) CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol 166:574–581
Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–2088
Prodhomme EJ, Tutt AL, Glennie MJ, Bugg TD (2003) Multivalent conjugates of poly-gamma-D-glutamic acid from Bacillus licheniformis with antibody F(ab′) and glycopeptide ligands. Bioconjug Chem 14:1148–1155
Seong SY, Matzinger P (2004) Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 4:469–478
Shi S, Nathan C, Schnappinger D, Drenkow J, Fuortes M, Block E, Ding A, Gingeras TR, Schoolnik G, Akira S, Takeda K, Ehrt S (2003) MyD88 primes macrophages for full-scale activation by interferon-gamma yet mediates few responses to Mycobacterium tuberculosis. J Exp Med 198:987–997
Takeda K, Kaisho T, Akira S (2003) Toll-like receptors. Annu Rev Immunol 21:335–376
Trinchieri G (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3:133–146
Uto T, Wang X, Sato K, Haraguchi M, Akagi T, Akashi M, Baba M (2007) Targeting of antigen to dendritic cells with poly(gamma-glutamic acid) nanoparticles induces antigen-specific humoral and cellular immunity. J Immunol 178:2979–2986
Youn HS, Lee JY, Fitzgerald KA, Young HA, Akira S, Hwang DH (2005) Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J Immunol 175:3339–3346
Zhang G, Ghosh S (2001) Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest 107:13–19
Acknowledgments
We thank Dr S. Akira and Dr S. Uemastu for MyD88−/− and TLR2−/− mice, Dr J. Y. Lee and Dr Kate Fitzgerald for IRF3 luciferase plasmid, and Dr H. Kuroda for helpful advices. This work was supported by grants of the Korea Health 21 R&D Project (A050562) and National R&D Program for Cancer Control (0720510), Ministry of Health & Welfare, Republic of Korea and a grant from KRIBB Initiative program to H. Poo.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, TY., Kim, YH., Yoon, SW. et al. Oral administration of poly-gamma-glutamate induces TLR4- and dendritic cell-dependent antitumor effect. Cancer Immunol Immunother 58, 1781–1794 (2009). https://doi.org/10.1007/s00262-009-0689-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-009-0689-4