Abstract
MUC1 tumor antigen is a target for immunotherapy of most human adenocarcinomas and some hematological malignancies. Expression of a MUC1-specific, MHC-unrestricted single-chain T cell receptor (scTCR) on cells of both innate and adaptive immune system through reconstitution of lethally irradiated mice by retroviral vector-transduced bone marrow cells, had been shown to effectively control the growth of MUC1+ tumors independent of their MHC type, suggesting that this receptor is a good candidate for broadly applicable gene therapy/immunotherapy. However, the translational application of this immuno-gene therapy modality was discouraged by the progressive transgene silencing in reconstituted T and B cells, as well as the potential of tumorogenesis intrinsic to oncoretroviral vectors. To overcome these problems and facilitate the future clinical use of this receptor, we have constructed a panel of novel self-inactivating lentiviral vectors (LVs) which harbor two independent internal promoters, one driving expression of the scTCR gene and the other of a fusion suicide gene, the HSV-TK–EGFP fusion gene, allowing the transduced cells to be destroyable by the pro-drug ganciclovir. Despite the large size of insert, these vectors were efficiently packaged into high titer virus that transferred the expression of transgene in both T cell lines and primary T cells. Sustained expression was maintained in a T cell line for over 4 months in vitro, suggesting its efficient resistance to transgene silencing. Both scTCR and HSV-TK–EGFP genes were functional in the transduced cells, as evidenced by their specific recognition of MUC1+ tumors and efficient eradication by ganciclovir.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial cell mucin (MUC1) is overexpressed in an aberrantly glycosylated form on epithelial adenocarcinomas [1–4] as well as on leukemia, lymphoma and multiple myeloma [5–8]. Previously we reported the cloning of a unique T cell receptor (TCR) from a MUC1-specific cytotoxic T cell line derived from a breast cancer patient, which recognized the aberrant tumor form of MUC1 in an MHC-unrestricted manner [9–11]. This property enabled the TCR to target MUC1-positive tumors regardless of their HLA type, making it a “universal” therapeutic agent for all patients with different adenocarcinomas. We reported that a single-chain TCR (scTCR) consisting of the Vα/Vβ/Cβ construct fused to a CD3ζ chain, in addition to T cells, can be expressed on the surface of other cells of both innate (macrophages, NK cells, granulocytes) and adaptive immune systems (B cells). Delivery of this scTCR gene into mouse bone marrow cells through the Moloney leukemia virus-based MFG oncoretroviral vector encoded its expression on the surface of cells of the innate and adaptive immune systems, and effectively controlled the growth of inoculated MUC1-positive tumors in the bone marrow transplanted mice [12]. However, we observed that the expression of the scTCR transgene in T and B cells was significantly reduced 3 months after bone marrow transplantation, while other cells continued high-level expression [12]. Problems with gene silencing in hematopoietic cells have been reported to be intrinsic to the native promoters of oncoretroviral vectors [13]. In addition, there has been an increasing concern about the safety of viral vectors in general that selectively integrate into transcription-active sites in the genome potentially leading to activation of proto-oncogenes [14–18].
In order to overcome transgene silencing in lymphocytes, and improve the safety and efficacy for clinical application of TCR gene therapy/immunotherapy, we chose HIV-derived lentiviral vectors (LVs). Although LVs can also be subject to transgene silencing, they are generally less susceptible than retroviral vectors [19]. Their biggest advantage, however, is that they can transduce a variety of slowly or nondividing cells, including unstimulated T cells [20–23], and clinical trials using LVs have been approved and conducted for HIV/AIDS gene therapy [24].
We have engineered these vectors to co-encode scTCR and a fusion suicide gene (HSV-TK–EGFP, TGL) [25–27], which makes the transduced cells trackable and destroyable. These two transgenes are under the control of two independent internal promoters, either EF1α [28] or MSCV U3 [29–30] controlling the scTCR gene expression, and PGK promoter [31] driving the expression of the TGL fusion suicide gene. These dual-promoter LVs were efficiently packaged to high-titer virus that transferred the expression of transgenes to mouse and human T cell lines as well as human primary T cells. All the transgenes were functional, suggesting they would be broadly applicable and highly safe reagents for cancer immunotherapy.
