Abstract
Lenalidomide (Revlimid®; CC-5013) and pomalidomide (CC-4047) are IMiDs® proprietary drugs having immunomodulatory properties that have both shown activity in cancer clinical trials; lenalidomide is approved in the United States for a subset of MDS patients and for treatment of patients with multiple myeloma when used in combination with dexamethasone. These drugs exhibit a range of interesting clinical properties, including anti-angiogenic, anti-proliferative, and pro-erythropoietic activities although exact cellular target(s) remain unclear. Also, anti-inflammatory effects on LPS-stimulated monocytes (TNF-α is decreased) and costimulatory effects on anti-CD3 stimulated T cells, (enhanced T cell proliferation and proinflammatory cytokine production) are observed These drugs also cause augmentation of NK-cell cytotoxic activity against tumour-cell targets. Having shown that pomalidomide confers T cell-dependant adjuvant-like protection in a preclinical whole tumour-cell vaccine-model, we now show that lenalidomide and pomalidomide strongly inhibit T-regulatory cell proliferation and suppressor-function. Both drugs inhibit IL-2-mediated generation of FOXP3 positive CTLA-4 positive CD25high CD4+ T regulatory cells from PBMCs by upto 50%. Furthermore, suppressor function of pre-treated T regulatory cells against autologous responder-cells is abolished or markedly inhibited without drug related cytotoxicity. Also, Balb/C mice exhibit 25% reduction of lymph-node T regulatory cells after pomalidomide treatment. Inhibition of T regulatory cell function was not due to changes in TGF-β or IL-10 production but was associated with decreased T regulatory cell FOXP3 expression. In conclusion, our data provide one explanation for adjuvant properties of lenalidomide and pomalidomide and suggest that they may help overcome an important barrier to tumour-specific immunity in cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lenalidomide has shown clinical activity in patients in a number of haematological malignancies. It is currently FDA-approved in the US for the treatment of patients with transfusion-dependent anaemia due to low-or- intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities. This approval was based on data from an expanded phase II study (manuscript submitted), that confirmed findings from a phase I clinical study [40]. This showed that among patients completing eight or more weeks of treatment with lenalidomide, 67% experienced an erythroid response (red blood cell transfusion independence for ≥8 weeks or rise in haemoglobin of 2 g/dl). Complete cytogenetic remissions were observed in 10 of 20 evaluable patients with abnormal karyotype, nine of whom had del (5) (q31.1). Achievement of cytogenetic remissions suggests a direct effect on the malignant clone.
Lenalidomide has also been FDA approved for use in combination with dexamethasone for treating patients with multiple myeloma who have received at least one prior therapy. This submission was based on data from two pivotal phase III trials in which lenalidomide plus dexamethasone was compared to dexamethasone alone [17]. Lenalidomide treatment also appears to be active in patients with chronic lymphocytic leukemia (CLL) [10], non-Hodgkin’s lymphoma (Wiernik et al. ASCO meeting abstract 2006) and cutaneous T cell lymphoma (CTCL) [49].
Pomalidomide has shown activity in MM [51] and is currently being developed for myelofibrosis [37] with myeloid metaplasia, sickle cell anaemia [15] (due to an ability to enhance foetal haemoglobin) and small cell lung carcinoma.
The ability of thalidomide to costimulate T cells was first reported by Haslett et al. [28] and confirmed with lenalidomide and pomalidomide for both CD4+ and CD8+ T cells [43]. This activity has also been shown to be important for the generation of anti-viral CD8+ T cell responses [27]. Furthermore, in a preclinical whole tumour cell vaccine model, pomalidomide was shown to augment tumour-specific immunity in association with enhanced Th1-type cytokine production [18]. It has been reported that select IMiDs® drugs can augment innate immunity by enhancing γδ T cell [23], NK cell [14] and NKT cell [11] activities. IL-2-primed peripheral blood mononuclear cells (PBMCs) treated with certain IMiDs® drugs demonstrate significantly increased lysis of MM cell lines with killing mediated by CD3-CD56+ cells, and cold target competition assays suggest that this is an NK rather than a LAK related phenomenon [14, 30]. Furthermore, in more recent studies, increased production of IL-2 from lenalidomide/pomalidomide-treated T cells has been shown to be responsible for the enhancement of NK cell activity [30].
The immunopotentiating aspects of lenalidomide and pomalidomide have more recently been demonstrated in patients by increased circulating activated/memory CD45RO+ T cells and increased serum levels of activation markers, cytokines and growth factors, such as soluble interleukin-2 (sIL-2) receptor, granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-12, tumour necrosis factor-α (TNF-α) and IL-8 [5, 51].
The ability of lenalidomide and pomalidomide to enhance immune function led us to investigate the possibility that these drugs may also inhibit the function of T regulatory cells. T regulatory cells are established as important controllers of the immune response and crucial in the control of autoimmune disease [38] and as suppressors of anti-tumour immunity [1, 2]. T regulatory cell numbers are increased during the establishment of tumours [25] and T regulatory cell depletion results in rejection of tumours in several murine tumour models [35, 55]. The presence of T regulatory cells in the tumour infiltrating lymphocyte population is indicative of a poor prognosis in patients with gastric and oesophageal cancers [34]. Furthermore, many solid tumours also have infiltrating T regulatory cells [12, 16, 41] and in ovarian cancer a high CD8:T regulatory cell ratio is a good prognostic indicator [50] (reviewed in reference [67]). T regulatory cells are significantly raised in patients with multiple myeloma [7], non-Hodgkin’s lymphoma [65] and chronic lymphocytic leukaemia [45], conditions which appear to respond to lenalidomide treatment. Interestingly, CTCL blasts in the skin may also resemble T regulatory cells [62]. T regulatory cells can also inhibit the cytolytic effects of NK cells and CD8+ cells, a potential factor in the progression of tumours [59] and which have been shown to be enhanced by lenalidomide and pomalidomide [14, 29].
Inhibition of T regulatory cell function using antibodies such as Ontak® (IL-2-diphtheria toxin fusion protein) [21, 22, 48] and CTLA4-IG [19, 42] is a developing clinical strategy for augmenting anti-tumour responses during cancer vaccination. Inhibition of T regulatory cells using CD25-specific monoclonal antibodies has been shown to promote the rejection of several transplantable murine tumour cell lines including melanoma, leukemia and colorectal carcinoma [35, 63] and to enhance vaccine-mediated anti-tumour immunity in renal cell carcinoma patients [13].
