Abstract
Purpose
Metastatic disease is a major cause of mortality in colorectal cancer patients. Even after complete resection of isolated liver metastases, recurrence develops in the majority of patients. Therefore, development of strategies to prevent recurrent liver metastases is of major clinical importance. The present prospectively randomised phase III trial investigates the efficiency of active specific immunotherapy (ASI) after liver resection for hepatic metastases of colorectal cancer.
Methods
Patients with histologically confirmed liver metastases from colorectal cancer were randomised to the vaccination or control group. After complete resection of liver metastases, patients randomised to the vaccination group received six doses of Newcastle disease virus (NDV) infected autologous tumour cell vaccine (ATV-NDV). The primary end-point was overall survival, secondary end-points were disease-free survival and metastases-free survival.
Results
Fifty-one patients were enrolled in the study with 50 patients available for analysis. The follow-up period was 116.1 ± 23.8 month in the vaccination arm and 112.4 ± 18.5 month in the control group. In the total patient group, no differences in the primary and secondary end-points were detected. Most interestingly, subgroup analysis revealed a significant advantage for vaccinated colon cancer patients with respect to overall survival [hazard ratio: 3.3; 95%, confidence interval (CI): 1.0–10.4; P = 0.042] and metastases-free survival (hazard ratio: 2.7; 95%, CI: 1.0–7.4; P = 0.047) in the intention-to-treat analysis.
Conclusion
Active specific immunotherapy in unselected colorectal cancer patients was not effective for prevention of recurrent metastatic disease. However, in colon cancer patients, ASI with ATV-NDV appears to be beneficial prolonging overall and metastases-free survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastases are the major cause of mortality in colorectal cancer patients [1]. The liver is the most frequent site for distant metastases, with up to 15–25% of patients suffering from synchronous, and another 20–50% developing metachronous liver metastases within the first 5 years following complete resection of the primary tumor [2]. Untreated liver metastases are associated with a median survival of 6–12 months [3, 4]. Surgical resection has proven to be a highly effective treatment option for singular liver metastases, achieving a median survival of 30–40 months and a 5-year survival of 20–40% [5–7]. However, even after complete resection, liver metastases recur in up to 75% of patients, with the highest risk of relapse within the first 2 years following liver resection [8]. Adjuvant therapies designed to reduce recurrence of liver metastases after potentially curative liver resection mainly include systemic or intrahepatic chemotherapy or combinations of both. Clinical trials evaluating those approaches showed conflicting and unsatisfactory results [9, 10].
Therefore, the search for additional strategies to prevent recurrent liver metastases is of major importance. Our group previously reported on the optimization of a protocol for adjuvant active specific immunization based on an autologous tumor cell vaccine modified by the non-lytic, low pathogenic Ulster strain of the Newcastle disease virus (NDV) [11]. In contrast to lytic strains of NDV, the antitumoral action of the Ulster strain results from its potent immune-stimulating properties that affect as well the innate as the adaptive immune system. Infection of tumor cells with live NDV results in a potent up-regulation of MHC class I and cell adhesion molecules on the tumor cell surface [12, 13]. Moreover, NDV infection of tumor cells leads to an improved tumor cell/T-cell interaction and an increased T cell co-stimulatory activity [14, 15]. Consequently, an increased cytotoxic potential of T cells in in-vitro co-incubation studies with NDV-infected tumor cells was observed [12, 14]. Expression of viral proteins at the tumor cell surface and presence of virus derived pathogen-associated molecular patterns (e.g. double-stranded RNA) results in breaking of host tolerance towards the tumor in vitro [14]. The T cell stimulatory action of dendritic cells pulsed with lysates of NDV infected tumor cells as well as the antitumor cytotoxicity of macrophages and monocytes is increased [16–18]. Finally, NDV induces increased production of various cytokines, e.g. interferon α as well as chemokines, e.g. RANTES, influencing the migration, the activational status and cytotoxic activity of various immune cells [19, 20]. Clinical phase I and II studies in various tumor entities have proven the safety of ASI with NDV-modified tumor cells. A detailed description of the mechanisms of action of ATV-NDV vaccine and results from other studies in cancer patients were reviewed recently [21]. In a clinical phase II trial we observed a recurrence rate of 61% in a group of 23 vaccinated colorectal cancer patients. This constituted a remarkable reduction compared to a 87% recurrence rate in a historical matched control group [22]. Based on these results, this prospectively randomized phase III trial investigates the efficacy of active specific immunotherapy (ASI) in patients following liver resection for hepatic metastases of colorectal cancer.