Materials and methods
Construction of lentiviral transfer vectors
The self-inactivating lentiviral vector system (transfer vector pTY linker (Fig. 1A), packaging plasmid pHP-dl-N/A, envelope plasmid pHEF-VSV-G, and accessory plasmid pCEP-tat) was kindly provided by Dr. Lung-Ji Chang at University of Florida through NIH AIDS Research & Reference Reagent Program (https://www.aidsreagent.org) [32, 33]. To increase the vector efficiency, the pTY linker was modified by cloning the Woodchuck hepatitis posttranscriptional regulatory element (WPRE) fragment [34, 35] (cut at the BamHI and KpnI sites from pWOX-TGL, a gift from Dr. Jarmo Wahlfors, University of Kuopio, Finland) into the pTY linker at the corresponding sites to generate pTY-WPRE. To further increase the vector efficiency, the resulting pTY-WPRE was cut using NheI and blunt-ligated with the cPPT/CTS fragment TRIP [36–38] (cut from pLenti6-To-TRIP-EGFP at EcoRI and ClaI sites, kindly provided by Dr. Yukai He, University of Pittsburgh) to generate pTY-TRIP-WPRE (Fig. 1A), which was used as a vector backbone for construction of a panel of LVs for current study and future clinical application. In order to construct vector pTY-TRIP-EF1a-scTCR-WPRE (pT3), the EF1a-scTCR fragment was cut from pEF4-scTCR [39] using MluI and NotI, and cloned into the pTY-TRIP-WPRE at SmaI site through blunt-ligation. To make the pTY-TRIP-TGL-WPRE, the HSV-TK–EGFP fusion gene (TGL) [25] was cut from pUC19-TGL, a kind gift from Dr. J Wahlfors of University of Kuopio, Finland, using XbaI, and then cloned into the pTY-TRIP-WPRE plasmid at SmaI site through blunt-ligation, to generate the vector pTY-TRIP-TGL-WPRE. The PGK promoter, cut from a commercial retroviral construct pMSCVpuro(Clontech) with HpaI and HindIII, was cloned into pTY-TRIP-TGL-WPRE at SalI site by blunt ligation to generate pTY-TRIP-PGK-TGL-WPRE (pE3). In order to make a dual-promoter lentiviral vector pTY-TRIP-EF1a-scTCR-PGK-TGL-WPRE (pB21), the EF1a-scTCR, which was cut from pEF4-scTCR using MluI and NotI, was cloned into pE3 at NheI site through blunt-ligation. In order to construct another dual-promoter lentiviral vector pTY-TRIP-MU3-scTCR-PGK-TGL-WPRE (pMTPT), MSCV U3 promoter (MU3) [28] was cut from pMSCVpuro (Clontech) using NheI and SmaI, and cloned into pTY-linker at the corresponding restriction sites to generate pTY-MU3. The pTY-MU3 was cut by using SmaI and blunt-ligated with scTCR fragment, which was cut from pEF4-scTCR with BamHI and NotI. The resulting pTY-MU3-scTCR was cut using NotI and KpnI, and the MU3-scTCR fragment was blunt-ligated with BamHI-linealized pTY-TRIP-WPRE to generate pTY-TRIP-MU3-scTCR-WPRE, which was linealized with NheI and blunt-ligated with PGK-TGL fragment (obtained from pE3 by NheI/BamHI digestin) to generate pTY-TRIP-MU3-scTCR-PGK-TGL-WPRE (pMTPT).
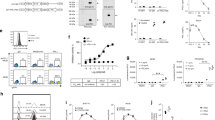
Schematic representation of the new LVs. The pTY linker (A) was modified to construct pTY-TRIP-WPRE (B) that includes cPPT/CTS and WPRE elements. C–E Mono-promoter LVs, F–I dual-promoter LVs, J bicistronic retroviral vector (pMFG-scTCR-IRES-EGFP, pMTE) with wild-type LTR encodes scTCR and EGFP. K pTY-EF-EGFP, unmodified mono-promoter LV encoding EGFP, LTR long terminal repeat, cPPT/CTS HIV-1 central polypurine tract (cPPT) and the central termination sequence (CTS), WPRE Woodchuck hepatitis posttranscriptional regulatory element, del U3 deletion of 3′U3, scTCR single-chain T cell receptor (scTCR) fused to mouse CD3ζ domain, scTCR-AGD-TmHz scTCR fused to human CD3ζ domain through the AGD linker. mscTCR mutated scTCR, TGL Herpes simplex virus thymidine kinase suicide gene (HSV-TK) and enhanced green fluorescence protein (EGFP) fusion gene, EF1α elongation factor 1α promoter, PGK human phosphoglycerate kinase promoter, MU3 U3 promoter region of the murine stem cell virus long terminal repeat
In order to construct a panel of LVs encoding the scTCR with human CD3ζ domain, EF1a-scTCR-AGD-TmHz fragment was cut from pEF6-scTCR-AGD-TmHz [39] using MluI and NotI and cloned into pTY-TRIP-WPRE at SmaI site to generate pTY-TRIP-EF1a-scTCR-AGD-TmHz-WPRE (pA17). The dual-promotor lentiviral vector pTY-TRIP-EF1a-scTCR-AGD-TmHz-PGK-TGL-WPRE (pTGC4) was constructed by cloning the EF1a-scTCR-AGD-TmHz fragment into pE3 at NheI site through blunt ligation.
In order to construct a dual-promoter LV encoding a site-mutated scTCR (mscTCR), which has shown an enhanced cell surface expression in human T cells (unpublished data), the EF1a-mscTCR was cut from pEF6-mscTCR using MluI and NotI, and cloned into pE3 at NheI through blunt ligation. The resulting plasmid is termed pB24.
Lentivirus production
293T cells (kindly provided by Dr. Yukai He, University of Pittsburgh) were cotransfected with four plasmids (the pTY transfer vector plus pHP-dl-N/A, pHEF-VSV-G, and pCEP-tat) [32, 33] using Lipofectamin 2000 kit (Invitrogen). All the plasmids were prepared using Qiagen endotoxin-free kit and quantitated by spectrophotometry at OD260/280. On day 1, transfected cells were placed in fresh medium and cultured for 24 h. Supernatants (first) were then harvested and fresh medium added for further culture for another 24 h, when the second supernatant was harvested and combined with the first. Virus containing supernatants were then filtered through a 0.45 μm Millex HV filter (Millipore, SLHV033RS) before used for transduction of target cells or stored at −80°C for later use.
Retrovirus production
293T cells were cotransfected with pMTE (pMFG-scTCR-IRES-EGFP)(12), helper plasmid pKAT [40] (a kind gift from Dr. Tao Cheng, University of Pittsburgh Cancer Institute) and envelope plasmid pVSV-G using the same protocol as in lentiviral vector production as described above. The viral supernatants were collected, filtered and stored at −80°C.
