Abstract
We have developed a high-throughput screen (HTS) to search for novel molecules that can synergize with TRAIL, thus promoting apoptosis of ACHN renal tumor cells in a combinatorial fashion. The HTS detects synthetic compounds and pure natural products that can pre-sensitize the cancer cells to TRAIL-mediated apoptosis, yet have limited toxicity on their own. We have taken into account the individual effects of the single agents, versus the combination, and have identified hits that are synergistic, synergistic-toxic, or additive when combined with TRAIL in promoting tumor cell death. Preliminary mechanistic studies indicate that a subset of the synergistic TRAIL sensitizers act very rapidly to promote cleavage and activation of caspase-8 following TRAIL binding. Caspase-8 is an apical enzyme that initiates programmed cell death via the extrinsic apoptotic pathway. Thus, these TRAIL sensitizers may potentially reduce resistance of tumor cells to TRAIL-mediated apoptosis. Two representative sensitizers were found to increase levels of p53 but did not inhibit the proteasome, suggesting that early DNA damage-sensing pathways may be involved in their mechanisms of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One strategy in developing new cancer therapeutics having better toxicity profiles compared with current cytotoxic drugs is to utilize molecularly-targeted therapies that selectively target cancer cells versus normal cells and can be used in minimal doses to reduce side effects [11, 32]. Death receptor ligands held initial promise in answering this need because they trigger programmed cell death in their target cancer cells. Unfortunately, Fas ligand (FasL) and tumor necrosis factor-alpha (TNFα), two of the best-studied death ligands, have proven to be too toxic for systemic use as anticancer agents in their native forms. However, the discovery of another death receptor ligand, tumor necrosis factor-α-related apoptosis-inducing ligand (TRAIL; Apo2L) [47, 71] and its receptors [7, 8, 29, 31, 44, 45, 58, 68], has renewed interest in this area of cancer research. Active TRAIL receptors, TR1 (DR4) and TR2 (DR5) [13], are often more highly expressed on cancer cells versus normal cells [29, 44]. Inactive TRAIL “decoy” receptors TR3 (DcR1) and TR4 (DcR2) are sometimes more prevalent on the surface of normal cells [7, 44, 58]. This complex and inversely related expression pattern for TRAIL receptors may be partly responsible for the selectivity of TRAIL ligand for tumor cells over normal cells, and its ability to preferentially cause apoptotic cell death in cancer cells [18, 43, 62] that may contribute to a more favorable safety profile.
TRAIL ligand exists in two forms: as a type II membrane protein expressed on the surface of certain lymphoid cells, and as a cleaved, soluble protein that is detectable in serum [5, 6]. The biological roles of TRAIL ligand in vivo have not been fully elucidated. However, recombinant soluble TRAIL causes apoptosis in sensitive tumor cells [47, 71], but not normal cells. Therefore, various TRAIL ligand formulations have been investigated for their therapeutic efficacy as well as possible toxicity in mice, cynomolgus monkeys and humans [1, 23, 69]. In response to the poor serum stability of recombinant TRAIL in vivo [23, 69], and in an attempt to create more targeted therapeutics with fewer side effects, humanized agonistic monoclonal TRAIL antibodies were developed that have either TR1 (HGS-ETR1, mapatumumab) or TR2 (HGS-ETR2, HGS-TR2 J, lexatumumab) specificity [15, 30, 49, 67, 73]. Phases I and II clinical trials were carried out using these antibodies as single agents in patients having solid tumors such as colorectal cancer, non-small cell lung cancer, and non-Hodgkin’s lymphomas [3, 10, 21, 30, 48, 64, 67]. In general, agonistic monoclonal antibodies to TR1 and TR2 have half-lives of days in vivo, good tolerability, low toxicity and are efficacious in promoting stable disease in about a third of certain patient groups, raising hopes that TRAIL-targeted therapies may have broad clinical applicability for cancer.
Although TRAIL has been reported to successfully target certain tumor cells which are resistant to traditional chemotherapies or radiation, TRAIL resistance has also been widely documented [4, 42, 65, 75]. Indeed, many cancer cells are quite resistant to TRAIL as a single agent [2]. It is now recognized that combination chemotherapeutic (or irradiative/chemotherapeutic) regimens may provide cancer patients with a more efficacious and longer-lasting treatment option [3, 10, 21] versus traditional cytotoxic mono-chemotherapy. Thus, many tumor cells may require prior sensitization with other agents in order for TRAIL to have direct anticancer effects.
This synergistic, combinatorial approach toward treating cancer with a compound in the presence of TRAIL has been successful in animal models in vivo, and clinical trials in humans are underway [3, 10, 21]. It has been difficult to choose which agents are best for sensitizing tumor cells to TRAIL, as most sensitizers actually only cause additive effects in conjunction with TRAIL, while less often true synergistic TRAIL-sensitizing effects are observed for compounds. Many of the sensitizing agents described to-date have also been identified based on in vitro assays. Often, very high concentrations of compounds are required for TRAIL sensitization in vitro (i.e., sodium butyrate [40], resveratrol [16], cisplatin [33], bisindolylmaleimide III [34], 5-fluorouracil [25]), and it is unlikely that such high concentrations could ever be attained in vivo. Sensitizers may target TRAIL pathway molecules that may or may not be present in or relevant across multiple tumor or tissue types. Development of assays that can distinguish between synergistic and additive effects of various compounds in the presence of TRAIL would help researchers prioritize compounds for the study of TRAIL-based combination chemotherapies. In addition, this may help identify compounds that could sensitize tumor cells to TRAIL at concentrations that might realistically be achievable in vivo.
The screen for TRAIL sensitizers was carried out in ACHN renal tumor cells and evaluated 16,480 pure synthetic and natural product compounds from both commercial sources and the National Cancer Institute’s Developmental Therapeutics Program libraries. Natural product libraries were included in order to enhance the potential chemical diversity pool from which we might derive novel TRAIL sensitizers. Compounds were initially tested for their ability to reduce cell numbers at one concentration in the presence of a fixed concentration of TRAIL ligand. Confirmed hit compounds were then tested for their dose-dependent ability to reduce cell numbers across a concentration range in the presence of a fixed concentration of TRAIL ligand. In the dose–response format, individual effects of both compound and TRAIL were measured in comparison with the effect of combining the two treatments. Out of 16,480 compounds initially tested, 18 synergistic compounds were confirmed, 14 pure natural products and four synthetic compounds. A related, but somewhat different, study by Schimmer et al. [56] primarily identified synthetic FasL sensitizers in PPC prostate cancer cells. Eight of the synthetic compounds reported were found to also sensitize cells to TRAIL in a secondary assay; however, no natural product TRAIL-sensitizers were reported. Our study instead focuses on detection of synergistic TRAIL-sensitizers as the primary screen, utilizes a renal cancer cell line, and includes libraries containing both natural products and synthetic compounds, which significantly increased the structural complexity and chemical diversity of the resultant hits versus those identified in the Schimmer study. Under our conditions, TRAIL-sensitizers may exhibit some cytotoxic effects when administered alone, but at a higher concentration than was required to potentiate the effect(s) of TRAIL. This category of TRAIL-sensitizers is referred to here as synergistic-toxic. Additive-type compounds did not potentiate the effect(s) of TRAIL, and the cytotoxic effect of combining an additive-type compound plus TRAIL was the sum of the effects of the two individual agents.
This report details the development and validation of the HTS, categorization of TRAIL sensitizer hits according to their dose–response curves ±TRAIL, evidence that a subset of synergistic TRAIL sensitizers rapidly potentiates the activation of the extrinsic apoptosis pathway, plus preliminary insights into possible mechanisms of action for two representative synergistic TRAIL-sensitizers.