Materials and methods
Patients
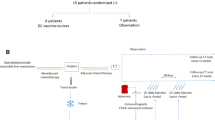
Fifty-one patients with histologically confirmed liver metastases of an adenocarcinoma of the colon or rectum were included in this trial. Eligible patients had undergone curative resection of the primary tumor and presented with metachronous metastases or were diagnosed with resectable synchronous liver metastases without evidence for further distant disease, local recurrence or secondary tumor. Patients with one of the following criteria were excluded from the trial: (1) other malignant diseases in their personal history, (2) inflammatory bowel disease or Turcot syndrome, (3) immunosuppressive treatment, autoimmune disease, protein intolerance, (4) history of severe cardiovascular disease, (5) history of chemotherapy or radiotherapy in a non-adjuvant or non-neoadjuvant setting, (6) liver dysfunction, (7) renal failure and hematopoietic insufficiency (8) carcinoembryonic antigen (CEA) level >5 ng/mL after resection of metastases and (9) incomplete surgical resection of metastatic disease. Ethical approval of the trial protocol was given by the medical boards of the two participating centers (Robert-Rössle-Klinik, Charité, University Medicine Berlin and University of Heidelberg). Before inclusion in the trial, informed consent for participation in the trial was obtained from all patients. Patients were prospectively randomized after stratification according to the following criteria: (1) sex; (2) primary colon or rectal cancer (3) synchronous or metachronous metastases; (4) solitary or multiple liver metastases.
Vaccine preparation
Single cells suspensions were prepared from the metastasis as previously described and stored in liquid nitrogen until further use [22]. On the day of vaccination, cell aliquots were rapidly thawed and incubated with 32 hemagglutinating units (HU) of the avirulent Ulster strain of NDV. Finally, cells were suspended in 250 μL HBSS and stored at 4°C on ice until vaccination.
Immunization protocol
Patients randomized to the ASI group received the first vaccination 14–21 days after the resection of metastases. The vaccination schedule is shown in Fig. 1. Vaccines containing 1 × 107 irradiated tumor cells infected with 32 HU NDV were injected intradermally (id) in the skin of the non-dominant forearm. A complete ASI cycle involved five consecutive vaccinations at 2-week intervals and one boost 3 months later. HBSS served as negative control. Patients were closely monitored for the occurrence of adverse reactions. Follow-up: patient follow-up was performed according to a standardized protocol: every 3 months during the first 3 years, every 6 months during the following 2 years and once yearly the following years. Documented histological diagnosis was required to confirm disease recurrence. Exceptionally, unequivocal findings in chest radiographies or CT scans were accepted for diagnoses of lung or liver metastases. When patients were lost for regular follow-up, the treating physician was contacted to obtain written information on the actual state of health of the patient.
The primary end-point of the trial was overall survival (OS). Secondary endpoints were disease-free survival (DFS) and metastases-free survival (MFS). OS, DFS and MFS were calculated from the date of metastases resection.
Statistics
The survival curves of DFS, MFS and OS in the treatment groups were estimated according to Kaplan–Meier and analyzed with Log-Rank-statistics (Breslow′s test for early follow-up intervals and Log-Rank-test for late follow-up intervals) as well as with the Cox’ proportional hazard regression. Median survivals and hazard ratios with 95% confidence intervals (CIs) were determined as usual. For comparison of categorical variables, χ 2 tests were applied. If prerequisites for the application of the χ 2 test were not fulfilled, the exact Fishers test was applied. For comparison of continuous variables, the t-test was performed after verification of Gaussian distribution by the Kolmogorov–Smirnov-test. If Gaussian distribution could not be assumed, the Mann Whitney-U test was adopted. A significance level of P < 0.05 was considered significant. Because of the exploratory character of the analysis, the reached P-values are to be understood as exploratory ones. For the same reason, no adjustments for multiple testing were undertaken.