Lentivirus titration
Determination of viral titers was based on the expression of TGL or scTCR in transduced 293T cells. 293T cells were seeded in a six-well plate at 2 × 105 cells per well. After overnight culture, the medium was removed and 1 ml of serially diluted viral supernatants (1/10, 1/100, 1/1,000, 1/10,000, 1/100,000, and mock), and polybrene (6 μg/ml, Millipore) added to the wells. 24 h later, supernatants were removed and 2 ml fresh medium were added. After further culture for 3 days, the cells were collected for FACS analysis. The titer was calculated using the following formula:\( {\text{TU}}/{\text{ml}} = \left( {P \times N/100 \times V} \right) \times 1/{\text{DF}}, \) where TU stands for transduction unit, P % GFP+ cells, N number of cells at time of transduction = 2 × 105, V volume of dilution added to each well = 1 ml, and DF dilution factor = 1 (undiluted), 10−1 (diluted 1/10), 10−2 (diluted 1/100), and so on [41].
Transduction of T cells
Human PBMCs isolated from healthy donors using Ficoll–Paque were activated for 3 days by coated anti-CD3 antibody (10 μg/ml) and soluble anti-CD28 antibody (1 μg/ml) in the presence of hIL-2 50 U/ml at 5–10 × 106 cells per well in a six-well plate. The activated PBMC or T cell lines (JRT3.5 or BWZ) were suspended in 1 ml of viral supernatant at 5 × 105 cells per ml in the presence of 6 μg/ml polybrene, in a six-well plate. The plate was centrifuged at 2,900 rpm for 50 min, followed by overnight incubation at 37°C, 5% CO2. The viral supernatants were carefully removed and fresh medium was added for culture and expansion.
Flow cytometry
The HPAF pancreatic cancer cells and MCF-7 breast cancer cells were stained with PE-labeled MUC1 antibody 4H5 (a gift from Dr. Jo Hilgers, Amsterdam), which is specific to tumor-type hypoglycosylated MUC1(the epitope APDTRPAP in the VNTR region of MUC1) [42], followed by two washes with PBS (2.5% FBS and 0.09% NaN3). Flow cytometry was used to measure the hypoglycosylated MUC1 expression on cell surface.
T cell functional assays
BWZ mouse T cells [12, 39] were transduced with LVs. After culture and expansion, 1 × 106 transduced cells were incubated at 37°C, 5% CO2 with 1 × 106 MUC1+ tumor cells, either HPAF pancreatic cancer cells, or MCF-7 breast cancer cells [12, 39], for 9 h. Brefeldin A (GolgiPlug, BD Bioscience) was added to the culture at the final concentration of 1 μg/ml. The cells were then permeabilized and fixed with BD Cytofix/Cytoperm kit (BD) following the instruction of manufacturer. After two times of washing with cytoperm buffer (BD), the cells were stained with APC-labeled anti-IL-2 antibody (BD) for 30 min at 4°C. The IL-2-producing cells were assessed by flow cytometry. The function of scTCR was also assessed by measuring the production of IL-2 in transduced T cells in response to the stimulation with BF1 antibody [12] using an ELISA kit (BD). A 24-well plate was coated with BF1 antibody (5 μg/ml in PBS) at 4°C overnight. The lentiviral vector transduced BWZ mouse T cells were then added to the wells and incubated at 37°C, 5% CO2 for 48 h. After brief centrifugation of the plate, the supernatants were collected and the IL-2 concentration was measured by ELISA.
Cytotoxicity by ganciclovir
BWZ cells transduced with LVs encoding the HSV-TK–EGFP fusion gene (TGL) were cultured at 37°C, 5% CO2 in RPMI1640 medium containing different concentration of ganciclovir or control medium. Every three to five days, the cells were split and resuspended in the same drug-containing medium or control medium. Two weeks later, cells were analyzed by flow cytometry to measure the disappearance of TGL-expressing cells. The selective killing rate was calculated using the following formula: \( {\text{selective}}\,{\text{killing}}\,{\text{rate}}\, ( {\text{\% )}} = (T_{0} - T_{n})/T_{0} \times 100, \) where T n is the percentage of TGL-expressing cells after treatment with difference doses of ganciclovir, T 0 is the percentage of TGL-expressing cells after treatment with medium only.
Statistical analysis
Statistical significance was determined by multifactorial ANOVA using SAS9.1 with GLM Procedure. The EC50 of the selective killing efficiency of GCV and the 95% Confidence Interval were calculated using GraphPad Prism (GraphPad Software Inc.).
Results
Dual-promoter self-inactivating LVs co-encode scTCR and a fusion suicide gene
We constructed the new LVs based on a minimal self-inactivating lentiviral vector pTY system [32]. The pTY self-inactivating (SIN) vector has a maximal deletion of the 3′U3 region, with only 24 nt remaining, compared with 55 nt 3′U3 in other SIN LVs. The 3′U5 is replaced with a bGHpA signal, and the 5′U3 is replaced with a CMV TATA enhancer [32]. The maximal deletion of 3′U3 region would make it less likely to be mobilized in case of HIV super-infection or HIV gag/pol transfection, where the CMV TATA enhancer and 3′bGHpA poloy A signal could increase virus production.