Materials and methods
Chemicals and reagents
Bortezomib was purchased from the National Institutes of Health Pharmacy. Sanguinarine hydrochloride was from Sigma-Aldrich. Pure compound libraries for screening were obtained from: Biomol International, L. P. (Plymouth Meeting, PA), Developmental Therapeutics Program (DTP, NCI-Frederick, Frederick, MD), Sigma-Aldrich Corp. (St. Louis, MO), and the Molecular Targets Development Program-Natural Products (MTDP-NP internal resource, NCI-Frederick, Frederick, MD). Pure natural products were obtained from the Drug Synthesis and Chemistry Branch, DTP, NCI-Frederick, (Frederick, MD). Calcium ionophore A23187, trichostatin A, actinomycin D, niclosamide, L-703,606 oxalate, and SU9516 were re-acquired from Sigma-Aldrich for follow-up assays to confirm activity. Similarly, doxorubicin was purchased from Fluka (Sigma-Aldrich); 5-iodotubercidin and MG-132 were from Calbiochem Biochemicals (EMD Chemicals, Inc., Gibbstown, NJ); and cucurbitacin D was from Chromadex, Inc. (Irvine, CA). Remaining test compounds were re-acquired from either internal MTDP resources or the NCI Natural Products Repository (NSC #s are listed in figures). Recombinant TRAIL ligand (168 amino acid TNF-homologous extracellular domain) was purchased from Peprotech, Inc. (Rocky Hill, NJ). 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT; NSC 601519) was provided by the Drug Synthesis and Chemistry Branch, DTP/NCI (Frederick, MD). JC-1 was obtained from Sigma, dissolved at 10 mg/mL in DMSO, and stored in aliquots light protected at −20°C.
Antibodies
For Western blots, the following primary antibodies were used: Anti-Cleaved Caspase-8 (Asp391) (18C8) Rabbit mAb #9496 from Cell Signaling Technologies, Inc. (Danvers, MA); Anti-Beta-actin Mouse mAb A2228 (clone AC-74) from Sigma-Aldrich (St. Louis, MO); Anti-p53 Mouse mAb (DO-1) sc-126 from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Cell culture conditions for the HTS, SRB and Western blot assays
Mycoplasma-free ACHN renal adenocarcinoma cells (National Cancer Institute, Frederick, MD) were utilized for the primary screen, dose–response testing of confirmed hits, as well as western blotting and caspase-8 activation experiments. ACHN is an adherent cell line and was cultured in the following maintenance media: red RPMI-1640 medium (Cellgro; Manassas, VA), supplemented with 10% (v/v) Hyclone defined FBS (Hyclone; Logan, UT; non heat-inactivated Defined FBS, lot #ARC26079), 2 mM l-alanyl-l-glutamine (Cellgro) or GlutaMAX™-I (Invitrogen; Carlsbad, CA; 1% v/v), 10 mM HEPES buffer (Sigma; 1%, v/v), 1X MEM non-essential amino acids (Sigma; 1% v/v), 1 mM sodium pyruvate (Sigma; 1% v/v), 55 μM β-mercaptoethanol (Gibco Invitrogen 21985-023; 0.1% v/v), and cholesterol solution (Sigma-Aldrich S5442; 0.04% v/v), without antibiotics. For screening and other test assays, clear RPMI-1640 medium without phenol red was substituted (Cellgro) and 100 U penicillin/100 μg/mL streptomycin was added (Sigma; 1% v/v). Cells were incubated in 5% CO2/saturated humidity at 37°C. ACHN cells were found to have a cholesterol requirement for uniform growth in 384-well microtiter plates and were also sensitive to cold thermal shock (data not shown). ACHN cells were maintained at sub-confluent densities.
High-throughput screening (HTS) assay development
See Fig. 1 for a graphical description of the time course used for the HTS. On the day prior to an assay, passage 14 to 25 ACHN cells were fed fresh red medium in the morning and harvested after ≥6 h into clear test medium. Cells were seeded at 3,500 cells per well into clear 384-well tissue culture treated plates (BD Biosciences; San Jose, CA) in 40 μL total volume of clear test medium using a sterilized μFill dispenser (BioTek Instruments, Inc.; Winooski, VT). Parallel sets of plates (two per library plate, designated “−TRAIL” and “+TRAIL”) were placed in the incubator without stacking and were left to attach to wells overnight (16–20 h).
The next day, test compounds and controls were diluted to 10× final concentration in clear test medium and 5 μL per well was added to each set of assay plates using a Biomek FX-384 equipped with a multichannel pod and Span-8 head (Beckman Coulter, Inc.; Fullerton, CA). The following controls were included per individual assay plate: DMSO background (blank); 40 nM bortezomib (final concentration; positive control for +TRAIL plate), and 5 μM sanguinarine chloride (final concentration; positive cytotoxicity control for −TRAIL plate), containing %DMSO matched to test sample content. Plates were returned to the incubator for 4 h ± 30 min. After pre-sensitization, 5 μL of clear test medium was added per well to the entire −TRAIL plate and 5 μL of TRAIL (40 ng/mL final concentration) in clear test medium was added per well to the entire +TRAIL plate. Plates were returned to the incubator and incubated overnight (20–21 h).
On the final day, viable cell numbers were assessed using the XTT assay [57]. Plates were allowed to develop for 4–5 h before being read for absorbance at 450 nm on a Wallac Victor2 1420 multilabel HTS counter plate reader (Waltham, MA).
Calculations and statistical analysis of HTS results
Percent reduction in cell numbers was calculated for test samples and positive controls based on either untreated (DMSO-only; −TRAIL plate) cells or TRAIL-only treated (+TRAIL plate) cells as negative controls. Percent reduction in cell number was calculated as follows:
-
−TRAIL plate:
-
% Reduction in cell number (RC) = [(Avg AbsSolvent-only cells − AbsSample)/Avg AbsSolvent-only cells] × 100,
-
and
-
+TRAIL plate:
-
%RC = [(Avg AbsTRAIL-only cells − AbsSample)/Avg AbsTRAIL-only cells] × 100, where Avg Abs = average absorbance and Abs = absorbance.
Two separate Z′-factor quality control parameters were calculated, for −TRAIL and +TRAIL plates, according to the method of Zhang et al. [74]:
-
Z′-factor (−TRAIL) = 1 − [3(Std dev %RCCells (−TRAIL) + Std dev %RCSanguinarine chloride (−TRAIL))/|(Avg %RCSanguinarine chloride (−TRAIL) − Avg %RCCells (−TRAIL))|]
-
and
-
Z′-factor (+TRAIL) = 1 − [3(Std dev %RCCells (+TRAIL) + Std dev %RCBortezomib (+TRAIL))/|(Avg %RCBortezomib (+TRAIL) − Avg %RCCells (+TRAIL))|], where Std dev = standard deviation of the indicated %RC values and Avg %RC = the average of all %RC values for that particular control.
Each plate was subject to two quality control cutoffs to ensure reliability of data: (1) cell controls (either DMSO-only or TRAIL-only) must be ≥1.2 absorbance at 450 nm and (2) calculated Z′-factors for each plate must be ≥0.4 (maximum = 1.0).
Initial hits were retested in quadruplicate at the same concentration used in screening to confirm their activity. Confirmation of activity was based on the consistency of the best three out of four test values for each set of results (−TRAIL and +TRAIL), to allow for random variation across plates.
SRB protein stain dose–response activity of hits ±TRAIL
As a second measure, in order to eliminate potential metabolic effects (XTT measures cell metabolism via reductase activity), SRB protein staining was used as an alternative endpoint for the dose–response assays. To determine probable synergistic activity of confirmed hits with TRAIL, a dose–response assay was carried out using the SRB protein staining protocol for cytotoxicity [60, 66], using the same cell line and similar conditions as those employed for the HTS (see Fig. 1). Controls were combined on each assay plate and included: SRB background absorbance (no cells), untreated (DMSO-only) cells, TRAIL-only cells, cytoxicity positive control −TRAIL (sanguinarine hydrochloride; one concentration), and synergy positive control +TRAIL (bortezomib; one concentration plus a dose–response range).
Briefly, compound dilutions were prepared in clear test medium and added to cells. A similar protocol and time course to the HTS was employed, except that TRAIL was added to a final concentration of 20 ng/mL and the final overnight incubation took place for 24–25 h. Cells were fixed to the bottom of the wells by direct addition of 1:1 volume (50 μL) of ice cold 20% (w/v) trichloroacetic acid (TCA) solution. Plates were incubated at 4°C for 30–60 min to fix cells and then plates were aspirated and rinsed 5× with deionized water using an Embla 96/384 plate washer (Molecular Devices, Sunnyvale, CA), and then allowed to dry at room temperature. Wells were stained for total protein content using 30 μL of SRB (1 g/L in 1% acetic acid) for 1 h at room temp. SRB was removed with a wrist flick and plates were rinsed with 1% acetic acid until excess dye was removed. Then plates were tapped onto paper towels and allowed to dry at room temperature. Dye was re-solubilized by addition of 10 mM TRIZMA base solution (30 μL) and re-suspended on a rotary shaker for a few min before reading the plates for absorbance at 520 nm using a Safire2 plate reader (Tecan Group Ltd., Männedorf, Switzerland).