Microsoft Access was used for data collection and the SPSS 12.0 software package (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Patient characteristics
From September 1991 to March 1998, 51 patients were recruited for this trial and randomized to the vaccination or control group. One patient from the control group suffering from metachronous liver metastases of an UICC Stage III ascending colon cancer was lost to follow-up and was therefore excluded from the statistical analysis.
The demographic patient data and the characteristics of the primary tumor of the remaining 50 patients are depicted in Table 1. The two treatment groups were similar with respect to age, sex as well as to histopathological characteristics and therapy of the primary tumor.
The majority of patients entered the trial with metachronous liver metastases [19 patients (76%) and 21 patients (84%) of the ASI and control group, respectively]. Detailed clinical and histological information on the liver metastases is summarized in Table 2. In order to assess the risk for recurrent liver metastases, a clinical score as described by Fong et al. was calculated [5]. There were no statistically significant differences concerning the risk for recurrent liver disease between both treatment groups.
Protocol violations
One patient in each group was treated according to the protocol of the opposite randomization group. Of these two patients, one patient initially randomized to the control group chose to be treated in the ASI group after randomization. This patient suffered from metachronous liver metastases diagnosed 12 months after an UICC stage II rectal carcinoma. He developed recurrent liver metastases 6 months after randomization and died 37 months later. Due to recurrent pleural effusions following liver resection, vaccination of one patient initially randomized to the ASI group was not initiated. This patient suffered from hepatic metastases diagnosed 30 months after resection of a stage III colon cancer. He was treated according to the protocol of the control group and developed lung and recurrent liver metastases 15 months after randomization and died 36 months later. Both patients were included in the intention-to-treat (ITT) analysis but excluded from the per-protocol (PP) analysis.
Quantitative parameters of immunotherapy
Eleven patients (44%) of the ASI group received the first vaccination between 14 and 21 days after liver resection. Due to technical and logistic reasons, ten patients were given the first vaccination earlier than 14 days, and four patients later than 21 days (range 9–53 days).
Eighteen (72%) patients completed a series of at least six vaccinations. Three patients exceeded the number of six vaccinations receiving seven, eleven and twelve vaccinations. Two, one and three patients received three, four and five vaccinations, respectively. Five of those patients developed disease recurrence before completion of the vaccination schedule. In one patient, tumor material was only sufficient for four vaccinations.
Immunotherapy-related morbidity
All vaccinations were well tolerated. In total, four (16%) patients treated with ASI experienced minor side effects. The predominant side effects were local erythema and itching at the injection site. Only one patient reported headaches on the day of the first vaccination that did not recur after subsequent vaccinations. Ulcerations or symptoms of autoimmune disease were not observed in the vaccinated patient group.
Recurrence pattern
In total, 18 patients (72%) from each randomization group developed recurrent disease during the observation period. The pattern of first recurrence after treatment of initial metastases was similar in both study arms. Synchronous development of local recurrence and distant metastases occurred in one patient (4%) of each treatment arm. Local recurrence was the initial site of disease recurrence in one patient of the ASI group (4%) and two patients from the control group (8%). Distant metastases as the initial manifestation of recurrent disease were found in 16 patients (64%) from the ASI group and 15 patients (60%) from the control group. Table 3 summarizes the sites of first recurrence. The pattern of first recurrence showed no differences between both treatment groups (P = 0.913).
Survival analysis
All patients
The follow-up period for all surviving patients was 116.1 ± 23.8 months in the ASI arm and 112.4 ± 18.5 months in the control arm (mean ± standard deviation).
Twelve (48.0%) and 16 (64.0%) patients died during the observation period in the ASI and control group, respectively. Neither the ITT nor the PP analysis detected a significant difference between the OS of the ASI and the control group (Fig. 2a). Vaccinated and control patients had a similar DFS both in the ITT and PP analysis (Fig. 2b). MFS showed no statistically significant differences in both treatment groups (Fig. 2c).