To make this SIN vector more efficient, we included cis elements cPPT/CTS and WPRE in the vector backbone. The resulting construct, pTY-TRIP-WPRE, was used to produce a panel of SIN vectors, schematically represented in Fig. 1, that co-encode scTCR and HSV-TK–EGFP (TGL). We chose dual-promoter LV because it was reported to efficiently co-express multiple genes in transduced hematopoietic cells [43, 44], and appeared a better choice than bicistronic expression mediated by IRES, where the transgene is not reliably expressed in all cell types [43, 45]. In our vectors, the scTCR gene is under the control of EF1α promoter, and the TGL fusion suicide gene under the control of PGK promoter. Both EF1α and PGK promoters are physiological promoters, which were reported to be less genotoxic [46] and highly efficient in hematopoietic cells [29, 30].
High-titer virus production despite large packaging size of dual-promoter LVs
It has been reported that lentivirus titers decrease semi-logarithmically with increasing vector length [47, 48]. Although vectors as large as 18 kb could be packaged to give very low but still measurable viral titers, the optimum reported packaging size appears to be less than 5 kb, as the titer drops approximately 100-fold for inserts between 6 and 12.5 kb in length [48]. We had around 7 kb of inserts in the dual-promoter LVs, and 4.1 kb of inserts in the mono-promoter LVs. That raised concerns that the viral titer might be greatly compromised. This turned out to be not as big a problem as we anticipated. As shown in Fig. 2, the virus titer of three dual-promoter LVs, pB24, pTGC4, and pB21, all of which encode the TGL gene and have an insert of 6.7 kb, was 1–2 × 107 U/ml. This is only a four-fold reduction compared to the virus titer of the mono-promoter LV pE3, which also encodes TGL gene but with a smaller insert size of 3.4 kb. This titer is considered sufficiently high for efficient transduction of hematopoietic stem cells or primary T cells in vitro.
High titer lentivirus production despite large packaging size of the LVs. 293T cells were transduced with indicated dilutions of viral supernatants produced from indicated vectors (see Fig. 1). Transduction efficiency was measured using flow cytometry. The titer was calculated by measuring either TGL (a) or scTCR positive cells (b) by flow cytometry. Numbers above arrows indicate percent positive cells. Calculation of virus titer is described in the Materials and Methods. TU stands for transduction unit. Insert size includes the cPPT/CTS element, internal promoter, transgene (scTCR and/or TGL), and WPRE
Transgene expression in T cell lines and primary T cells transduced by LVs
Transduction efficiency and transgene expression by mono-promoter LV (pE3 or pTY-EF-EGFP) or dual-promoter LV (pTGC4) were tested in T cell lines and primary T cells. As shown in Fig. 3, high transduction efficiency and expression level was achieved in T cell lines, for both mono-promoter and dual-promoter LVs. In human primary T cells, while mono-promoter LV transferred high transduction efficiency (up to 40%) and transgene expression, the dual-promoter LV achieved lower performance, both in transduction efficiency (around 5%) and in transgene expression level. These percentage of antigen-specific cells is similar to levels observed in vivo in response to other antigens and thus would be expected to be therapeutic.
Transduction efficiency of mono- and dual-promoter LVs in a T cell line and human primary T cells. JRT3.5 cells (a) were transduced with single-promoter LV pE3 encoding the TGL fusion gene, or dual-promoter LV pTGC4 that co-encodes scTCR and TGL (see Fig. 1). The CD3/CD28 activated human primary T cells were transduced with undiluted or tenfold diluted viral supernatants of mono-promoter LV pTY-EF-EGFP (b). The same primary T cells were also transduced with tittered mono-promoter LV pTY-EF-EGFP or dual-promoter LV (pTGC4) (c). Transgene expression was analyzed by flow cytometry
Resistance to gene silencing in dual-promoter LV transduced cells
The pMFG-scTCR-IRES-EGFP [pMTE, Fig. 1J] that we previously used in a preclinical gene therapy study [12] is Moloney murine leukemia virus (MMLV)-based oncoretroviral vector with wild-type LTR. Lethally irradiated mice that were transplanted with the pMTE oncoretroviral vector-transduced bone marrow cells successfully reconstituted the innate and adaptive immune system and efficiently controlled the growth of MUC1-positive tumors. However, the transgene expression in reconstituted immune cells, especially in T and B cells, was progressively reduced and silenced, which raised the concern for the long-term antitumor efficacy of this gene therapy strategy. Although SIN lentiviral vector was also reported to be subjected to gene silencing, it is generally agreed that LV is more resistant to gene silencing than retroviral vector. Figure 4 shows that after a long-term in vitro expansion of transduced Jurkat cells, high expression level of the transgene was maintained in LV-transduced cells up to 4 months, and was always higher than in retroviral vector transduced cells (Fig. 4).