Calculation of SRB dose–response results and statistical measures
After subtraction of background absorbance (SRB, no cells) from raw data, percent reduction in cell number values for samples were calculated in relation to solvent-only treated cells and this time the effect of the TRAIL reagent alone was determined:
-
%RC TRAIL = [(Avg AbsSolvent-only cells − AbsTRAIL-only cells)/Avg AbsSolvent-only cells] × 100.
-
Z′-factors were calculated similarly to the HTS, except that the controls for the (+TRAIL) portion of the assay were: untreated cells = cells (−TRAIL) and positive control = cells with bortezomib (+TRAIL). Calculations were normalized to untreated cells (−TRAIL) in all instances.
The average value for %RC by TRAIL reagent alone (per plate) was used to generate a “predicted” curve for the expected additive activity of test compounds +TRAIL using the following calculation:
-
Avg %RCCompound(−TRAIL) + Avg %RCCells(+TRAIL) = Predicted %RCCompound(+TRAIL)
Results were plotted in SigmaPlot as three %RC curves per compound’s dose–response: (1) compound/extract effect −TRAIL (cytotoxicity), (2) actual compound/extract effect +TRAIL (synergy effect), and (3) predicted effect of compound/extract +TRAIL (expected additive effect).
Luciferase assay for caspase-8 enzyme activation
The Caspase-Glo™ 8 Assay (Promega Corporation, Madison, WI; G8200) was performed, according to the manufacturer’s instructions, on selected hits from the TRAIL HTS. (Note: Kits were used that contained the proteasome inhibitor MG-132 to help reduce nonspecific background signal contributed by proteasome activity present in the cell lysates.) On the day prior to the assay, passage 14 to 25 ACHN cells were plated at 7,000 cells/well in 25 μL clear test medium in white luminescence tissue culture-treated 384-well plates (Corning #3704). Separate plates were used per time point and cells were allowed to attach to plates at 37°C overnight (16–20 h).
The next day, compounds or DMSO solvent controls were added at 5 μL/well. Plates were returned to the incubator for 4 h. Clear test media blank or TRAIL (40 ng/mL) was added to cells at 5 μL/well. Plates were returned to the incubator for the appropriate period of time while any zero hour plates were processed immediately. For processing, plates were removed from the incubator, allowed to cool to room temperature for 10 min, enzyme controls were added if appropriate, and then 35 μL of the Glo lysis buffer was added per well (1:1, v:v). Plates were shaken on an orbital rotator for 30 s and then covered with aluminum foil and the luminescence signal was allowed to develop at room temperature for 2–4 h. Plates were read for luminescence on a Wallac Victor2 1420 multilabel HTS counter. When background signal had decayed to a stable state, that data set was used (usually 3 h).
For the time course experiment, 10 units/well final concentration of purified caspase-8 enzyme (Biomol International, L. P., Plymouth Meeting, PA; SE-172) and 40 μM of the pan-caspase inhibitor Z-VAD-FMK (Biomol; P-416) were used in various combinations as controls, either in the presence or absence of cells. Enzyme dilution buffer contained 10 mM HEPES, 0.1% Prionex reagent, and was adjusted to pH 7.4. Controls were added to the plate after the 10 min cooling period and just prior to addition of the Glo lysis reagent. See Fig. 1 for a graphical description of the time course used for this assay.
Assessment of changes in mitochondrial membrane potential
The ratiometric fluorescent dye JC-1 [61] was used to monitor relative mitochondrial membrane potential. Cells were plated at 7,000 cells per well (25 μL/well in black wall, clear bottom 384-well plates) in clear test medium, allowed to attach overnight, then treated for 4, 8, or 24 h with compounds (10 μM final concentration, or DMSO vehicle control) or 4 h with compound followed by addition of TRAIL (40 ng/mL final) and additional incubation for 4 h. Immediately before use, JC-1 stock (10 mg/mL) was diluted to 0.2 mg/mL in DMSO then further diluted to 10 μg/mL in phosphate-buffered saline (PBS). JC-1 in PBS was immediately added to assay plates containing an equal volume of medium to give a final concentration of 5 μg/mL. After incubation for 30 min at 37°C, cells were washed 6 times with PBS followed by addition of 50 μL PBS per well. Fluorescence intensity was determined using the Tecan Safire2 plate reader fluorescence plate reader (bottom read mode); 488 nm excitation, 538 nm (green) and 597 nm (red) emission. The fluorescence ratio was taken as a measure of mitochondrial potential [61].
Western blots for caspase-8 proteolytic processing and p53 expression
ACHN cells were seeded into 6-well tissue culture plates at 59.5 × 104 cells/well in clear test medium and the next day compound was added to a final concentration of 10 μM. Cells were sensitized for 4 h followed by addition of TRAIL to 40 ng/mL final concentration. Cells were harvested in lysis buffer at short intervals after addition of the TRAIL reagent using ~165 μL per well. Lysis buffer contained: 50 mM Tris–Cl (pH 8.0), 300 mM NaCl, 5 mM ethylenediamine tetraacetic acid (EDTA), 0.5% Triton X-100, 40 μM Z-VAD-FMK, plus one mini-complete protease inhibitor tablet (Roche, Mannheim, Germany; 04 693 124 001) or 100 μL Halt Protease Inhibitor Single-Use Cocktail (Pierce Biotechnology, Inc., Rockford, IL; 78430) per 7 mL buffer. Lysates were clarified by centrifugation at 4°C for 15 min at 15,000 rpm and protein content normalized using the bicinchonic acid (BCA) assay (Pierce Biotechnology, Inc.).
Lysates were run under reducing conditions at 20 μg total protein per lane, in NuPAGE 4-12% Bis–Tris gels (1.0 mm × 12 well; Invitrogen), using MES SDS running buffer and added antioxidant in the upper chamber. Protein bands were transferred to PVDF membranes using the manufacturer’s transfer buffer containing added antioxidant (Invitrogen).
PVDF membranes (0.2 μm pore size) were blocked with 0.1% milk in TBS with 0.5% Tween 20 (0.2 μm filtered), washed briefly 2× with TBS + 0.5% Tween 20 then incubated with the primary antibody in TBS + 5% BSA + 0.1% Tween 20 (0.2 μm filtered) overnight. The blots were then washed 4× with TBS + 0.5% Tween 20 for 15 min each. Goat anti-rabbit HRP (Pierce Biotechnology, Inc.) was added in blocking buffer at 1:500 and incubated with the blots at room temperature for 45 min, the blots were washed 6× with TBS + 0.5% Tween 20 for 15 min each, developed with Pierce SuperSignal West Femto Maximum Sensitivity Substrate, and then exposed to Kodak BioMax MR film.
Long-term survival of renal cancer cell lines
ACHN, A498, and UO31 renal cancer cells were maintained in complete red RPMI-1640 medium and plated in clear test medium at 2.5 × 104 cells/well (ACHN, UO-31) or 5.0 × 104 cells/well (A498) in Costar 24-well plates. Cells were allowed to attach overnight. The next day, compounds were added, followed by medium blank or TRAIL (500 ng/mL final concentration; Peprotech) 3 h later. Cells were incubated with compound +TRAIL overnight. The following day, plates were washed 2× with warm serum-free/additive-free RPMI-1640 medium and then re-fed with 2 mL/well complete growth medium. Cells were returned to the incubator for 5 days to allow for recovery and expansion. On day seven, plates were washed with 2 mL warm PBS and then fixed in 100% methanol. Plates were dried overnight and stained the following day with Crystal Violet for 10 min, washed with water, and allowed to dry. Surviving tumor cells could then be visualized following the staining.