In order to evaluate whether specific patient groups benefit from ASI, a subgroup analysis of colon and rectal cancer patients was performed as planned in the trial protocol.
Colon cancer
Four colon cancer patients (30.8%) from the ASI group and nine colon cancer patients (78.6%) from the control group died. OS rates revealed a significant advantage for the ASI group in the PP analysis as well in the early (Breslow: P = 0.031) as in the later follow-up period (Log-Rank: P = 0.016). In the ITT analysis, this effect was only significant after longer observation periods (Breslow: P = 0.061; Log-Rank: P = 0.031) (Fig. 3a). The hazard ratios were 4.3; 95%, CI: 1.2–15.4; P = 0.026 in favor of the ASI group in the PP analysis and 3.3; 95%, CI: 1.0–10.4; P = 0.042 in favor of the ASI group in the ITT analysis, respectively.
Disease-free survival was significantly higher in ASI patients in the PP analysis after long term follow-up (Log-Rank: P = 0.043). However, in the ITT analysis, this difference was no longer significant (Breslow: P = 0.11; Log-Rank: P = 0.068, Fig. 3c).
Metastases-free survival in vaccinated patients was longer than in patients of the control group. These differences were significant in the PP as well as in the ITT analysis (Fig. 3d). However, whereas this difference was significant in early (Breslow: P = 0.045) and long term follow-up (Log-Rank P = 0.022) in the PP analysis, the difference turned out to be significant only after longer follow-up (Log-Rank: P = 0.039) in the ITT analysis. The hazard ratio is 3.3; 95%, CI: 1.1–9.4; P = 0.029 in favor of the ASI group in the PP analysis and 2.7; 95%, CI: 1.0–7.4; P = 0.047 in favor of the ASI group in the ITT analysis, respectively.
Rectal cancer
In contrast to colon cancer patients, vaccinated and control rectal cancer patients showed no significant differences in OS (Fig. 3b) and DFS in either PP or ITT analysis.
Discussion
The present randomized phase III trial revealed a significant long-term benefit with respect to OS as well as MFS for ATV-NDV vaccine treated colon cancer patients following resection of liver metastases. In the total treatment group comprising colon and rectal cancer patients, this treatment regime did not confer a survival benefit to vaccinated patients.
These results contrast with those of our phase II trial reported in 1992 [22]. In contrast to the present trial that includes a roughly equal ratio of rectal cancer and colon cancer patients (see Table 1), the patient groups of the phase II trial predominantly comprised colon cancer patients (70%). Most interestingly, the observed prolongation of MFS in colon cancer patients in the present trial is in keeping with the prolonged recurrence-free interval of vaccinated patients of our 1992 trial [22]. Moreover, in accordance with the observed trend towards prolonged overall survival in the phase II trial, vaccinated colon cancer patients in the present trial showed a significantly prolonged OS. This is in line with reports by other investigators assessing the value of ASI in an adjuvant setting after surgical therapy of the primary tumor. Using an autologous tumor-cell/Bacillus Calmette–Guerin (BCG) vaccine, Vermorken et al. reported a significant prolongation of recurrence-free survival and a trend towards improved overall survival in a phase III trial [23]. A randomized phase III trial by Hoover et al. [24] assessing the value of active specific vaccination with a tumor cell/BCG vaccine showed a significant overall survival and disease-free survival benefit only in colon cancer patients, whereas no benefit was seen in patients with rectal cancer.