Sustained transgene expression in T cells transduced with LVs. JRT3.5 cells were transduced with LVs pB21 or pMTPT (see Fig. 1) or the retroviral vector pMTE and transgene expression monitored by flow cytometry at indicated time points up to 4 months (a). b Percent positive cells and mean fluorescence intensity (MFI) normalized to the initial time point (day 20)
Recognition of MUC1-positive tumor cell lines by LV encoded scTCR
Our previous results showed that BWZ T cells transduced with pMFG-based vector encoding scTCR gene specifically recognized different human MUC1-positive tumor cell lines as measured by their IL-2 production using ELISA or intracellular cytokine staining. We first confirmed the ability of the scTCR expressed on the surface of the BWZ cells transduced with the dual-promoter LV pB21 to signal properly, by cross-linking it with the BF1 monoclonal antibody specific to human TCR β chain. As shown in Fig. 5a, transduced BWZ cells efficiently produced IL-2, while mock-transduced cells or control vector pE3 (not encoding scTCR) transduced cells failed to produce the cytokine. In order to confirm that the specificity of the scTCR was not altered during the construction of the new vectors, two human tumor cell lines, pancreatic cancer cell line HPAF and breast cancer cell line MCF-7, which express hypoglycosylated MUC1 (data not shown), were co-cultured either with BWZ cells transduced with two different LVs (mono-promoter LV pT3 and dual-promoter LV pB21) encoding scTCR, or with BWZ cells transduced with mock or mono-promoter LV encoding TGL gene only (pE3). The result shows that upon stimulation with two MUC1-positive tumor cells, a significantly higher percentage of scTCR-LV-transduced BWZ cells produced IL-2 compared to BWZ controls (Fig. 5b, c) (P = 0.0019, multiple factorial ANOVA analysis by SAS GLM Procedure) There were no significant difference between the two MUC1+ tumor cell lines (P = 0.8728), and no significant difference between the mono-promoter and dual-promoter LVs (P = 0.4444). Furthermore, no interaction between the tumor cell line and LV promoter was observed (P = 0.8146). This confirms the unaltered MHC unrestricted MUC1 specificity of this TCR.
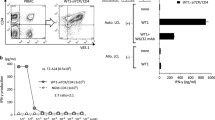
LV encoded MUC1 specific scTCR signals in transduced T cells and specifically recognizes MUC1+ tumor cells. Mouse BWZ T cells were transduced with indicated vectors or vector control (see Fig. 1). a The transduced cells were stimulated with plate-bound anti-TCR antibody ΒF1 for 48 h. IL-2 in the supernatants was measured by ELISA. b Transduced cells were co-cultured with MUC1+ pancreatic cancer cell line HPAF or breast cancer cell line MCF7 for 9 h in the presence of GolgiPlug. Cells were permeabilized and fixed, followed by intracellular staining with anti-mouse IL-2 antibody. IL-2-producing cells were determined by flow cytometry. c Differences in recognition of different tumor cells by BWZ cells transduced with different LVs were analyzed by multifactorial ANOVA with SAS GLM procedure
Selective eradication by the pro-drug ganciclovir of LV-transduced T cells
In order to further improve the safety of the scTCR encoding LVs, we included an HSV-TK suicide gene allowing in vivo elimination of transduced cells. To test the function of this transgene, we treated transduced JRT3.5 cells with the prodrug ganciclovir. We took advantage of the EGFP gene fused to the HSV-TK to measure by fluorescence the percentage of surviving cells at different times after treatment and with different concentrations of the drug. As shown in Fig. 6, BWZ cells transduced with mono-promoter LV (pE3) or dual-promoter LVs (pB21 and pTGC4) are selectively eradicated by ganciclovir, with EC50 of 0.091 μM (95% confidence interval (CI): 0.060–0.137 μM, R 2 = 0.9929) in pE3-transduced cells, 0.102 μM (95% CI: 0.035–0.300 μM, R 2 = 0.9261) in pB21-transduced cells, and 0.037 μM (95% CI 0.022–0.063 μM, R 2 = 0.9861) in pTGC4-transduced cell, respectively.
Selective killing of LV-transduced cells by ganciclovir. Mouse BWZ T cells transduced with LVs pB21 and pTGC4 (see Fig. 1). After 2 weeks, remaining transduced (green) cells were measured by flow cytometry (a). Selective killing rate of ganciclovir was calculated using the formula described in M&M, and 50% effective concentration (EC50) and its 95% confidence interval were determined giving the dose-effect curve (b)
Discussion
We have constructed several new dual promoter LVs for gene therapy/immunotherapy of MUC1+ tumors. These vectors are self-inactivating and encode the MUC1-specific, MHC-unrestricted scTCR gene and the HSV-TK–EGFP fusion suicide gene that confers a high safety profile important for future clinical applications. These vectors efficiently transfer high-level and sustained expression of scTCR and HSV-TK transgenes to T cells. Transduced cells, either soon after or long after transduction, can be selectively eradicated by the prodrug ganciclovir. Furthermore, these SIN LVs have minimal mobilization activity adding to their safety (X. Chen et al. manuscript in preparation), as mobilization by HIV gag/pol was recently detected in SIN LVs and raised a new concern for LV safety profile [49–51].
In order for these vectors to be eventually used in the clinic, the virus titer should be high enough so the target cells (hematopoietic stem cells or primary T cells) can be efficiently transduced. Our results show that in spite of the large size of the inserts, the viral titers obtained with the dual-promoter LVs are unexpectedly high. This may be due to the unique design of the pTY SIN vector in that CMV/TATA enhancer was placed upstream of 5′LTR, and bGHpA signal was used to replace 3′U5. In addition, cPPT/CTS and WPRE were included in the vector to further enhance virus production. The other possible reason for the high virus production is that we modified the protocol using 3:1 ratio of packaging plasmid to transfer plasmid instead of 1:1 ratio used by others. This high amount of packaging plasmid may have improved the encapsidation of large viral RNA, increasing the viral titers.
Since MUC1 is aberrantly over-expressed in over half of all human cancers [52], targeting this molecule through gene therapy/immunotherapy based on MUC1-specific, MHC-unrestricted scTCR would be applicable to a large number of cancer patients irrespective of their HLA types. More importantly, it would be applicable to the efforts of eradicating the minimal residual diseases, since cancer stem cells also express hypoglycosylated MUC1 [53] and are thus a potential target of this scTCR gene therapy strategy.