The current study demonstrates that lenalidomide and pomalidomide can inhibit the proliferation of FOXP3+ CTLA-4+CD4+CD25high T regulatory cells in vitro. Furthermore, these drugs can also inhibit the suppressor function of the T regulatory cells against autologous responder cells in vitro. This inhibitory activity is associated with reduction of FOXP3 and OX40 expression. Finally, we show that pomalidomide is able to reduce lymph node T regulatory cell numbers following challenge with live tumour cells. These results suggest a mechanism by which these drugs may overcome T regulatory cell suppressive effects and increase anti-tumour immunity independently of their documented direct T cell costimulatory activity.
Materials and methods
Reagents
Thalidomide, lenalidomide and pomalidomide were obtained from the Celgene Corp (Summit, New Jersey, USA), and dissolved in DMSO to create 10 mM stock solutions that were maintained at −20°C for no longer than 1 week. For in vivo studies, the drugs were dissolved in at a concentration of 5 mg/ml in 0.5% DMSO in PBS and stored at 4°C for the duration of the experiment.
Phenotyping of activated PBMCs
PBMCs were obtained from fresh buffy coats and seeded at 1 × 106 cells/ml in 24-well plates in RPMI culture medium (RPMI containing 10% foetal calf serum, 100 units of penicillin and streptomycin and 100 units of glutamine), to which 150 U/ml of IL-2 (Chiron UK) was added. In addition cultures were treated with lenalidomide, pomalidomide or thalidomide (all 10 μM) or the equivalent concentration of DMSO (0.1% v/v). During culture aliquots of cells were taken regularly over a period of 12 days, and surface stained with anti-CD25 FITC and anti-CD4 PerCP followed by intracellular staining with anti-CTLA-4 (CD152) PE (Becton Dickinson) and anti-FOXP3 (clone PCH101 from Ebioscience). Expression of CD25highCD4+ cells expressing CTLA-4 and FOXP3 in the PBMC population was analysed using a FACSCalibur. The expression of T regulatory cells in PBMC cultures was also examined using varying concentrations of the pomalidomide, lenalidomide, and thalidomide, after a period of incubation which was shown to give a maximal inhibition of T regulatory cell expression.
Measurement of T regulatory cell proliferation in PBMC populations
Freshly isolated PBMC were stained using the CellTrace™ CFSE Cell Proliferation Kit (Molecular Probes, Eugene, USA). PBMC were re-suspended in pre-warmed PBS/0.1% BSA at a final concentration of 1 × 106 cells/ml plus CFSE (1 μM). The cells were incubated with the dye at 37°C for 10 min and the staining was quenched by addition of five volumes of ice-cold RPMI culture medium and 5-min incubation on ice. The cells were pelleted by centrifugation and washed. The stained PBMC were treated for 7 days in RPMI culture medium with lenalidomide, pomalidomide (both 10 μM) and thalidomide (10 and 100 μM), plus IL-2 (500 IU/ml). Cells were surface stained with anti-CD4-PerCP and anti-CD25-APC (BD Pharmingen), followed by intracellular staining with anti-FOXP3-PE (clone PCH101, Ebioscience). The expression of CD4+CD25high T cells expressing FOXP3 and showing changes in CFSE staining was measured using a FACSCalibur.
Isolation of T regulatory cells from buffy coats
Buffy coats were obtained from the National Health Blood Service and used to prepare PBMCs. The PBMCs were used for the isolation of CD25+CD4+ cells using a Dynal CD4+CD25+ isolation kit either immediately, or after 7 days of culture with 500 IU u/ml IL-2 and either 10 μM of pomalidomide or lenalidomide, 100 μM thalidomide or DMSO. Briefly, PBMCs were treated with a mixture of CD14, CD56, CD19, CD8 and CD235a (glycophorin A), followed by depletion dynabeads to prepare a population of isolated CD4+ cells. These cells were then treated with CD25 dynabeads, and the CD25− population was separated out and stored (at 37°C) for later use, while the CD25+ population was detached from the beads using DETACHaBEAD™. CD45RA expressing cells were depleted, providing a population of CD45RO positive CD4+CD25+ T cells (to which regulatory activity is restricted [36, 53, 56]). The purity of this population was typically 95%. The T regulatory cells isolated immediately from PBMCs were treated overnight with pomalidomide, lenalidomide or DMSO control without exogenous IL-2. After incubation, one aliquot of the cells were washed, and surface-stained with anti-CD25 APC, and anti-CD4 PERCP (BD Pharmingen), followed by intracellular staining with anti-CTLA-4 (CD152) (BD Pharmingen) and FOXP3-FITC (clone PCH101 from Ebioscience). A second aliquot was used to stain for CD134 (OX-40) expression, and the staining included anti-CD4 PERCP, anti-CD25 APC, anti-CTLA-4 PE, and the anti-CD134 FITC (BD Pharmingen). A further aliquot was used for performing an annexin 5/7AAD apoptosis assay. Briefly, cells were stained with annexin 5-PE and 7AAD PERCP, and the expression of these markers on the cells was analysed using a FACscalibur. The percentage of early apoptotic (annexin 5 positive), late apoptotic (7AAD positive) and dead cells (annexin 5/7AAD positive cells) was then determined. The remaining T regulatory cells were used for the proliferation assay as described below.
Analysis of T regulatory cell TGF-β receptor, surface TGF-β, intracellular IL-10, GITR and OX40
T regulatory cells were isolated by the procedure above and treated for 24, 48 or 72 h with pomalidomide, lenalidomide or thalidomide (10 μM). Cells were then surface stained with anti-CD4 PerCP and anti-CD25 APC antibodies (BD Pharmingen), and mouse antibodies to TGF-β or biotinylated TGF-β (R&D), followed by staining with anti-mouse IgG FITC or Streptavidin FITC, respectively. For IL-10 staining, cells were treated for 3 h with brefeldin A followed by surface staining with anti-CD4 PERCP and anti-CD25 APC antibodies, and then cells were fixed and permeabilized before staining with FITC-conjugated anti-IL-10 (R&D). For GITR and CD134 (OX-40) analysis, cells were incubated for 24 h with various concentrations of pomalidomide, lenalidomide, thalidomide or DMSO control, then surface stained with anti-CD4 PerCP and anti-CD25 APC antibodies (BD Pharmingen), followed by permeabilization and intracellular staining with anti-GITR-PE (BD Pharmingen) and anti-CD134-FITC. Flow cytometric data are expressed as percentage of the mean channel fluorescence (MCF) values obtained with the DMSO control in the CD4+ population using a CD25+ gate and as percentage values in comparison with the DMSO control % expression in the purified T regs.