Proteasome inhibition assay
The Proteasome-Glo™ Chymotrypsin-Like Cell-Based Assay (G8661) from Promega was used according to the manufacturer’s instructions. Briefly, ACHN cells were plated at 1 × 105 cells/mL at 50 μL/well in 96-well white luminescence and clear tissue culture plates and allowed to attach overnight at 37°C. The following day, compounds were added as 4× stock solutions to 1, 5, and 20 nM (bortezomib and antibiotic M259) or 25, 50, and 100 nM (cyanocycline A) final concentrations. Compounds were incubated either 4 h or overnight in parallel plates for concurrent evaluation of growth inhibition via MTS (metabolic activity) measurement. Plates for proteasome inhibition evaluation were pre-cooled, Proteasome-Glo reagent was added in 100 μL volume per well, plates were covered and mixed at 700 rpm for 2 min, and then allowed to develop at room temperature for 10 min prior to reading on a luminometer. MTS plates were read for absorbance after 1.5 h color development at 37°C. Proteasome inhibition results were corrected for cell growth inhibition by the compound treatments.
Definition of synergy
A synergistic TRAIL-sensitizer was defined as a compound that potentiates the effect(s) of TRAIL such that the actual combined inhibitory effect on tumor cells of the compound plus TRAIL is at least 20% greater than the sum of their individual measured effects in a dose–response context using a fixed concentration of TRAIL ligand.
Results
TRAIL HTS development, reproducibility and validation
ACHN cells are not sensitive to recombinant TRAIL ligand at concentrations of up to 10 μg/mL (results not shown), but they can be sensitized to 40 ng/mL of TRAIL by pre-exposure to certain chemical sensitizers, including the proteasome inhibitor bortezomib (Table 1). We screened pure compounds in the presence (“+TRAIL”) and absence (“−TRAIL”) of 40 ng/mL recombinant TRAIL ligand and selected compounds as hits which exhibited low cytotoxicity by themselves, but significantly reduced numbers of tumor cells when combined with TRAIL. In the HTS, bortezomib served as the positive control +TRAIL. Bortezomib (40 nM) routinely reduces cell number by ~20–40%, whereas in the presence of 40 ng/mL TRAIL, cell numbers are reduced by ~75–85%. Order of addition experiments confirmed that sensitization to TRAIL-mediated growth inhibition was maximal when cells were pretreated with a chemical sensitizer followed by recombinant TRAIL ligand, rather than using simultaneous or reverse-order addition of reagents (data not shown). A presensitization lead time of 4 ± 0.5 h was sufficient for compounds to sensitize cells to the effects of TRAIL (results not shown) when cells were continuously exposed to both agents for 20 h after adding TRAIL. Sanguinarine hydrochloride alone at 5 μM reduces cell numbers by 75–85% and was used as the cytotoxic positive control for the −TRAIL portion of the HTS. The ACHN cells’ response in the TRAIL HTS was somewhat variable depending on the lot of FBS (data not shown), and therefore one lot of FBS was utilized throughout the entire screen to ensure uniform response of the cells. Additionally, ACHN cells were found to 1) have a cholesterol requirement for uniform growth in 384-well plates and 2) be sensitive to cold thermal shock (data not shown). These limitations were overcome by supplementing red growth and clear test media with cholesterol and handling single plates one at a time without stacking.
Pure compounds were assayed in single wells at a final concentration of 10 μM. Based on initial results, TRAIL hits were defined as compounds reducing cell numbers by ≤30% in the absence of TRAIL and ≥75% in the presence of TRAIL, as measured by XTT absorbance value changes. The effect of TRAIL alone at 40 ng/mL was found to be variable, so the +TRAIL screening plate was normalized to TRAIL-only treated cells.
Reproducibility of the TRAIL HTS was investigated by repeated testing of one chemical library that contained four hit compounds (MTDP-NP). The library was tested in triplicate sets of plates 1 day, and then hits were individually “cherry-picked” and retested in quadruplicate within one set of plates on a separate day. Intraday, intraplate and interday statistics were calculated (Table 2). Intraday, intraplate and interday %CVs (not shown) for the +TRAIL portion of the assay were all <5% when percent reduction in cell number (%RC) activity was high, indicating good reproducibility of the assay under these conditions. Intraday Z′-factors for the −TRAIL and +TRAIL portions of the HTS were 0.55–0.88 and 0.55–0.58, respectively, indicating an excellent ability of the assay to detect both cytotoxic and TRAIL-sensitizing compounds and extracts. We observed that satisfactory Z′-factors (the range across the entire screen was 0.40–0.88) usually correlated with an average XTT absorbance value of ≥1.2 for the untreated cell controls.
During the course of screening, we discovered some pure compounds that have previously been reported to sensitize cancer cells to TRAIL. Some of these compounds are presented in Table 1, and their mechanisms of action are listed.
SRB dose–response profiles of confirmed hits
Using a dose–response assay to follow up on confirmed hits from the HTS, we were able to define four categories of compounds: S (synergistic), S-T (synergistic-toxic), A (additive), and I (inactive). A synergistic TRAIL-sensitizer can be defined as a compound that potentiates the effect(s) of TRAIL such that the combined inhibitory effect on tumor cells of the compound plus TRAIL is significantly greater than the sum of their individual effects. Synergistic TRAIL-sensitizers may exhibit some cytotoxicity when administered alone, but at a higher concentration than that required to potentiate the effect(s) of TRAIL. This category of TRAIL-sensitizers is referred to as synergistic-toxic. In contrast, additive TRAIL-sensitizers do not potentiate the effect(s) of TRAIL, and the cytotoxic effect of combining an additive compound plus TRAIL is the sum of the two individual effects. Figure 2 shows examples from each category. Synergistic compounds exhibited ≤40% reduction of cell number at most concentrations tested −TRAIL and synergistic-toxic compounds exhibited cytotoxicity which exceeded this level but still had a significant differential activity ±TRAIL at most concentrations at the high end of the dose–response curve. In this way, we prioritized categories of hits for further investigation, focusing mainly on the S/S-T group. From the initial 16,480 pure compounds tested, 70 confirmed hits were identified and, of the 36 pure compounds we were able to re-acquire, we identified 18 pure compounds as S/S-T: antibiotic M259 (NSC 51954), cyanocycline A (NSC 349644), mithramycin A (NSC 24559), tetrocarcin A (NSC 333856), cucurbitacin B (NSC 49451), MG-132, cucurbitacin D, trichopolyn B (NSC 301460), triangulyne A (NSC 693001), actinomycin D, 5-iodotubercidin, mitomycin derivative T58 (NSC 123114), compound 99A145A (uncharacterized triangulyne), triangulyne G (NSC 693007), calcium ionophore A23187, doxorubicin, niclosamide, and marcellomycin (NSC 265211).
SRB dose–response profiles of selected hits ±TRAIL. S-T synergistic-toxic, S synergistic, A additive, I inactive. Ability of compounds to activate caspase-8 enzyme and perturb JC-1 readout of the mitochondrial membrane potential in the presence of TRAIL is represented by ±, respectively; nd not determined
Caspase-8 enzyme activation by synergistic TRAIL sensitizers
Of the 18 S/S-T hit compounds listed above, the following twelve compounds (10 μM) activated caspase-8 in the presence, but not absence, of TRAIL (40 ng/mL): antibiotic M259, cyanocycline A, mithramycin A, tetrocarcin A, cucurbitacin B, MG-132, cucurbitacin D, trichopolyn B, triangulyne A, actinomycin D, 5-iodotubercidin and mitomycin derivative T58 (results not shown; see Fig. 1 for a summary).
Modulation of mitochondrial membrane potential by synergistic TRAIL sensitizers
Of the 12 S/S-T hit compounds that activated caspase-8 only in the presence of TRAIL (Fig. 1), two compounds altered the mitochondrial membrane potential (MMP), as measured by the JC-1 assay, in the presence but not absence of TRAIL: cucurbitacin D and trichopolyn B. Four compounds were not tested in this assay. Since compounds that activate the intrinsic apoptosis pathway can act via disruption of the mitochondrial membrane potential, this result suggests that these compounds may be acting via a TRAIL-dependent, intrinsic mechanism(s) of action. Six compounds had no effect on MMP, either with or without TRAIL present: antibiotic M259, cyanocycline A, mithramycin A, tetrocarcin A, cucurbitacin B, and MG-132, suggesting that these compounds may act mainly via the extrinsic apoptosis pathway. TRAIL alone did not have a significant effect on MMP after 4 h (94.8% of control) or 24 h (119.1% of control).