TCR-based gene therapy against cancer can be achieved through transduction of hematopoietic stem cells [12] or differentiated T cells [54, 55]. With our TCR, transduction of the hematopoietic stem cells resulted in not only antigen-specific T cells but also antigen specific B cells, NK cells, macrophages, granulocytes and other cells of the hematopoietic origin, directing innate and adaptive immunity to the tumor site simultaneously [12, 56]. Planned preclinical experiments in animal models and Phase I clinical trials will determine the benefit and toxicity of such an approach.
HSCs are highly desirable recipient cells for gene therapy because of their self-renewal and multilineage differentiation capacities. Stable engraftment of genetically modified HSCs leads to long term expression of transgene in stem and progenitor cells [57]. It was reported that oncoretroviral delivery of a tumor antigen-specific TCR gene into mouse hematopoietic stem cells provided long-term antigen-specific T cell immunity against cancer [58]. In our previous work, the MUC1-specific, MHC-unrestricted scTCR gene was delivered into mouse bone marrow cells using a MFG-based oncoretroviral vector, which resulted in the long-term scTCR transgene expression in repopulated progeny immune cells, both innate and adaptive, and effective control of the growth of MUC1-positive pancreatic cancer cells as well as mouse leukemia CLL in recepient mice [12]. This strategy represents a novel approach for cancer immunotherapy termed “instructive immunotherapy” [58] that re-programs the immune system to fight cancer by genetically engineering the hematopoietic stem cells.
Engineering and manipulation of lymphocytes through gene therapy strategy for adoptive transfer is another effective approach to eradicate tumor cells in vivo [59–61]. T cells transduced by oncoretroviral and lentivial vectors to express tumor-specific TCR can be expanded in vitro to a large number and adoptively transferred to animals and patients for killing tumor cells [54, 55, 60, 61]. The MHC-unrestricted feature of our MUC1-specific scTCR is particularly applicable to this strategy because tumors downregulate MHC expression as a means to evade host immunosurvailance [62]. This unique MHC-unrestricted function enables the immune cells expressing this scTCR to recognize and kill tumor cells irrespective of their MHC type and expression level, making this a “universal” TCR therapeutic gene for any individual with MUC1-positive tumor.
References
Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ (2004) MUC1 immunobiology: from discovery to clinical applications. Adv Immunol 82:249–293
Girling A, Bartkova J, Burchell J, Gendler S, Gillett C, Taylor-Papadimitriou J (1989) A core protein epitope of the polymorphic epithelial mucin detected by the monoclonal antibody SM-3 is selectively exposed in a range of primary carcinomas. Int J Cancer 43(6):1072–1076
Gendler S, Taylor-Papadimitriou J, Duhig T, Rothbard J, Burchell JA (1988) A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem 263(26):12820–12823
Siddiqui J, Abe M, Hayes D, Shani E, Yunis E, Kufe D (1988) Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proc Natl Acad Sci USA 85(7):2320–2323
Brossart P, Schneider A, Dill P, Schammann T, Grunebach F, Wirths S, Kanz L, Buhring HJ, Brugger W (2001) The epithelial tumor antigen MUC1 is expressed in hematological malignancies and is recognized by MUC1-specific cytotoxic T-lymphocytes. Cancer Res 61(18):6846–6850
Teruya-Feldstein J, Donnelly GB, Goy A, Hegde A, Nanjangud G, Qin J, Thaler H, Gilles F, Dyomin VG, Lloyd KO, Zelenetz AD, Houldsworth J, Chaganti RS (2003) MUC-1 mucin protein expression in B-cell lymphomas. Appl Immunohistochem Mol Morphol 11(1):28–32
Duperry C, Klein B, Durie BG M, Zhang X, Fourdan M, Poncelet R, Favier F, Vincent C, Brochier J, Lenoir G, Bataille R (1989) Phenotype analysis of human myeloma cell lines. Blood 73(2):566–572
Takahashi T, Makiguchi Y, Hinoda Y, Kakiuchi H, Nakagawa N, Imai K, Yachi A (1994) Expression of MUC1 on myeloma cells and induction of HLA-unrestricted CTL against MUC1 from a multiple myeloma patient. J Immunol 153(5):2102–2109
Barnd DL, Lan MS, Metzgar RS, Finn OJ (1989) Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci USA 86(18):7159–7163
Jerome KR, Domenech N, Finn OJ (1993) Tumor-specific cytotoxic T cell clones from patients with breast and pancreatic adenocarcinoma recognize EBV-immortalized B cells transfected with polymorphic epithelial mucin complementary DNA. J Immunol 151(3):1654–1662
Magarian-Blander J, Ciborowski P, Hsia S, Watkins SC, Finn OJ (1998) Intercellular and intracellular events following the MHC-unrestricted TCR recognition of a tumor-specific peptide epitope on the epithelial antigen MUC1. J Immunol 160(7):3111–3120
Alajez NM, Schmielau J, Alter MD, Cascio M, Finn OJ (2005) Therapeutic potential of a tumor-specific, MHC-unrestricted T-cell receptor expressed on effector cells of the innate and the adaptive immune system through bone marrow transduction and immune reconstitution. Blood 105(12):4583–4589