As yet, it is unknown why ASI may induce a less effective immune response in rectum cancer than in colon cancer patients. In an experimental setting where ASI was given to the thigh, Hoover et al. suspected that pelvic radiotherapy could negatively affect the local lymph nodes that are required for an efficient induction of immune response. However, in our experimental setting, vaccination was given in the forearm. The local lymph nodes draining the application site of the vaccine were left intact, excluding the above mentioned explanation [24]. Several alternative mechanisms may account for the generation of a differential immune response. First, rectum carcinoma may be less immunogenic per se. Second, the immune response generated by the tumor itself, especially the induction of tolerance, may differ according to the localization of the primary tumor. Subsets of dendritic cells which have been shown to be implicated in the generation of an antitumoral immune response as well as in the induction of oral tolerance to alimentary antigens or commensal bacteria, are differentially distributed along the digestive tract, thus creating local differences in the inducibility of tolerance and immunity [25, 26]. Third, quantitative differences in microscopic residual disease and its location in rectum and colon cancer patients may account for the observed differences. Finally, molecular mechanisms leading to tumorigenesis and tumor progression may occur with different frequencies in the colon and the rectum. Thus, sporadic tumors presenting a CpG island methylator phenotype occur more frequently in the right colon [27]. Different degrees of genetic instability may lead to loss of antigenicity and a higher propensity for immune escape. However, a formal correlation between different mechanisms of genetic instability and propensity for immune escape has not yet been experimentally or epidemiologically proven. Further basic research is required to elucidate the molecular and cellular origin of the observed differences in the immune response in those two tumor entities.
In the present trial, no severe adverse reactions have been observed and the ATV-NDV vaccine was well tolerated by all patients. This compares favorably with other vaccine strategies relying on whole cancer cells combined with alternative adjuvants like BCG that have been described to cause moderate to severe side effects in a considerable percentage of treated patients [23, 24].
We recognize that the small number of participants reduces the statistical power of the present trial and restricts the generalization of the results. Rapid patient accrual was hampered by the presence of multiple exclusion criteria’s in a large portion of patients, a concurrent chemotherapeutic trial aiming at the same patient population and also by patients refusal of randomization.
However, the magnitudes of the effect of ASI with this vaccine in the colon cancer group—in terms of overall survival (48% above the control group) and duration of the immunization-induced protection period (about 10 years)—make the results of this exploratory study remarkable. It is reminiscent of the first results obtained 20 years ago with this vaccine in a metastasizing animal tumor when applying it postoperatively in an adjuvant setting [28]. The promising outcome of this trial should initiate further clinical research validating the promising results in larger group of colon cancer patients.
In conclusion, this trial could not show efficacy of ASI with NDV-infected autologous tumor cells in the total group of colon and rectal cancer patients after resection of liver metastases. However, in colon cancer patients, the present data show that ASI may be beneficial, prolonging overall survival as well as the interval to metastases recurrence.
References
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ (2005) Cancer statistics. CA Cancer J Clin 55:10–30
Ballantyne GH, Quin J (1993) Surgical treatment of liver metastases in patients with colorectal cancer. Cancer 71:4252–4266
Bengmark S, Hafstrom L (1969) The natural history of primary and secondary malignant tumors of the liver, I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer 23:198–202
de Brauw LM, van de Velde CJ, Bouwhuis-Hoogerwerf ML, Zwaveling A (1987) Diagnostic evaluation and survival analysis of colorectal cancer patients with liver metastases. J Surg Oncol 34:81–86
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–318
Foster JH (1978) Survival after liver resection for secondary tumors. Am J Surg 135:389–394
Scheele J, Stangl R, Altendorf-Hofmann A (1990) Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 77:1241–1246