Effect of two synergistic TRAIL sensitizers on long-term survival of renal cancer cell lines
M259 and cyanocycline A inhibited long-term survival of ACHN, A498 and UO31 renal cancer cells at 10 and 40 nM, respectively (Fig. 4), in the presence, but not absence of TRAIL. These results support our initial characterization of cyanocycline A and M259 as synergistic-toxic TRAIL sensitizers, and also suggest that these compounds are predominantly cytotoxic, rather than cytostatic, in the presence of TRAIL.
Caspase-8 activation and proteolytic cleavage time course for two synergistic TRAIL sensitizers
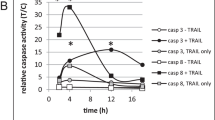
As antibiotic M259 and cyanocycline A had potent dose–response activity as S-T hits (Fig. 2), a short time course of action for these compounds before and after addition of TRAIL was examined to determine whether they were causing both rapid enzyme activation and proteolytic cleavage of caspase-8. Because the HTS used a long time course that likely allowed for activation of multiple downstream and possibly non-extrinsic pathways (see Fig. 1), we wanted to investigate whether the HTS was selecting for a subset of compounds that activated the extrinsic pathway earlier in time. Figure 3 illustrates the ability of antibiotic M259 and cyanocycline A to activate and cleave caspase-8 at 3 and 2 h, respectively, after presensitization (4 h) of cells followed by addition of TRAIL and subsequent observation (0.5–4 h post-TRAIL addition; up to 8 h total observation).
Time course experiments on antibiotic M259 (a) and cyanocycline A (b). Top Western blots show the appearance of the p43/41 fragment of cleaved caspase-8 and the table (c) illustrates the enzymatic activation of caspase-8, using a luminescent substrate for the readout and equivalent treatment conditions in 384-well plates. ACHN cells were treated with compound alone (10 μM) for 4 h and then TRAIL was added to a final concentration of 40 ng/mL; cells were measured for caspase-8 cleavage or activation at intervals after the addition of TRAIL. Treatment conditions included: 1 untreated cells; 2 compound alone, 8 h; 3 TRAIL alone, 1 h; 4 TRAIL alone, 4 h; 5 compound + TRAIL, 4 h + 1 h; 6 compound + TRAIL, 4 h + 1.5 h; 7 compound + TRAIL, 4 h + 2 h; 8 compound + TRAIL, 4 h + 3 h; 9 compound + TRAIL, 4 h + 4 h
p53 expression time course for antibiotic M259 and cyanocycline A
Both M259 and cyanocycline A caused a significant increase in p53 protein levels by 1 h in ACHN cells treated with 10 μM compound (Fig. 5). Protein levels appear to slowly and steadily increase through 8 h in cells exposed to either compound.
Effect of antibiotic M259 and cyanocycline A on the proteasome
Neither M259 nor cyanocycline A inhibited proteasome activity in ACHN cells at 1, 5, and 20 nM or 25, 50, and 100 nM, respectively, at either 4 h or overnight exposure (Fig. 5). However, the proteasome inhibitor bortezomib gave a concentration-dependent inhibitory response over 1, 5 and 20 nM under the same conditions, indicating that the two test compounds do not appear to be acting as proteasome inhibitors.
Discussion
The focus of the current study was on the development of a high-throughput screening (HTS) assay to detect compounds that are relatively nontoxic as single agents but significantly reduce tumor cell number in the presence of the TRAIL death receptor ligand. It was beyond the scope of the current study to test multiple dose combinations of each compound-TRAIL combination screened (i.e., isobologram analysis). We identified 70 confirmed active pure compounds, out of 16,480 initially tested, and carried these forward into subsequent assays. Of this group of 70 confirmed hits, we could re-acquire only 36 due to resupply issues and these were tested for their dose–response activity. Of the 36 re-acquired compounds, 18 were found to be synergistic or synergistic-toxic in combination with TRAIL ligand, 11 were additive with TRAIL, and the remaining seven were inactive. Of the 18 synergistic or synergistic-toxic TRAIL sensitizers found, 14 were pure natural products and four were synthetic compounds, confirming the utility of including natural product libraries in our screening effort.
In the HTS, several pure compounds were discovered that exhibited a strong dose-dependent reduction of tumor cell numbers in the presence, but not absence, of TRAIL. Many previously reported TRAIL-sensitizers have not been characterized as to whether they have synergistic or additive effects with TRAIL on tumor cells in vitro (i.e., resveratrol [16], cisplatin [33], and bisindolylmaleimide III [34]). Therefore, we decided to characterize the types of TRAIL sensitizers we found in our HTS. Some of our synergistic TRAIL sensitizer hits (i.e., antibiotic M259, cyanocycline A, and mithramycin A) exhibited activity in the sub-μM range in the presence of TRAIL, suggesting that these TRAIL-sensitizers may be reasonably expected to produce in vivo effects at physiologically achievable serum concentrations.
The synergistic or synergistic-toxic TRAIL sensitizers were observed to have a relatively rapid mechanism(s) of action that resulted in apparent cell death after 4 h presensitization with compound followed by 20–25 h co-treatment with TRAIL ligand. Six of these compounds (antibiotic M259, cyanocycline A, mithramycin A, tetrocarcin A, cucurbitacin B, and MG-132) exhibited a synergistic dose–response effect on reducing cell numbers in the presence of a fixed dose of TRAIL ligand, and activated caspase-8 only in the presence of TRAIL within a short time course (4 h post-treatment with TRAIL). They also did not affect the MMP within the same time frame, suggesting that this group of compounds may specifically and rapidly act to enhance the extrinsic, but not intrinsic, apoptosis signaling pathway. Longer-term tumor cell survival studies showed little colony re-growth present in wells treated with the combination of antibiotic M259 or cyanocycline A plus TRAIL (Fig. 4), indicating that these compound-TRAIL combinations were cytotoxic rather than cytostatic. This observation may be important in the context of preventing emergence of TRAIL resistance in tumors treated with TRAIL sensitizers plus TRAIL.
Long-term survival of a ACHN, b A498, and c UO31 renal cancer cells after treatment with cyanocycline A or antibiotic M259 ± 500 ng/mL TRAIL. Compounds were added, followed 3 h later by TRAIL, and incubated overnight. The following day, compounds were washed off and fresh medium was added. Cells recovered for 5 days before being fixed and stained for analysis
We chose a caspase-8 activation assay as an immediate downstream assay from the SRB dose–response evaluation because caspase-8 enzyme activation is the earliest point at which one can functionally measure an increase in activation of the extrinsic apoptosis pathway [34]. Also, it is possible that the synergistic TRAIL-sensitizers plus TRAIL can activate pathways that converge at the level of caspase-8 activation [46]. Surprisingly, none of the compounds tested significantly increased caspase-8 activity in the absence of TRAIL. In the Western blot experiments, observation of cells immediately before harvesting indicated that cells treated with compound +TRAIL were rounding up off the plate and exhibited shrinking cytoplasm by 4 h post-addition of TRAIL (data not shown). This time course is faster than most researchers report for cells undergoing intrinsically mediated apoptosis [72], suggesting that our screen may be selecting for hits that target the extrinsic pathway. Rapid proteolytic processing of caspase-8 (within ~30–60 min) has been observed in the context of anoikis (cell detachment-induced apoptosis) [17], and treatment of cells with antibiotic M259 for 4 h followed by TRAIL resulted in significant numbers of cells detaching from the plate within the 4 h post-TRAIL period observed (data not shown). This suggests that anoikis may be one possible mechanism of action by which some synergistic TRAIL-sensitizers act to cause cell death.