Pannell D, Ellis J (2001) Silencing of gene expression: implications for design of retrovirus vectors. Rev Med Virol 11:205–217
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, t Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M (2003) LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302(5644):415–419
Kohn DB, Sadelain M, Glorioso JC (2003) Occurrence of leukaemia following gene therapy of X-linked SCID. Nat Rev Cancer 3(7):477–488
Nienhuis AW, Dunbar CE, Sorrentino BP (2006) Genotoxicity of retroviral integration in hematopoietic cells. Mol Ther 13(6):1031–1049
Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F (2002) HIV-1 integration in the human genome favors active genes and local hotspots. Cell 110(4):521–529
Cattoglio C, Facchini G, Sartori D, Antonelli A, Miccio A, Cassani B, Schmidt M, von Kalle C, Howe S, Thrasher AJ, Aiuti A, Ferrari G, Recchia A, Mavilio F (2007) Hot spots of retroviral integration in human CD34+ hematopoietic cells. Blood 110(6):1770–1778
Mohamedali A, Moreau-Gaudry F, Richard E, Xia P, Nolta J, Malik P (2004) Self-inactivating lentiviral vectors resist proviral methylation but do not confer position-independent expression in hematopoietic stem cells. Mol Ther 10(2):249–259
Chinnasamy D, Chinnasamy N, Enriquez MJ, Otsu M, Morgan RA, Candotti F (2000) Lentiviral-mediated gene transfer into human lymphocytes: role of HIV-1 accessory proteins. Blood 96(4):1309–1316
Costello E, Munoz M, Buetti E, Meylan PR, Diggelmann H, Thali M (2000) Gene transfer into stimulated and unstimulated T lymphocytes by HIV-1-derived lentiviral vectors. Gene Ther 7(7):596–604
Verhoeyen E, Dardalhon V, Ducrey-Rundquist O, Trono D, Taylor N, Cosset FL (2003) IL-7 surface-engineered lentiviral vectors promote survival and efficient gene transfer in resting primary T lymphocytes. Blood 101(6):2167–2174
Zhou X, Cui Y, Huang X, Yu Z, Thomas AM, Ye Z, Pardoll DM, Jaffee EM, Cheng L (2003) Lentivirus-mediated gene transfer and expression in established human tumor antigen-specific cytotoxic T cells and primary unstimulated T cells. Hum Gene Ther 14(11):1089–1105
Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, Binder GK, Slepushkin V, Lemiale F, Mascola JR, Bushman FD, Dropulic B, June CH (2006) Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA 103(46):17372–17377
Pasanen T, Hakkarainen T, Timonen P, Parkkinen J, Tenhunen A, Loimas S, Wahlfors J (2003) TK-GFP fusion gene virus vectors as tools for studying the features of HSV-TK/ganciclovir cancer gene therapy in vivo. Int J Mol Med 12(4):525–531
Pellinen R, Hakkarainen T, Wahlfors T, Tulimäki K, Ketola A, Tenhunen A, Salonen T, Wahlfors J (2004) Cancer cells as targets for lentivirus-mediated gene transfer and gene therapy. Int J Oncol 25(6):1753–1762
Li X, Mukai T, Young D, Frankel S, Law P, Wong-Staal F (1998) Transduction of CD34 + cells by a vesicular stomach virus protein G (VSV-G) pseudotyped HIV-1 vector. Stable gene expression in progeny cells, including dendritic cells. J Hum Virol 1(5):346–352
Ramezani Ali, Hawley Teresa S, Hawley Robert G (2003) Performance- and safety-enhanced lentiviral vectors containing the human interferon-β scaffold attachment region and the chicken β-globin insulator. Blood 101(12):4717–4724
Gao Z, Golob J, Tanavde VM, Civin CI, Hawley RG, Cheng L (2001) High levels of transgene expression following transduction of long-term NOD/SCID-repopulating human cells with a modified lentiviral vector. Stem Cells 19(3):247–259
Cui Y, Golob J, Kelleher E, Ye Z, Pardoll D, Cheng L (2002) Targeting transgene expression to antigen-presenting cells derived from lentivirus-transduced engrafting human hematopoietic stem/progenitor cells. Blood 99(2):399–408
Hasegawa Y, Emi N, Shimokata K, Abe A, Kawabe T, Hasegawa T, Kirioka T, Saito H (1993) Gene transfer of herpes simplex virus type I thymidine kinase gene as a drug sensitivity gene into human lung cancer cell lines using retroviral vectors. Am J Respir Cell Mol Biol 8(6):655–661
Iwakuma T, Cui Y, Chang LJ (1999) Self-inactivating lentiviral vectors with U3 and U5 modifications. Virology 261(1):120–132
Chang LJ, Urlacher V, Iwakuma T, Cui Y, Zucali J (1999) Efficacy and safety analyses of a recombinant human immunodeficiency virus type 1 derived vector system. Gene Ther 6(5):715–728
Zufferey R, Donello JE, Trono D, Hope TJ (1999) Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol 73(4):2886–2892
Higashimoto T, Urbinati F, Perumbeti A, Jiang G, Zarzuela A, Chang LJ, Kohn DB, Malik P (2007) The woodchuck hepatitis virus post-transcriptional regulatory element reduces readthrough transcription from retroviral vectors. Gene Ther 14(17):1298–1304
Charneau P, Clavel F (1991) A single-stranded gap in human immunodeficiency virus unintegrated linear DNA defined by a central copy of the polypurine tract. J Virol 65(5):2415–2421
Charneau P, Alizon M, Clavel F (1992) A second origin of DNA plus-strand synthesis is required for optimal human immunodeficiency virus replication. J Virol 66(5):2814–2820