Measurement of suppressor function of T regulatory cells using a proliferation assay with CD25−CD4+ cells
T regulatory cells were isolated by the procedure outlined in “isolation of T regulatory cells from buffy coats” either immediately from the fresh buffy coat or after 7 days of culture of the buffy coat PBMCs with 500 units/ml IL-2 and either 10 μM of lenalidomide, pomalidomide, thalidomide or a DMSO control. When isolated immediately from buffy coats they were treated overnight with varying concentrations of pomalidomide, lenalidomide, thalidomide or DMSO control. The cells were washed thoroughly (three times with RPMI culture medium) after incubation with the IMiDs, DMSO and IL-2 and then incubated with CD4+CD25− cells obtained from the same buffy coat at a 1:1 ratio, in a 3-day proliferation assay using 96-well plates pre-coated with 0.1 μg/well CD3. After 72 h thymidine (0.5 μCi) was added to each well overnight, and cells were harvested to obtain counts per minute (cpm) per well. Under these conditions, the CD4+CD25+ (T regulatory cell) population inhibits proliferation of the CD4+CD25− population of cells. The inhibitory effects of certain IMiDs® drugs on the regulatory T cell suppressor function were assessed by calculating the increase in cpm detected in mixed CD4+CD25+:CD4+CD25− cultures when the CD4+CD25+ cells were pre-treated with drugs versus DMSO control.
Established tumour cell lines and animals
CT26 mouse colon carcinoma cells were grown in DMEM + 10% FCS + 2 mM l-glutamine and maintained in a sub-confluent monolayer at 37°C in humidified atmosphere containing 5% CO2 and subcultured every 3–5 days using Accutase. Balb/c female mice were purchased from Harlan (UK) and used at approximately 12–15 weeks old.
Effects of certain ImiDs® drugs on expression of murine T regulatory cells in vivo from spleen and lymph nodes
The effect of pomalidomide on T regulatory cell expansion in a CT26 colorectal cancer murine model was investigated. Balb/c mice were injected subcutaneously with 5 × 105 live CT26 cells and then mice were treated intraperitoneally with pomalidomide (50 mg/kg) or PBS daily for 6 days. On day 7 spleen and draining lymph nodes were collected. Lymphocytes and splenocytes were washed and red cells were lysed with red cell lysis solution (Gentra, USA). Cells were washed with staining buffer (FACSflow, Becton Dickinson) containing 1% normal mouse serum (Sigma) and 0.01% azide (Sigma), in a 96-well round bottom plate, and then incubated with 10 μl of the pre-titrated monoclonal antibodies anti-CD4 FITC, anti-CD25 PercP, anti-CTLA-4 PE and anti-FOXP3 APC or their respective isotype controls for 20 min at 4°C. They were washed once with staining buffer. Cells were washed once with FACs buffer and twice with Cytofix (BD Cytofix/Cytoperm kit). Then they were permeabilized with Perm wash (BD Cytofix/Cytoperm kit) for 10 min at room temperature. The intracellular antibody was added in combination with Perm wash and incubates for 15 min at 4°C. Cells were washed once with FACs buffer and resuspended in 200 μl of 1% paraformaldehyde (Sigma) and stored in the dark at 4°C until analysis. Analysis was carried out using a FACScan (Becton Dickinson). All antibodies were from BD Pharmingen (Oxford, UK).
Statistical analyses
Statistical significance of the results was measured by one-way ANOVA followed by post hoc bonferonni calculations or paired t tests as appropriate to the experiment used using the program Graphpad PRIZM. The numbers of experiments and the data points collected were calculated in order to ensure that enough data points were available for significant statistical calculations.
Results
Pomalidomide and lenalidomide inhibit the expansion of CD25 high CD4+CTLA4+FOXP3+ T regulatory cells in activated PBMCs
To determine whether lenalidomide or pomalidomide can alter the expansion of T regulatory cells in a PBMC population we examined the expression of FOXP3+CTLA-4highCD25highCD4+ cells in PBMC maintained in IL-2 over the course of 7 days. Figure 1 shows that there is an inhibitory effect of lenalidomide or pomalidomide on the expression of CD4+CD25highCTLA-4+FOXP3+ cells. In cells treated with IL-2 alone, there is a marked increase in the percentage of CD4+CD25highCD152+FOXP3+ T regulatory cells in a population of CD4+CD25high cells over a period of 7 days. Incubation with lenalidomide or pomalidomide significantly decreases expression of the T regulatory cell population after 7 days of culture (Fig. 1a). The drugs decrease the percentage of CD4+CD25high cells expressing both CTLA-4 and FOXP3 from 25 to 12% (P < 0.01 using one way ANOVA and post-hoc bonferroni test). In contrast, thalidomide has no significant effect on the percentage of T regulatory cells in the PBMC population during the time course. The inhibition occurs to a similar extent in both CTLA-4+ and CTLA-4− populations. The decrease in the expression of these cells is not due to an increase in apoptosis of these cells (Fig. 1c) but Lenalidomide and pomalidomide inhibit the proliferation of Tregs in the PBMC population after 7 days as measured by CFSE staining (Fig. 2a, b). CD4+CD25− cells did not proliferate in response to IL-2. There was no effect of any of the drugs on the expression of CD25−CD4+ cells during the 7-day cultures. Since the drugs do not affect numbers of CD25−CD4+ cells in these assay conditions, we believe there is no evidence that there is conversion of these cells into Tregs.