Fernandez-Trigo V, Shamsa F, Sugarbaker PH (1995) Repeat liver resections from colorectal metastasis. Repeat hepatic metastases registry. Surgery 117:296–304
Figueras J, Valls C, Rafecas A, Fabregat J, Ramos E, Jaurrieta E (2001) Resection rate and effect of postoperative chemotherapy on survival after surgery for colorectal liver metastases. Br J Surg 88:980–985
Biasco G, Derenzini E, Grazi G, Ercolani G, Ravaioli M, Pantaleo MA, Brandi G (2006) Treatment of hepatic metastases from colorectal cancer: many doubts, some certainties. Cancer Treat Rev 17:214–228
Lehner B, Schlag P, Liebrich W, Schirrmacher V (1990) Postoperative active specific immunization in curatively resected colorectal cancer patients with a virus-modified autologous tumor cell vaccine. Cancer Immunol Immunother 32:173–178
Haas C, Ertel C, Gerhards R, Schirrmacher V (1998) Introduction of adhesive and costimulatory immune functions into tumor cells by infection with Newcastle disease virus. Int J Oncol 13:1105–1115
Washburn B, Schirrmacher V (2002) Human tumor cell infection by Newcastle disease virus leads to upregulation of HLA and cell adhesion molecules and to induction of interferons, chemokines and finally apoptosis. Int J Oncol 21:85–93
Termeer CC, Schirrmacher V, Brocker EB, Becker JC (2000) Newcastle disease virus infection induces B7-1/B7-2-independent T-cell costimulatory activity in human melanoma cells. Cancer Gene Ther 7:316–323
Ertel C, Millar NS, Emmerson PT, Schirrmacher V, von Hoegen P (1993) Viral hemagglutinin augments peptide-specific cytotoxic T cell responses. Eur J Immunol 23:2592–2596
Bai L, Koopmann J, Fiola C, Fournier P, Schirrmacher V (2002) Dendritic cells pulsed with viral oncolysates potently stimulate autologous T cells from cancer patients. Int J Oncol 21:685–694
Schirrmacher V, Bai L, Umansky V, Yu L, Xing Y, Qian Z (2000) Newcastle disease virus activates macrophages for anti-tumor activity. Int J Oncol 16:363–373
Washburn B, Weigand MA, Grosse-Wilde A, Janke M, Stahl H, Rieser E, Sprick MR, Schirrmacher V, Walczak H (2003) TNF-related apoptosis-inducing ligand mediates tumoricidal activity of human monocytes stimulated by Newcastle disease virus. J Immunol 170:1814–1821
Zeng J, Fournier P, Schirrmacher V (2002) Induction of interferon-alpha and tumor necrosis factor-related apoptosis-inducing ligand in human blood mononuclear cells by hemagglutinin-neuraminidase but not F protein of Newcastle disease virus. Virology 297:19–30
Lokuta MA, Maher J, Noe KH, Pitha PM, Shin ML, Shin HS (1996) Mechanisms of murine RANTES chemokine gene induction by Newcastle disease virus. J Biol Chem 271:13731–13738
Schirrmacher V (2005) Clinical trials of antitumor vaccination with an autologous tumor cell vaccine modified by virus infection: improvement of patient survival based on improved antitumor immune memory. Cancer Immunol Immunother 54:587–598
Schlag P, Manasterski M, Gerneth T, Hohenberger P, Dueck M, Herfarth C, Liebrich W, Schirrmacher V (1992) Active specific immunotherapy with Newcastle-disease-virus-modified autologous tumor cells following resection of liver metastases in colorectal cancer, First evaluation of clinical response of a phase II-trial. Cancer Immunol Immunother 35:325–330
Vermorken JB, Claessen AM, van Tinteren H, Gall HE, Ezinga R, Meijer S, Scheper RJ, Meijer CJ, Bloemena E, Ransom JH, Hanna MG Jr., Pinedo HM (1999) Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet 353:345–350
Hoover HC Jr, Brandhorst JS, Peters LC, Surdyke MG, Takeshita Y, Madariaga J, Muenz LR, Hanna MG Jr (1993) Adjuvant active specific immunotherapy for human colorectal cancer: 6.5-year median follow-up of a phase III prospectively randomized trial. J Clin Oncol 11:390–399
Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M (2005) Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol 6:507–514
Johansson C, Kelsall BL (2005) Phenotype and function of intestinal dendritic cells. Semin Immunol 17:284–294
Hawkins N, Norrie M, Cheong K, Mokany E, Ku SL, Meagher A, O’Connor T, Ward R (2002) CpG island methylation in sporadic colorectal cancers and its relationship to microsatellite instability. Gastroenterology 122:1376–1387
Heicappell R, Schirrmacher V, von Hoegen P, Ahlert T, Appelhans B (1986) Prevention of metastatic spread by postoperative immunotherapy with virally modified autologous tumor cells. I. Parameters for optimal therapeutic effects. Int J Cancer 37:569–577
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schulze, T., Kemmner, W., Weitz, J. et al. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial. Cancer Immunol Immunother 58, 61–69 (2009). https://doi.org/10.1007/s00262-008-0526-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0526-1