Cleavage of caspase-8 into 43/41, 18, and 10 kDa fragments is reported to correlate with simultaneous functional activation of this enzyme [26, 59]. Therefore, we expected to observe simultaneous cleavage and enzymatic activation of caspase-8 by the representative TRAIL sensitizers antibiotic M259 and cyanocycline A over a short time course ±TRAIL. The caspase-8 enzyme activation assay may not necessarily be definitive for caspase-8 alone [12], as it could include a substrate cleavage contribution from activated caspase-3. Due to this possibility, we chose to include results from the more specific western blot evaluation of caspase-8 cleavage to corroborate results from the enzyme assay. We found that cleavage of caspase-8 did appear to correlate with its functional enzymatic activation (Fig. 3). After 4 h presensitization with M259 followed by 3 h TRAIL, average treatment/control luminescence values from the caspase-8 enzyme activation assay were as follows: TRAIL alone = 1.87; M259 alone = 0.67; M259 + TRAIL = 5.76. These results indicate a significant (i.e., more than additive) increase in substrate cleavage at the 3 h post-TRAIL time point for M259. The corresponding protein blot indicates a strong band present at 41/43 KDa, representing cleaved caspase-8 protein, at the same time point. In the case of cyanocycline A, activation and cleavage of caspase-8 appear to occur slightly earlier. Enzyme activation values for cyanocycline A at the 2 h post-TRAIL time point are as follows: TRAIL only = 2.49; cyanocycline A = 0.37; cyanocycline A + TRAIL = 6.94. A strong band for cleaved caspase-8 appears in the corresponding blot at 2 h post-addition of TRAIL. Although experimental differences between the two types of assays (6-well plates for protein lysates versus 384-well plates for the enzymatic activation experiment) could introduce minor amounts of variability to the results, a trend is observed for the time course of caspase-8 enzyme activation to correlate with caspase-8 protein cleavage. Both M259 and cyanocycline A appear to affect caspase-8 at very early time points after TRAIL addition.
Of the six synergistic TRAIL sensitizers, it is interesting to note that five are reported to target DNA and/or RNA in one manner or another: antibiotic M259 (DNA and RNA synthesis inhibition) [53], cyanocycline A (DNA and RNA synthesis inhibition; DNA-binding) [19, 20], mithramycin A (DNA fragmentation; DNA minor groove binding) [27, 28], 23,24-dihydrocucurbitacin B (closely related to cucurbitacin B; causes DNA fragmentation) [72] and tetrocarcin A (DNA synthesis inhibition; DNA fragmentation) [36, 39], suggesting that nucleic acid oxidation-, damage-, or synthesis-sensing mechanisms may feed into the TRAIL signaling pathway at early time points. This insight may lead to further exploration of pathways involving RNA/DNA synthesis inhibition, reactive oxygen species generation, p53, p21, and CDK regulation and their potential interaction(s) with the TRAIL death receptor signaling system.
p53 is a tumor suppressor that can be rapidly activated in response to DNA damage or other chemical insults to a cell, such as the generation of reactive oxygen species, and its prolonged activation can result in programmed cell death [52]. Increase in levels of p53 protein expression in ACHN cells by selected hit compounds would be suggestive of the involvement of DNA damage-related pathways in their mechanism(s) of action. Both antibiotic M259 and cyanocycline A increased levels of p53 in the absence of TRAIL, beginning at 1 h and peaking at or after 8 h in cells (Fig. 5).
In the course of its turnover in a cell, p53 is polyubiquitinylated, which acts as a tag to shunt it into the proteasome for proteolytic inactivation and disposal. Inhibiting the proteasome would be expected to indirectly increase levels of p53 by prohibiting its degradation, giving the same result as one might see for mechanisms acting upstream to directly increase p53 levels. Therefore, we also tested M259 and cyanocycline A for their ability to inhibit the proteasome. Figure 5 illustrates that, even at extended time points as were used in the screening assay, M259 and cyanocycline A did not inhibit the proteasome, whereas bortezomib, a known proteasome inhibitor, robustly inhibited proteasome activity across an equivalent concentration range. Taken in context, these two results suggest that both M259 and cyanocycline A act to rapidly induce an increase in p53 via an upstream and early mechanism that potentially involves DNA damage, generation of reactive oxygen species, or another target(s) that causes an immediate feedback signal into the p53 pathway, rather than by prohibiting the degradation of p53 protein via the proteasome. Further studies are needed to characterize the exact mechanisms of action of M259 and cyanocycline regarding how the p53 and caspase-8 pathways may or may not be interacting to sensitize resistant cells to the effects of TRAIL.
The rapid activation of caspase-8 by the DNA/RNA-inhibitory compounds mentioned above suggests the involvement of multiple and simultaneously occurring mechanisms that converge to cause rapid cell death in addition to direct nucleic acid effects. In fact, cyanocycline A is reported to inhibit both RNA and DNA synthesis with IC50 values of ~0.02 and ~0.2 μg/mL, respectively, after treating cells for only 2 h [19]. Cyanocycline A was active at 100 nM (~0.04 μg/mL) in the presence of TRAIL in our assays. Interestingly, M259 has also been reported to inhibit the incorporation of radio-labeled guanine into both RNA and DNA after 6 h treatment of cells [53]. Additionally, 23,24-dihydrocucurbitacin B (related to cucurbitacin B) causes DNA fragmentation in cells treated with 3.6 μM for 6 h [72]. The inhibition of nucleic acid synthesis may occur via the targeting of RNA and DNA polymerases, an enzymatic effect that can be expected to occur on a faster time scale versus genomic regulatory effects resulting from accumulation of DNA damage. During assay validation we discovered several other known nucleic acid synthesis inhibitors that also synergized with TRAIL (Table 1): actinomycin D, doxorubicin, mithramycin A, and mitomycin derivative T58, supporting this hypothesis. Results presented in Fig. 4 suggest that these nucleic acid-inhibitory agents sensitize other cancer cell lines besides ACHN to TRAIL-mediated killing. If these results are also found for additional DNA-acting agents tested across various tumor cell lines in the presence of TRAIL, it may imply that certain established DNA-targeting chemotherapies will find new utility as TRAIL sensitizers at lower dosing regimens than are needed to cause their DNA-mediated effects. In fact, two antineoplastic DNA synthesis inhibitors, gemcitabine and cisplatin, are presently being studied for their effects in combination with TRAIL in humans [3, 10, 21].
Further studies on these DNA-acting agents are necessary to confirm their exact targets, such as RNA and DNA polymerase, cell cycle regulatory proteins, etc., plus non-DNA-related mechanisms such as induction of endoplasmic reticulum and/or mitochondrial stress responses and changes in membrane dynamics of DR4/DR5 receptors such as their oligomerization, endocytosis and signaling. It will be important to understand whether inhibiting these or additional targets would provide signaling feedback that rapidly activates caspase-8 in the TRAIL pathway. However, non-nucleic acid-inhibiting TRAIL-synergizing hits (proteasome inhibitor MG-132, triangulyne polyacetylenes) were also discovered in our HTS, suggesting that there exist additional unique and novel-acting chemical classes of TRAIL sensitizers.
Sensitivity to TRAIL may be governed by an apoptotic signaling threshold, or set point [46, 63]. In this case, one cannot expect to overcome TRAIL resistance in tumor cells that do not possess functional TRAIL pathway signaling machinery. However, in cells where the signaling is present, but attenuated via regulatory mechanisms, it may be possible to re-sensitize them to TRAIL by pre-administering compounds that enhance death receptor signaling. When administered in conjunction with TRAIL, TRAIL synergizers may be considered as potential combinatorial therapeutics. The efficacy and safety of TRAIL synergizer-TRAIL regimens are currently being evaluated in clinical trials, with results pending [3, 10, 21]. The clinical success of synergistic multi-drug therapies is dependent on three conditions: (1) the careful choice of individual treatments one wishes to combine, (2) the use of optimized doses, and (3) selecting the proper timing of administration(s) [50]. From a drug discovery point of view, it is desirable to develop synergistic assays that properly bias the lead output toward the subset of compounds that will be useful as combinatorial therapies. Dosing regimens and toxicity of therapeutic protocols utilizing TRAIL sensitizers is a research area that is only beginning to be explored and one can expect many new insights to emerge over time. The availability of new compounds having potentially novel mechanisms of action will also contribute to an increased understanding of the biological pathways that contribute to TRAIL-mediated apoptosis.