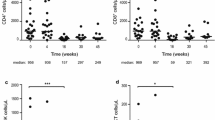
Effect of IL-2 and pomalidomide, lenalidomide and thalidomide treatment on expression of T-regulatory cells in a PBMC population. PBMC were treated with IL-2 (150 U/ml) and either lenalidomide, pomalidomide, thalidomide (all 10 μM) or DMSO alone every 2 days. Cells were then harvested, stained with anti-CD4 PERCP, anti-CD25 APC, anti-CTLA-4 PE (BD Pharmingen) and anti-FOXP3 FITC (Ebioscience) and analysed using a FACSCalibur as described in “Materials and methods”. a Shows results in a 7-day time course, expressed as the mean percentage of the expression of CD4+CD25high CTLA-4+ FOXP3+ cells in the CD4+CD25+ population derived from three separate experiments and error bars indicate standard error. Asterisks indicate significant differences (P < 0.05) between cell expression in the DMSO control versus lenalidomide or pomalidomide using repeated measures ANOVA. b Shows results with varying concentrations of lenalidomide, pomalidomide or thalidomide cultured under the conditions above with harvesting of cells on day 7. There is no significant increase in the apoptosis of FOXP3+CD4+CD25+ cells on day 7 after treatment of the PBMC with 10 μM lenalidomide, pomalidomide or 100 μM thalidomide (c)
Effect of the IMiDs on proliferation of FOXP3+ cells in the CD4+ T cell population as assessed using CFSE staining. CFSE staining of PBMCs pretreated with lenalidomide, pomalidomide or thalidomide on day 7 is shown as a representative flow cytometry plot in (a), and the percentage inhibition of proliferation is shown in a bar-chart in (b), with results taken from three experiments with range indicated by error bars
To determine IC50s for inhibition of T regulatory cell expansion, PBMCs were cultured for 7 days with IL-2 (150 units/ml) and varying concentrations of lenalidomide, pomalidomide and thalidomide. IC50s, obtained from the day 7 data, were ~10 μM for lenalidomide, ~1 μM for pomalidomide with no effect of thalidomide up to 200 μM (Fig. 1b).
Lenalidomide and pomalidomide abolish or markedly inhibit the suppression of anti-CD3mAb stimulated CD25−CD4+ cell proliferation by T regulatory cells
The effects of lenalidomide and pomalidomide on the suppressor function of purified T regulatory cells were examined by determining their effect when added to autologous CD25−CD4+ cells stimulated on anti-CD3 mAb-coated plates. The addition of isolated CD25+CD4+ T regulatory cells to the autologous CD25− responder population at a 1:1 ratio inhibits proliferation of the CD25− cells (Fig. 3a). However, pretreatment of the CD25+CD4+ T regulatory cells with either lenalidomide or pomalidomide (at 10 μM) completely overcomes this inhibition. These drugs do not affect the proliferation of the isolated CD25+CD4+ T regulatory cells during the course of the 3-day assay (data not shown). When CD4+CD25+ cells from PBMCs treated with IL-2 for 7 days are isolated using the Dynal treg cell kit, these cells are suppressive against autologous CD3 treated CD4+CD25− cells in the proliferation assay using a 1:1 ratio of CD4+CD25+:CD4+CD25−cells and treatment of the PBMC cultures with lenalidomide or pomalidomide inhibits this suppressive action (Fig. 3b).
Effect of pomalidomide, lenalidomide and thalidomide on the ability of T regulatory cells to suppress anti-CD3 stimulated CD25−CD4+ cells. Purified T regulatory cells were isolated as described in “Materials and methods”. Cells were either freshly isolated from PBMCs and treated overnight with varying concentrations of lenalidomide or pomalidomide or thalidomide or isolated after 7 days of culture with 500 units/ml IL-2 and either DMSO or 10 μM lenalidomide, thalidomide or pomalidomide. The cells were then washed three times with PBS, and then incubated at a 1:1 ratio with CD25−CD4+ cells (75,000 cells of CD25− cells and CD25+ cells per well). In a results with freshly isolated tregs are shown and are expressed as the counts per minute (cpm) obtained with the CD4+CD25− cells incubated with CD4+CD25+ cells pretreated with the DMSO control. a Shows the inhibition of results expressed as the mean of three separate experiments (error bars indicate the standard error). An asterisk signifies a significant difference in proliferation compared to the control population of CD4+CD25− cells incubated with DMSO treated CD4+CD25+ cells (P < 0.05 by one way ANOVA). b Shows suppression of CD4+CD25− cells by T regulatory cells purified from the PBMC population after 7 days of culture with 500 units/ml of IL-2 and either DMSO, Thalidomide, lenalidomide or pomalidomide as described in “Materials and methods”. The cells were incubated at a 1:1 ratio with CD25−CD4+ cells (75,000 cells of CD25− cells and CD25+ cells per well). Results are taken from three separate experiments, and expressed as the % of cpms obtained with the DMSO control CD25− cell population, (due to the variability of the cpms obtained in the individual experiments) with the range indicated by error bars. c Shows flow cytometric analysis of annexin-V/7AAD staining of the CD4+CD25+ cells isolated under the aforementioned conditions and treated with lenalidomide, pomalidomide or thalidomide. These cells do not undergo a greater extent of apoptosis compared to untreated or DMSO treated cells as shown in three repeated experiments
Lenalidomide and pomalidomide inhibit T regulatory cell FOXP3 expression but do not affect T regulatory cell survival/apoptosis
We set out to determine whether the suppressive function of the drugs on purified T regulatory cells was due to a cytotoxic or apoptotic effect or whether there was down regulation of FOXP3, a marker which is strongly associated with the inhibitory function of these cells [6, 33, 52]. Our data suggest that neither lenalidomide nor pomalidomide had any significant effect on T regulatory cell survival/apoptosis after 24 h of culture with the drugs. (Fig. 3c). However, there was an inhibition of FOXP3 expression in CD25+CD4+CTLA-4+ cells up to 50% at the highest concentration of pomalidomide used (10 μM) (Fig. 4a, b). There was no significant effect of thalidomide on FOXP3 expression even at 200 μM.
Effect of pomalidomide, lenalidomide and thalidomide on FOXP3 expression on purified T regulatory cells. FOXP3 expression in the purified T regulatory cell population (gated on the FACs as CD4+CD25+ CTLA-4+ cells) is decreased at the highest two doses of lenalidomide and pomalidomide as indicated by a representative flow cytometry plot (a), and as an inhibition curve of FOXP3 expression (b), with results averaged from five experiments with the range indicated by error bars
Effect of lenalidomide and pomalidomide on T regulatory cell expression of surface TGF-β, TGF-β receptor, intracellular TNF-α, intracellular IL-10 and OX40
Lenalidomide and pomalidomide can co-stimulate T cells to produce TNF-α after 72 h culture on αCD3 coated wells. Since other members of the TNF family of receptors such as GITR and OX-40 have been shown to be important in the function of T regulatory cells, we wished to determine whether the compounds were differentially affecting TNF-α secretion, GITR expression or OX-40 expression in either purified T regulatory cells after short-term culture or in PBMC treated with IL-2 after 7 days of culture.