Sirven A, Pflumio F, Zennou V, Titeux M, Vainchenker W, Coulombel L, Dubart-Kupperschmitt A, Charneau P (2000) The human immunodeficiency virus type-1 central DNA flap is a crucial determinant for lentiviral vector nuclear import and gene transduction of human hematopoietic stem cells. Blood 96(13):4103–4110
Alajez NM, Eghtesad S, Finn OJ (2006) Cloning and expression of human membrane-bound and soluble engineered T cell receptors for immunotherapy. J Biomed Biotechnol 2006(2):68091
Qi R, An H, Yu Y, Zhang M, Liu S, Xu H, Guo Z, Cheng T, Cao X (2003) Notch1 signaling inhibits growth of human hepatocellular carcinoma through induction of cell cycle arrest and apoptosis. Cancer Res 63(23):8323–8329
Tiscornia G, Singer O, Verma IM (2006) Production and purification of lentiviral vectors. Nat Protoc 1(1):241–245
Beatty PL, Plevy SE, Sepulveda AR, Finn OJ (2007) Cutting edge: transgenic expression of human MUC1 in IL-10 −/− mice accelerates inflammatory bowel disease and progression to colon cancer. J Immunol 179(2):735–739
Yu X, Zhan X, D’Costa J, Tanavde VM, Ye Z, Peng T, Malehorn MT, Yang X, Civin CI, Cheng L (2003) Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol Ther 7(6):827–838
Semple-Rowland SL, Eccles KS, Humberstone EJ (2007) Targeted expression of two proteins in neural retina using self-inactivating, insulated lentiviral vectors carrying two internal independent promoters. Mol Vis 13:2001–2011
Mizuguchi H, Xu Z, Ishii-Watabe A, Uchida E, Hayakawa T (2000) IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol Ther 1:376–382
Zychlinski D, Schambach A, Modlich U, Maetzig T, Meyer J, Grassman E, Mishra A, Baum C (2008) Physiological Promoters Reduce the Genotoxic Risk of Integrating Gene Vectors. Mol Ther 16:718–725
Kumar M, Keller B, Makalou N, Sutton RE (2001) Systematic determination of the packaging limit of lentiviral vectors. Hum Gene Ther 12(15):1893–1905
al Yacoub N, Romanowska M, Haritonova N, Foerster J (2007) Optimized production and concentration of lentiviral vectors containing large inserts. J Gene Med 9(7):579–584
Logan AC, Haas DL, Kafri T, Kohn DB (2004) Integrated self-inactivating lentiviral vectors produce full-length genomic transcripts competent for encapsidation and integration. J Virol 78(16):8421–8436
Hanawa H, Persons DA, Nienhuis AW (2005) Mobilization and Mechanism of Transcription of Integrated Self-Inactivating Lentiviral Vectors. J Virol 79:8410–8421
Lucke S, Grunwald T, Uberla K (2005) Reduced Mobilization of Rev-Responsive Element-Deficient Lentiviral Vectors. J Virol 79:9359–9362
Acres B, Limacher JM (2005) MUC1 as a target antigen for cancer immunotherapy. Expert Rev Vaccines 4(4):493–502
Engelmann K, Shen H, Finn OJ (2008) MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Res 68(7):2419–2426
Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA (2005) Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol 174(7):4415–4423
Tsuji T, Yasukawa M, Matsuzaki J, Ohkuri T, Chamoto K, Wakita D, Azuma T, Niiya H, Miyoshi H, Kuzushima K, Oka Y, Sugiyama H, Ikeda H, Nishimura T (2005) Generation of tumor-specific, HLA class I-restricted human Th1 and Tc1 cells by cell engineering with tumor peptide-specific T-cell receptor genes. Blood 106(2):470–476
Micucci F, Zingoni A, Piccoli M, Frati L, Santoni A, Galandrini R (2006) High-efficient lentiviral vector-mediated gene transfer into primary human NK cells. Exp Hematol 34(10):1344–1352
Budak-Alpdogan T, Banerjee D, Bertino JR (2005) Hematopoietic stem cell gene therapy with drug resistance genes: an update. Cancer Gene Ther 12(11):849–863
Yang L, Baltimore D (2005) Long-term in vivo provision of antigen-specific T cell immunity by programming hematopoietic stem cells. Proc Natl Acad Sci USA 102(12):4518–4523
Dudley ME, Rosenberg SA (2003) Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer 3(9):666–675
Engels B, Noessner E, Frankenberger B, Blankenstein T, Schendel DJ, Uckert W (2005) Redirecting human T lymphocytes toward renal cell carcinoma specificity by retroviral transfer of T cell receptor genes. Hum Gene Ther 16(7):799–810
de Witte MA, Jorritsma A, Kaiser A, van den Boom MD, Dokter M, Bendle GM, Haanen JB, Schumacher TN (2008) Requirements for Effective Antitumor Responses of TCR Transduced T Cells. J Immunol 181(7):5128–5136
Bubeník J (2004) MHC class I down-regulation: tumour escape from immune surveillance? Int J Oncol 25(2):487–491
Acknowledgments
We are grateful to all the investigators around the world who generously provided us with their materials. We also thank Hong Ding (Dept of Clinical Pharmacology, Covance Laboratories Inc., Madison, WI) for SAS statistical analysis, and Nehad M. Alajez (Ontario Cancer Institute, Toronto, Ontario, Canada) for discussion and communication throughout the experiments. This work was supported by NIH CA 56103 grant to OJF and Woeber Fund for Breast Cancer Research to XC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, X., Gao, W., Gambotto, A. et al. Lentiviral vectors encoding human MUC1-specific, MHC-unrestricted single-chain TCR and a fusion suicide gene: potential for universal and safe cancer immunotherapy. Cancer Immunol Immunother 58, 977–987 (2009). https://doi.org/10.1007/s00262-008-0624-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0624-0