Abbreviations
- TRAIL:
-
Tumor necrosis factor-α-related apoptosis-inducing ligand
- HTS:
-
High-throughput screen
- TNFα:
-
Tumor necrosis factor-alpha
- TR1/TR2/TR3/TR4:
-
TRAIL death receptors-1/-2/-3/-4
- XTT:
-
2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide
- SRB:
-
Sulforhodamine B
- TCA:
-
Trichloroacetic acid
- DMSO:
-
Dimethylsulfoxide
- FBS:
-
Fetal bovine serum
- −TRAIL:
-
In the absence of TRAIL ligand
- +TRAIL:
-
In the presence of TRAIL ligand
- %GI:
-
Percent growth inhibition
- MMP:
-
Mitochondrial membrane potential
References
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH (1999) Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 104(2):155–162
Brooks AD, Ramirez T, Toh U, Onksen J, Elliott PJ, Murphy WJ, Sayers TJ (2005) The proteasome inhibitor bortezomib (Velcade) sensitizes some human tumor cells to Apo2L/TRAIL-mediated apoptosis. Ann NY Acad Sci 1059:160–167
Buchsbaum DJ, Forero-Torres A, LoBuglio AF (2007) TRAIL-receptor antibodies as a potential cancer treatment. Future Oncol 3(4):405–409
Cheng J, Hylander BL, Baer MR, Chen X, Repasky EA (2006) Multiple mechanisms underlie resistance of leukemia cells to Apo2 Ligand/TRAIL. Mol Cancer Ther 5(7):1844–1853
Choi JW (2005) Relationships between tumor necrosis factor (TNF)-related apoptosis- inducing ligand (TRAIL) and hematopoietic activity in healthy adults. Ann Hematol 84(11):728–733
Choi JW, Song JS, Pai SH (2004) Associations of serum TRAIL concentrations, anthropometric variables, and serum lipid parameters in healthy adults. Ann Clin Lab Sci 34(4):400–404
Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG (1997) The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL- mediated apoptosis, yet retains an incomplete death domain. Immunity 7(6):813–820
Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA (1997) Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med 186(7):1165–1170
Dolcet X, Llobet D, Pallares J, Rue M, Comella JX, Matias-Guiu X (2005) FLIP is frequently expressed in endometrial carcinoma and has a role in resistance to TRAIL- induced apoptosis. Lab Invest 85(7):885–894
Duiker EW, Mom CH, de Jong S, Willemse PH, Gietema JA, van der Zee AG, de Vries EG (2006) The clinical trail of TRAIL. Eur J Cancer 42(14):2233–2240
Falschlehner C, Emmerich CH, Gerlach B, Walczak H (2007) TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol 39(7–8):1462–1475
Fukumori T, Takenaka Y, Oka N, Yoshii T, Hogan V, Inohara H, Kanayama HO, Kim HR, Raz A (2004) Endogenous galectin-3 determines the routing of CD95 apoptotic signaling pathways. Cancer Res 64(10):3376–3379
Fulda S, Wick W, Weller M, Debatin KM (2002) Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med 8(8):808–815
Ganten TM, Koschny R, Haas TL, Sykora J, Li-Weber M, Herzer K, Walczak H (2005) Proteasome inhibition sensitizes hepatocellular carcinoma cells, but not human hepatocytes, to TRAIL. Hepatology 42(3):588–597
Georgakis GV, Li Y, Humphreys R, Andreeff M, O’Brien S, Younes M, Carbone A, Albert V, Younes A (2005) Activity of selective fully human agonistic antibodies to the TRAIL death receptors TRAIL-R1 and TRAIL-R2 in primary and cultured lymphoma cells: induction of apoptosis and enhancement of doxorubicin- and bortezomib-induced cell death. Br J Haematol 130(4):501–510
Gill C, Walsh SE, Morrissey C, Fitzpatrick JM, Watson RW (2007) Resveratrol sensitizes androgen independent prostate cancer cells to death-receptor mediated apoptosis through multiple mechanisms. Prostate 67(15):1641–1653
Grossmann J, Walther K, Artinger M, Kiessling S, Scholmerich J (2001) Apoptotic signaling during initiation of detachment-induced apoptosis (“anoikis”) of primary human intestinal epithelial cells. Cell Growth Differ 12(3):147–155
Hao C, Beguinot F, Condorelli G, Trencia A, Van Meir EG, Yong VW, Parney IF, Roa WH, Petruk KC (2001) Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apotosis in human malignant glioma cells. Cancer Res 61(3):1162–1170
Hayashi T, Okutomi T, Suzuki S, Okazaki H (1983) Inhibition of nucleic acid biosynthesis in procaryotic and eucaryotic cells by cyanocycline A. J Antibiot (Tokyo) 36(9):1228–1235
Hill GC, Wunz TP, MacKenzie NE, Gooley PR, Remers WA (1991) Computer simulation of the binding of naphthyridinomycin and cyanocycline A to DNA. J Med Chem 34(7):2079–2088
Huang Y, Sheikh MS (2007) TRAIL death receptors and cancer therapeutics. Toxicol Appl Pharmacol 224(3):284–289
Jin X, Wu XX, Abdel-Muneem Nouh MA, Kakehi Y (2007) Enhancement of death receptor 4 mediated apoptosis and cytotoxicity in renal cell carcinoma cells by subtoxic concentrations of doxorubicin. J Urol 177(5):1894–1899
Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA (2001) Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-relatedapoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther 299(1):31–38
Kim YS, Schwabe RF, Qian T, Lemasters JJ, Brenner DA (2002) TRAIL-mediated apoptosis requires NF-kappaB inhibition and the mitochondrial permeability transition in human hepatoma cells. Hepatology 36(6):1498–1508
Lacour S, Micheau O, Hammann A, Drouineaud V, Tschopp J, Solary E, Dimanche- Boitrel MT (2003) Chemotherapy enhances TNF-related apoptosis-inducing ligand DISC assembly in HT29 human colon cancer cells. Oncogene 22(12):1807–1816
Lavrik I, Krueger A, Schmitz I, Baumann S, Weyd H, Krammer PH, Kirchhoff S (2003) The active caspase-8 heterotetramer is formed at the CD95 DISC. Cell Death Differ 10(1):144–145
Lee TJ, Jung EM, Lee JT, Kim S, Park JW, Choi KS, Kwon TK (2006) Mithramycin A sensitizes cancer cells to TRAIL-mediated apoptosis by down-regulation of XIAP gene promoter through Sp1 sites. Mol Cancer Ther 5(11):2737–2746
Lombo F, Menendez N, Salas JA, Mendez C (2006) The aureolic acid family of antitumor compounds: structure, mode of action, biosynthesis, and novel derivatives. Appl Microbiol Biotechnol 73(1):1–14
MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES (1997) Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem 272(41):25417–25420
Marini P (2006) Drug evaluation: lexatumumab, an intravenous human agonistic mAb targeting TRAIL receptor 2. Curr Opin Mol Ther 8(6):539–546
Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P, Ashkenazi A (1997) A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol 7(12):1003–1006
Merino D, Lalaoui N, Morizot A, Solary E, Micheau O (2007) TRAIL in cancer therapy: present and future challenges. Expert Opin Ther Targets 11(10):1299–1314
Mirandola P, Sponzilli I, Gobbi G, Marmiroli S, Rinaldi L, Binazzi R, Piccari GG, Ramazzotti G, Gaboardi GC, Cocco L, Vitale M (2006) Anticancer agents sensitize osteosarcoma cells to TNF-related apoptosis-inducing ligand downmodulating IAP family proteins. Int J Oncol 28(1):127–133
Mitsiades N, Mitsiades CS, Poulaki V, Anderson KC, Treon SP (2002) Intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human multiple myeloma cells. Blood 99(6):2162–2171
Mori S, Murakami-Mori K, Nakamura S, Ashkenazi A, Bonavida B (1999) Sensitization of AIDS-Kaposi’s sarcoma cells to Apo-2 ligand-induced apoptosis by actinomycin D. J Immunol 162(9):5616–5623
Morimoto M, Fukui M, Ohkubo S, Tamaoki T, Tomita F (1982) Tetrocarcins, new antitumor antibiotics. 3. Antitumor activity of tetrocarcin A. J Antibiot (Tokyo) 35(8):1033–1037
Muhlethaler-Mottet A, Flahaut M, Bourloud KB, Auderset K, Meier R, Joseph JM, Gross N (2006) Histone deacetylase inhibitors strongly sensitise neuroblastoma cells to TRAIL-induced apoptosis by a caspases-dependent increase of the pro- to anti- apoptotic proteins ratio. BMC Cancer 6:214
Nagy K, Szekely-Szuts K, Izeradjene K, Douglas L, Tillman M, Barti-Juhasz H, Dominici M, Spano C, Luca Cervo G, Conte P, Houghton JA, Mihalik R, Kopper L, Petak I (2006) Proteasome inhibitors sensitize colon carcinoma cells to TRAIL-induced apoptosis via enhanced release of Smac/DIABLO from the mitochondria. Pathol Oncol Res 12(3):133–142