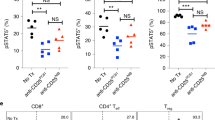
We found that GITR expression on purified T regulatory cells was not affected either after short-term culture (1–3 days) or in CD4+CD25high cells after 7 days’ culture (data not shown). However, we found strong inhibition of OX-40 receptor expression in purified T regulatory cells after overnight culture with lenalidomide and pomalidomide at concentrations as low as 0.01 μM. Thalidomide was inhibitory only at 100 μM (Fig. 5a, b). In contrast, OX-40 expression on CD4+CD25high cells was not significantly affected in 7-day IL-2 treated cultures in the presence of other lymphocytes (data not shown).
Effect of pomalidomide, lenalidomide and thalidomide on GITR, CD134 (OX-40), TGF-β receptor, TGF-β and intracellular IL-10 expression in T regulatory cells. CD4+CD25+ T regulatory cells were isolated as described under “Materials and methods” and were incubated for 24 h with either PBS with DMSO or varying concentrations of lenalidomide, pomalidomide or thalidomide. Lenalidomide and pomalidomide inhibit CD134 expression at levels as low as 0.01 μM, whereas thalidomide inhibits expression only at 100 μM. a Is a representative FACs plot showing inhibition of OX40 expression in purified CD4+CD25high cells (shown to have suppressive activity against CD25−CD4+ cells) which are gated on the FOXP3+ population. The quantitation of the inhibition is shown in (b), which is a plot averaged from three separate experiments. Cells were also stained with FACs antibodies against TGF-β receptor, surface TGF-β, intracellular IL-10 expression or intracellular TNF-α expression as described under “Materials and methods”. No changes were seen in the expression of these markers with 10 μM of drug treatment as measured by flow cytometric analysis (c). Results in the two panels in Fig. 3c are expressed as percentages of (1) expression of the cellular markers as compared to the DMSO control and (2) mean channel fluorescence (MCF) of the markers as compared to the DMSO control. Values are expressed as percentages of the DMSO control due to the variability of the raw values (TGF: 6–32% positive expression, MCF = 10–25; TGF receptor: 25–100% positive expression, MCF = 58–600; IL-10: 12–93% positive expression, MCF = 8.2–86.3; TNF-α: 2–19% positive expression, MCF: 12–30). The results are the means of the percentage of the DMSO control taken from three separate experiments with the range indicated by error bars
We next assessed the effect of lenalidomide and pomalidomide on the T regulatory cell expression of TGF-β, TGF-β receptor and intracellular IL-10. Using purified T regulatory cells we found that neither of these drugs significantly affected the percent expression or MCF readings of cell surface TGF-β, TGF-β receptor, intracellular IL-10 or TNF-α (Fig. 5c). These values (MCF, also known as mean fluorescence intensity or MFI, and the percentage expression of the marker) are expressed as percentages of the DMSO control for each experiment. This is due to the fact that it is difficult to show absolute data in the graph because the % expression and MFIs vary in the three experiments done as shown in the legend to Fig. 5. There was also no effect on secreted TGF-β or IL-2 from purified T regulatory cells (data not shown).
Effect of pomalidomide on the expression of T regulatory cells in vivo using whole tumour cell challenge in the CT26 murine model
We investigated the effect of pomalidomide on the % of T regulatory cells in the spleen and lymph nodes of mice after challenge of the mice with CT26 tumours. After tumour challenge, a regimen of 6 days of treatment with pomalidomide at 50 mg/kg significantly decreased the number of CD4+/CD25+/CTLA-4/FOXP3+ T regulatory cells in the draining lymph nodes by 40% compared to the PBS DMSO control (P < 0.05 by paired t test) (Fig. 6). However, pomalidomide did not have a significant inhibitory effect on the number of CD4+/CD25+/CTLA-4/FOXP3+ T regulatory cells in the spleens of these mice although a decrease was seen. There was no effect of pomalidomide on the numbers of CD25−CD4+ cells or the total numbers of CD4+ lymphocytes in the lymph nodes or spleen (data not shown).
Effect of pomalidomide on T regulatory cell number within lymph nodes and spleen. BALB/C mice were injected subcutaneously with 5 × 105 live CT26 and then injected intraperitoneally with pomalidomide (50 mg/kg), or PBS. On day 7, spleen and draining lymph nodes were collected. Splenocytes and lymphocytes were stained with the following antibodies: CD4, CD25, CTLA-4, FOXP3 and their respective controls. The results are averaged from three separate experiments using five animals per condition in each experiment. Each asterisk indicates significance compared to the PBS control (containing DMSO) (P < 0.05 by paired t test)
Discussion
Lenalidomide and pomalidomide have shown impressive activity in the treatment of patients with multiple myeloma [24], although the binding target(s) of these drugs have not yet been elucidated. However, a number of mechanisms appear to be important in the anti-MM effect; these include pro-apoptotic [44] and anti-angiogenic effects [39]. Based on previous studies of the immunological effects of lenalidomide and pomalidomide and the success of pomalidomide in a pre-clinical cancer vaccine model for which T regulatory cells are known to provide a barrier to efficacy, we wanted to determine whether these compounds had any effect on the T regulatory cell population. Our findings have shown two distinct effects of these drugs on T regulatory cells. The first effect is the ability to decrease the expansion of T regulatory cells (FOXP3+CTLA-4+CD4+CD25high cells) within PBMC populations maintained in IL-2. PBMC treated with IL-2 show an expansion of these cells after 7 days. These cells, when isolated from PBMCs 7 days after IL-2 treatment, are able to suppress proliferation of CD25−CD4+ T cells and pomalidomide and lenalidomide can inhibit this suppression (Fig. 2b). There are only a small percentage of such cells in the PBMC population at the start of the incubation period under the gating conditions we use, as we restrict the population to CD25high cells to rule out any possibility that activatory cells are being assessed. Several publications show that CD25highCD4+CTLA-4+FOXP3+ cells within a PBMC population are regulatory T cells and that IL-2 leads to expansion of these cells in patients [31, 32, 64, 66]. Treatment of patients with IL-2 is a common regimen in cancer, and the levels of IL-2 detectable in the patients’ blood can fall in the range we have used in our in vitro experiments. Therefore it is relevant to assess the effects of the drugs on populations of Tregs that arise during IL-2 treatment. Under these conditions, in the presence of the drugs, whereas the expression of CD25highCD4+CTLA-4+FOXP3+ cells is decreased (Fig. 1a), and the proliferation of these cells is also decreased as measured by CFSE staining (Fig. 2a) there is no concomitant effect on proliferation of non-T regulatory (CD25−CD4+FOXP3-CTLA-4−) cells in the PBMC population (although we did not investigate proliferation of other T cell phenotypes) nor is there any significant effect on cell survival/apoptosis of the T regulatory cells, either in the PBMC population or as purified cells, ruling out a toxic effect on T cell populations. Also, thalidomide (at concentrations up to 200 μM) does not inhibit the expansion of the T regulatory cells in PBMCs treated with IL-2.
We found that lenalidomide and pomalidomide affect the function of purified T regulatory cells, as shown by the ability to prevent the inhibition of proliferation seen upon addition of purified T regulatory cells to the autologous CD4+CD25− responder cell population. This inhibitory effect on T regulatory cell function is apparent after 24-h incubation with lenalidomide or pomalidomide. In contrast, thalidomide does not inhibit the function of T regulatory cells under these assay conditions, even up to 200 μM. Thalidomide is far less active in its immunomodulatory effects (T cell costimulation and. production) and its inhibitory effects on LPS induced monocyte TNF production than the second generation IMiDs, pomalidomide and lenalidomide, and therefore it is not unexpected that there is a lack of activity on T regulatory cells. It also has a lack of activity in other in vitro functional assays such as NK-mediated killing of mAb-coated tumour cells by antibody-dependent cellular cytotoxicity and is also less effective therapeutically than pomalidomide and lenalidomide. Structurally, pomalidomide and lenalidomide possess an additional four-amino group, not present in thalidomide, and this accounts for differences in pharmacological behaviour. The stability of the drugs is similar with half-lives ranging from 3.9 h for lenalidomide [20] to 6 h for pomalidomide [54] and thalidomide [58], so differences between the efficacy of lenalidomide and pomalidomide compared with thalidomide are not due to differences in their biological stability. The possibility that pomalidomide and lenalidomide are affecting contaminating activatory cells within the regulatory T cell population is very unlikely as the drugs do not affect CD4+ or CD8+ effector cells (potential minor contaminants) in the absence of a primary stimulus, and the effector CD4+CD25− cells are not exposed to the drugs under these assay conditions. Proliferation of purified conventional T cells is increased with lenalidomide and pomalidomide in the presence of anti-CD3 alone or αCD3/αCD28. This has been shown in a previous paper [43]. The same effect was also seen in purified CD8+ T cells with thalidomide [28]. Therefore, the effect on conventional T cells and the effect on Tregs are clearly distinct (thalidomide had no effect on Treg in our paper). We observed inhibitory effects on Treg function that led to increased T cell activation but without exposing the conventional T cells to the drugs (extensive washing followed incubation with Treg prior to mixing the cell populations).
The inhibition of T regulatory cell function by pomalidomide and lenalidomide appears to be related to down-regulation of the important T regulatory cell transcription factor FOXP3, which is critical for the functioning of regulatory T cells [6] and not to alterations in the expression of the TNF family protein GITR, which is critical in the function of T regulatory cells [9, 46] or TGF-β (cell surface or secreted), TGF-β receptor, IL-10 or TNF-α. Preliminary data from our group suggest that pomalidomide downregulates the expression of FOXP3 mRNA in Tregs and we are now backing this up with QPCR and Western blotting data.
TNF-related agonists such as OX-40 (CD134) can abolish the suppressive activity of T regulatory cells, e.g. mice whose T regulatory cells are deficient in CD134 have dysfunctional T regulatory cells, and T regulatory cells that are CD134 positive are more highly suppressive towards activated T cells than T regulatory cells lacking CD134 [47, 57, 60] We have found that lenalidomide and pomalidomide inhibit the expression of CD134 by T regulatory cells by up to 50% at concentrations as low as 0.01 μM. In contrast, thalidomide inhibits expression only at 100 μM. Thus, modulation of CD134 on T regulatory cells is another potential mechanism to explain the suppressive effects of lenalidomide and pomalidomide on T regulatory cells.
We have utilized the CT26 murine colorectal cancer model to study the effects of selected IMiDs® drugs on the expression of T regulatory cells in vivo. As an initial experiment towards a separate paper on the effects of the IMiDs on treg function in vivo, we looked at the effect of pomalidomide (our most effective drug in vitro) on the level of T regulatory cells within the draining lymph nodes and spleens of Balb/c mice after CT26 tumour challenge, which more closely represents a clinical treatment setting. We treated the animals for 7 days with pomalidomide after tumour challenge and analysed the numbers of Tregs at this time as this time point had given an expansion of Tregs to a level at least 100% greater than at day 0 in the human in vitro assay. It has previously been shown that T regulatory cells are important in the inhibition of tumour rejection in CT26 challenged BALB/C mice and that the numbers of T regulatory cells in the spleen and draining lymph nodes increase with CT26 tumour challenge [61]. The number of tregs in the lymph nodes of non-tumour challenged animals was on average 11% of the CD4 population (which represented 30–40% of the lymph node cells and did not change significantly in tumour challenged compared to tumour free animals after 7 days). The number of T regulatory cells increased to 18% of the CD4 population in the draining lymph nodes of animals challenged with CT26 tumours compared to the unchallenged animals (P < 0.05 by one way ANOVA and post-hoc bonferroni analyses). These results are similar to those previously reported by Valzasina et al. [61]. We administered pomalidomide for 7 days and found that the percentage of T regulatory cells in the draining lymph nodes decreased by up to 40% in compared to the PBS control in the tumour challenged animals (P < 0.05 by one-way ANOVA and post-hoc bonferroni analyses). Pomalidomide therefore decreases the number of tregs to levels found in the lymph nodes of non tumour challenged animals. This is a significant finding as Tregs are important for the inhibition of anti-tumour immunity in this mouse model and depletion of tregs induces immunity to CT26 [26]. Prior to day 7, the numbers of Tregs in spleen and lymph nodes were not significantly different in challenged versus unchallenged animals and pomalidomide also has no effect on the Treg numbers (data not shown). Drug efficacy on tumour growth was not assessed in this particular experiment since the tumour load was too small to measure at day 7: the focus was on treg expansion in response to CT26 and the ability of pomalidomide to decrease this expansion in draining lymph nodes, which we have demonstrated. We saw no significant difference in the T regulatory cell expression in the spleens of the pomalidomide treated versus PBS treated animals. This difference may be due to a greater expansion of T regulatory cells in the lymph nodes, where T cell priming occurs, compared to the spleen.