Nakajima H, Sakaguchi K, Fujiwara I, Mizuta M, Tsuruga M, Magae J, Mizuta N (2007) Apoptosis and inactivation of the PI3-kinase pathway by tetrocarcin A in breast cancers. Biochem Biophys Res Commun 356(1):260–265
Nakata S, Yoshida T, Horinaka M, Shiraishi T, Wakada M, Sakai T (2004) Histone deacetylase inhibitors upregulate death receptor 5/TRAIL-R2 and sensitize apoptosis induced by TRAIL/APO2-L in human malignant tumor cells. Oncogene 23(37):6261–6271
Ng CP, Zisman A, Bonavida B (2002) Synergy is achieved by complementation with Apo2L/TRAIL and actinomycin D in Apo2L/TRAIL-mediated apoptosis of prostate cancer cells: role of XIAP in resistance. Prostate 53(4):286–299
O’Kane HF, Watson CJ, Johnston SR, Petak I, Watson RW, Williamson KE (2006) Targeting death receptors in bladder, prostate and renal cancer. J Urol 175(2):432–438
Ozoren N, El-Deiry WS (2002) Defining characteristics of Types I and II apoptotic cells in response to TRAIL. Neoplasia 4(6):551–557
Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM (1997a) An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277(5327):815–818
Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM (1997b) The receptor for the cytotoxic ligand TRAIL. Science 276(5309):111–113
Petak I, Houghton JA (2001) Shared pathways: death receptors and cytotoxic drugs in cancer therapy. Pathol Oncol Res 7(2):95–106
Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A (1996) Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 271(22):12687–12690
Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, Barrett M, Judson I, Kaye S, Fox NL, Halpern W, Corey A, Calvert H, de Bono J (2007) Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res 13(20):6187–6194
Pukac L, Kanakaraj P, Humphreys R, Alderson R, Bloom M, Sung C, Riccobene T, Johnson R, Fiscella M, Mahoney A, Carrell J, Boyd E, Yao XT, Zhang L, Zhong L, von Kerczek A, Shepard L, Vaughan T, Edwards B, Dobson C, Salcedo T, Albert V (2005) HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. Br J Cancer 92(8):1430–1441
Ramakrishnan R, Antonia S, Gabrilovich DI (2008) Combined modality immunotherapy and chemotherapy: a new perspective. Cancer Immunol Immunother. doi:10.1007/s00262-008-0531-4
Reddy RM, Yeow WS, Chua A, Nguyen DM, Baras A, Ziauddin MF, Shamimi-Noori SM, Maxhimer JB, Schrump DS (2007) Rapid and profound potentiation of Apo2L/TRAIL-mediated cytotoxicity and apoptosis in thoracic cancer cells by the histone deacetylase inhibitor Trichostatin A: the essential role of the mitochondria- mediated caspase activation cascade. Apoptosis 12(1):55–71
Riley T, Sontag E, Chen P, Levine A (2008) Transcriptional control of human p53- regulated genes. Nat Rev Mol Cell Biol 9(5):402–412
Sato K, Lepage GA (1965) Metabolic effects of an antibiotic, Nsc-51954, on susceptible and resistant tumor cells. Cancer Res 25:477–483
Saulle E, Petronelli A, Pasquini L, Petrucci E, Mariani G, Biffoni M, Ferretti G, Scambia G, Benedetti-Panici P, Cognetti F, Humphreys R, Peschle C, Testa U (2007) Proteasome inhibitors sensitize ovarian cancer cells to TRAIL induced apoptosis. Apoptosis 12(4):635–655
Sayers TJ, Brooks AD, Koh CY, Ma W, Seki N, Raziuddin A, Blazar BR, Zhang X, Elliott PJ, Murphy WJ (2003) The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAIL-mediated apoptosis by reducing levels of c-FLIP. Blood 102(1):303–310
Schimmer AD, Thomas MP, Hurren R, Gronda M, Pellecchia M, Pond GR, Konopleva M, Gurfinkel D, Mawji IA, Brown E, Reed JC (2006) Identification of small molecules that sensitize resistant tumor cells to tumor necrosis factor-family death receptors. Cancer Res 66(4):2367–2375
Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR (1988) Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res 48(17):4827–4833
Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A (1997) Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277(5327):818–821
Singh R, Pervin S, Chaudhuri G (2002) Caspase-8-mediated BID cleavage and release of mitochondrial cytochrome c during Nomega-hydroxy-l-arginine-induced apoptosis in MDA-MB-468 cells. Antagonistic effects of l-ornithine. J Biol Chem 277(40):37630–37636
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay foranticancer-drug screening. J Natl Cancer Inst 82(13):1107–1112
Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD Jr, Chen LB (1991) Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA A88(9):3671–3675
Song JH, Song DK, Pyrzynska B, Petruk KC, Van Meir EG, Hao C (2003) TRAIL triggers apoptosis in human malignant glioma cells through extrinsic and intrinsic pathways. Brain Pathol 13(4):539–553
Tibbetts MD, Zheng L, Lenardo MJ (2003) The death effector domain protein family: regulators of cellular homeostasis. Nat Immunol 4(5):404–409
Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, Hill M, Mays T, McCoy T, Fox NL, Halpern W, Corey A, Cohen RB (2007) Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis- inducing ligand receptor-1. J Clin Oncol 25(11):1390–1395
Van Geelen CM, de Vries EG, de Jong S (2004) Lessons from TRAIL-resistance mechanisms in colorectal cancer cells: paving the road to patient-tailored therapy. Drug Resist Updat 7(6):345–358
Vichai V, Kirtikara K (2006) Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 1(3):1112–1116
Vulfovich M, Saba N (2005) Technology evaluation: mapatumumab, Human Genome Sciences/GlaxoSmithKline/Takeda. Curr Opin Mol Ther 7(5):502–510
Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour , Gerhart MJ, Schooley KA, Smith CA, Goodwin RG, Rauch CT (1997) TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J 6(17):5386–5397
Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH (1999) Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 5(2):157–163
Wang MJ, Liu S, Liu Y, Zheng D (2007) Actinomycin D enhances TRAIL-induced caspase-dependent and -independent apoptosis in SH-SY5Y neuroblastoma cells. Neurosci Res 59(1):40–46
Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA et al (1995) Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3(6):673–682
Yang L, Wu S, Zhang Q, Liu F, Wu P (2007) 23, 24-Dihydrocucurbitacin B induces G2/M cell-cycle arrest and mitochondria-dependent apoptosis in human breast cancer cells (Bcap37). Cancer Lett 256(2):267–278
Zeng Y, Wu XX, Fiscella M, Shimada O, Humphreys R, Albert V, Kakehi Y (2006) Monoclonal antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) induces apoptosis in primary renal cell carcinoma cells in vitro and inhibits tumor growth in vivo. Int J Oncol 28(2):421–430
Zhang JH, Chung TD, Oldenburg KR (1999) A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4(2):67–73
Zhang L, Fang B (2005) Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther 12(3):228–237
Zhu H, Guo W, Zhang L, Wu S, Teraishi F, Davis JJ, Dong F, Fang B (2005) Proteasome inhibitors-mediated TRAIL resensitization and Bik accumulation. Cancer Biol Ther 4(7):781–786
Zhu H, Zhang L, Huang X, Davis JJ, Jacob DA, Teraishi F, Chiao P, Fang B (2004) Overcoming acquired resistance to TRAIL by chemotherapeutic agents and calpain inhibitor I through distinct mechanisms. Mol Ther 9(5):666–673
Acknowledgments
Jennifer Wilson generously assisted with numerous XTT assays during the development of the TRAIL HTS; Heidi R. Bokesch conducted LC-MS and NMR quality control evaluations on compounds provided by the NCI Natural Products Repository; and Antony M. Wamiru and Nancy B. Shulley were instrumental in the design and trouble-shooting of Beckman 384-FX robot programs for use in the screen. The authors thank Kirk R. Gustafson for his time to read and comment on the manuscript. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Booth, N.L., Sayers, T.J., Brooks, A.D. et al. A cell-based high-throughput screen to identify synergistic TRAIL sensitizers. Cancer Immunol Immunother 58, 1229–1244 (2009). https://doi.org/10.1007/s00262-008-0637-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0637-8