We propose that the inhibitory effects of lenalidomide and pomalidomide on T regulatory cell expansion and function play a potentially important role in clinical efficacy during the treatment of immunocompromised patients, such as those with cancer. The enhanced expansion and function of T regulatory cells has been reported in a number of solid and haematological cancers including MM [3, 8, 50, 64]. Our results suggest that lenalidomide and pomalidomide, but not thalidomide, may enhance anti-tumour immunity by inhibiting the suppressive effects of this population. This activity is in addition to direct costimulatory effects observed on purified and sub-optimally activated T cells and the subsequent generation of Th1-type immunity including enhanced effector NK cell function [4]. Both properties fit in with clinical studies which have highlighted a general immunostimulatory effect in solid tumour patients treated with lenalidomide [5] and in MM patients treated with pomalidomide [51]. In conclusion, we believe that the inhibitory effects on T regulatory cells may be a crucial component in the adjuvant-like properties of lenalidomide and pomalidomide in the context of tumour-mediated immunosuppression, including the tumour vaccine setting.
References
Antony PA, Paulos CM, Ahmadzadeh M, Akpinarli A, Palmer DC, Sato N, Kaiser A, Hinrichs CS, Klebanoff CA, Tagaya Y, Restifo NP (2006) Interleukin-2-dependent mechanisms of tolerance and immunity in vivo. J Immunol 176:5255–5266
Antony PA, Restifo NP (2002) Do CD4+CD25+ immunoregulatory T cells hinder tumor immunotherapy? J Immunother 25:202–206
Baecher-Allan C, Anderson DE (2006) Immune regulation in tumor-bearing hosts. Curr Opin Immunol 18:214–219
Bartlett JB, Dredge K, Dalgleish AG (2004) The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer 4:314–322
Bartlett JB, Michael A, Clarke IA, Dredge K, Nicholson S, Kristeleit H, Polychronis A, Pandha H, Muller GW, Stirling DI, Zeldis J, Dalgleish AG (2004) Phase I study to determine the safety, tolerability and immunostimulatory activity of thalidomide analogue CC-5013 in patients with metastatic malignant melanoma and other advanced cancers. Br J Cancer 90:955–961
Bettelli E, Dastrange M, Oukka M (2005) Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci USA 102:5138–5143
Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, Classen S, Schultze JL (2006) In vivo peripheral expansion of naive CD4+CD25high FOXP3+ regulatory T cells in patients with multiple myeloma. Blood 107:3940–3949
Beyer M, Schultze JL (2006) Regulatory T cells in cancer. Blood 108:804–811
Calmels B, Paul S, Futin N, Ledoux C, Stoeckel F, Acres B (2005) Bypassing tumor-associated immune suppression with recombinant adenovirus constructs expressing membrane bound or secreted GITR-L. Cancer Gene Ther 12:198–205
Chanan-Khan A, Miller KC, Takeshita K, Koryzna A, Donohue K, Bernstein ZP, Mohr A, Klippenstein D, Wallace P, Zeldis JB, Berger C, Czuczman MS (2005) Results of a phase 1 clinical trial of thalidomide in combination with fludarabine as initial therapy for patients with treatment-requiring chronic lymphocytic leukemia (CLL). Blood 106:3348–3352
Chang DH, Liu N, Klimek V, Hassoun H, Mazumder A, Nimer SD, Jagannath S, Dhodapkar MV (2006) Enhancement of ligand dependent activation of human natural killer T cells by Lenalidomide: therapeutic implications. Blood 108:618–621
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949
Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J (2005) Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 115:3623–3633
Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, Lin B, Podar K, Gupta D, Chauhan D, Treon SP, Richardson PG, Schlossman RL, Morgan GJ, Muller GW, Stirling DI, Anderson KC (2001) Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 98:210–216
de Parseval LM, Brady H, Glezer E, Richard N, Muller G, Morris CL, Stirling D, Chan K (2004) Novel immunomodulatory drugs (IMiDs(R)): a potential, new therapy for {beta}-hemoglobinopathies. ASH Annu Meet Abstr 104:3740
Diederichsen ACP, Zeuthen J, Christensen PB, Kristensen T (1999) Characterisation of tumour infiltrating lymphocytes and correlations with immunological surface molecules in colorectal cancer. Eur J Cancer 35:721–726
Dimopoulos MA, Spencer A, Attal M, Prince M, Harousseau JL, Dmoszynska A, Yu Z, Olesnyckyj M, Zeldis J, Knight R (2005) Study of lenalidomide plus dexamethasone versus dexamethasone alone in relapsed or refractory multiple myeloma (MM): results of a phase 3 study (MM-010). ASH Annu Meet Abstr 106:6
Dredge K, Marriott JB, Todryk SM, Muller GW, Chen R, Stirling DI, Dalgleish AG (2002) Protective antitumor immunity induced by a costimulatory thalidomide analog in conjunction with whole tumor cell vaccination is mediated by increased Th1-type immunity. J Immunol 168:4914–4919
Egen JG, Kuhns MS, Allison JP (2002) CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 3:611–618
Fine HA, Kim L, Albert PS, Duic JP, Ma H, Zhang W, Tohnya T, Figg WD, Royce C (2007) A phase I trial of lenalidomide in patients with recurrent primary central nervous system tumors. Clin Cancer Res 13:7101–7106
Foss F (2006) Clinical experience with denileukin diftitox (ONTAK). Semin Oncol 33:S11–S16
Foss FM (2000) DAB(389)IL-2 (denileukin diftitox, ONTAK): a new fusion protein technology. Clin Lymphoma 1 Suppl 1:S27–S31
Galustian C, Klaschka D, Labarthe MC, Bartlett JB, Dalgleish AG (2004) The immunomodulatory drug (IMID (R)) CC-4047 enhances the proliferation and anti-tumor function of gamma delta T cells. J Immunother 27:S50
Galustian C, Labarthe MC, Bartlett JB, Dalgleish AG (2004) Thalidomide-derived immunomodulatory drugs as therapeutic agents. Expert Opin Biol Ther 4:1963–1970
Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F (2004) CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 34:336–344
Golgher D, Jones E, Powrie F, Elliott T, Gallimore A (2002) Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol 32:3267–3275
Hanekom WA, Hughes J, Haslett PA, Apolles P, Ganiso V, Allin R, Goddard E, Hussey GD, Kaplan G (2001) The immunomodulatory effects of thalidomide on human immunodeficiency virus-infected children. J Infect Dis 184:1192–1196
Haslett PA, Corral LG, Albert M, Kaplan G (1998) Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med 187:1885–1892
Haslett PA, Hanekom WA, Muller G, Kaplan G (2003) Thalidomide and a thalidomide analogue drug costimulate virus-specific CD8+ T cells in vitro. J Infect Dis 187:946–955
Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, Kumar S, Chauhan D, Treon SP, Richardson P, Anderson KC (2005) Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol 128:192–203
Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS (2006) The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol 177:4488–4494
Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M (2004) Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood 104:895–903
Hori S, Sakaguchi S (2004) Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect 6:745–751
Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H (2003) Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res 9:4404–4408
Jones E, hm-Vicker M, Simon AK, Green A, Powrie F, Cerundolo V, Gallimore A (2002) Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun 2:1
Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH (2001) Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med 193:1285–1294
Koh KR, Janz M, Mapara MY, Lemke B, Stirling D, Dorken B, Zenke M, Lentzsch S (2005) Immunomodulatory derivative of thalidomide (IMiD CC-4047) induces a shift in lineage commitment by suppressing erythropoiesis and promoting myelopoiesis. Blood 105:3833–3840
Lan RY, Ansari AA, Lian ZX, Gershwin ME (2005) Regulatory T cells: development, function and role in autoimmunity. Autoimmun Rev 4:351–363
Lentzsch S, LeBlanc R, Podar K, Davies F, Lin B, Hideshima T, Catley L, Stirling DI, Anderson KC (2003) Immunomodulatory analogs of thalidomide inhibit growth of Hs Sultan cells and angiogenesis in vivo. Leukemia 17:41–44
List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, Rimsza L, Heaton R, Knight R, Zeldis JB (2005) Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med 352:549–557
Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS et al (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169: 2756–2761 (Baltimore, Md: 1950)
Loke P, Allison JP (2004) Emerging mechanisms of immune regulation: the extended B7 family and regulatory T cells. Arthritis Res Ther 6:208–214
Marriott JB, Clarke IA, Dredge K, Muller G, Stirling D, Dalgleish AG (2002) Thalidomide and its analogues have distinct and opposing effects on TNF-alpha and TNFR2 during co-stimulation of both CD4(+) and CD8(+) T cells. Clin Exp Immunol 130:75–84
Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, Munshi NC, Treon SP, Anderson KC (2002) Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood 99:4525–4530
Motta M, Rassenti L, Shelvin BJ, Lerner S, Kipps TJ, Keating MJ, Wierda WG (2005) Increased expression of CD152 (CTLA-4) by normal T lymphocytes in untreated patients with B-cell chronic lymphocytic leukemia. Leukemia 19:1788–1793
Nocentini G, Riccardi C (2005) GITR: a multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. Eur J Immunol 35:1016–1022
Nolte-’t Hoen EN, Wagenaar-Hilbers JP, Boot EP, Lin CH, Arkesteijn GJ, van EW, Taams LS, Wauben MH (2004) Identification of a CD4+CD25+ T cell subset committed in vivo to suppress antigen-specific T cell responses without additional stimulation. Eur J Immunol 34:3016–3027
Olsen E, Duvic M, Frankel A, Kim Y, Martin A, Vonderheid E, Jegasothy B, Wood G, Gordon M, Heald P, Oseroff A, Pinter-Brown L, Bowen G, Kuzel T, Fivenson D, Foss F, Glode M, Molina A, Knobler E, Stewart S, Cooper K, Stevens S, Craig F, Reuben J, Bacha P, Nichols J (2001) Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol 19:376–388
Querfeld C, Kuzel TM, Guitart J, Rosen ST (2005) Preliminary results of a phase II study of CC-5013 (lenalidomide, revlimid (R)) in patients with cutaneous T-cell lymphoma. Blood 106:936A–937A
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. PNAS 102:18538–18543
Schey SA, Fields P, Bartlett JB, Clarke IA, Ashan G, Knight RD, Streetly M, Dalgleish AG (2004) Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. J Clin Oncol 22:3269–3276
Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF (2001) Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem 276:37672–37679
Shevach EM (2002) CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol 2:389–400
Streetly MJ, Gyertson K, Daniel Y, Zeldis JB, Kazmi M, Schey SA (2008) Alternate day pomalidomide retains anti-myeloma effect with reduced adverse events and evidence of in vivo immunomodulation. Br J Haematol 141:41–51
Sutmuller RP, van Duivenvoorde LM, van EA, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ (2001) Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med 194:823–832
Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S (1998) Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol 10:1969–1980
Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, Sugamura K, Ishii N (2004) Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol 172:3580–3589
Teo SK, Colburn WA, Tracewell WG, Kook KA, Stirling DI, Jaworsky MS, Scheffler MA, Thomas SD, Laskin OL (2004) Clinical pharmacokinetics of thalidomide. Clin Pharmacokinet 43:311–327
Trzonkowski P, Szmit E, Mysliwska J, Dobyszuk A, Mysliwski A (2004) CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol 112:258–267
Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP (2005) Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood 105:2845–2851
Valzasina B, Piconese S, Guiducci C, Colombo MP (2006) Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25− lymphocytes is thymus and proliferation independent. Cancer Res 66:4488–4495
Walsh PT, Benoit BM, Wysocka M, Dalton NM, Turka LA, Rook AH (2006) A role for regulatory T cells in cutaneous T-cell lymphoma; induction of a CD4+CD25+Foxp3+ T-cell phenotype associated with HTLV-1 infection. J Invest Dermatol 126:690–692
Wei WZ, Morris GP, Kong YC (2004) Anti-tumor immunity and autoimmunity: a balancing act of regulatory T cells. Cancer Immunol Immunother 53:73–78
Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 9:606–612
Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM (2006) Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood 107:3639–3646
Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, Frank DA, Ritz J (2006) IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 108:1571–1579
Zou W (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6:295–307
Author information
Authors and Affiliations
Corresponding author
Additional information
Marie-Christine Labarthe and Keith Dredge contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Galustian, C., Meyer, B., Labarthe, MC. et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother 58, 1033–1045 (2009). https://doi.org/10.1007/s00262-008-0620-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0620-4