Abstract
The protein-bound polysaccharide isolated from basidiomycetes (PSK), a biological response modifier, has been used as immunotherapeutic agent for the treatment of cancers. It has been demonstrated previously that PSK activates various types of immune cells in vitro, and orally administrated PSK activates anti-tumor CD4+ T cell response in mesenteric lymph nodes (MLNs). The detailed mechanism of action of PSK, however, has not been elucidated yet. The objective of the present study was to clarify the molecular mechanism of immunopotentiating effects of PSK using primary culture of the MLN CD4+ T cells. T cell receptor (TCR) stimulation-induced interleukin-2 production from MLN CD4+ T cells was significantly augmented by PSK in a concentration-dependent manner, and the augmentation was reflected at mRNA level. Furthermore, PSK augmented transcriptional activities of nuclear factor of activated T cells and activator protein 1, and phosphorylation of extracellular signal-regulated kinase 1/2 and linker for activation of T cells induced by TCR stimulation, whereas PSK had no influences without TCR stimulation. Collectively, the results indicate that PSK augments activation of MLN CD4+ T cells, probably by modulating the TCR signaling, and provide important knowledge for the elucidation of the true target molecule(s) of PSK.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The anti-cancer agent Krestin (Protein-bound Polysaccharide K; PSK), a biological response modifier isolated from Coriolus versicolor, has been reported to prolong the overall survival or the disease-free survival or the duration of response in patients with gastric cancer, colorectal cancer and small cell lung cancer [4, 17, 19]. Various mechanisms of the anti-cancer effect of PSK have been reported, including reduction of immunosuppressive substances [15], induction of various cytokines [9], stimulation of various immune cells such as activation of cytotoxic T cells [24] and maturation of dendritic cells [11], and direct action on cancer cells [27, 28]. Therefore, it is suggested that anti-cancer effects of PSK are mainly by acting on the host immune system.
Since oral administration of PSK has been reported to augment the intestinal immunity [16], the immune tissue, that the orally administrated PSK first encounters is presumably the gut-associated lymphoid tissue (GALT). In addition, previous report has shown that PSK significantly suppresses proliferation of cancer cells implanted into the cecal wall of mice as a model of colon cancer and augments tumor-specific proliferation of mesenteric lymph node (MLN) cells ex vivo, suggesting an involvement of MLN CD4+ T cells in the anti-tumor effect of PSK [7]. Furthermore, in vitro studies have demonstrated that PSK augments the proliferation and cytotoxic activity of murine T cells [8], human peripheral blood lymphocytes and tumor-infiltrating lymphocytes [18], suggesting that PSK acts directly on T cells. However, the detailed mechanisms of the effects of PSK on T cells in the GALT remain largely unknown.
In the present study, we intended to clarify the effect of PSK on the GALT-derived CD4+ T cell function using primary culture of immunomagnetically separated CD4+ T cells isolated from MLNs of naive mice. We found that PSK significantly augmented T cell receptor (TCR) stimulation-induced interleukin (IL)-2 production and intracellular signaling activation. These results show that PSK augments activation of MLN CD4+ T cells, probably by modulating the TCR signaling.
Materials and methods
Animals
Eight to fourteen week-old female BALB/c mice were purchased from Japan Charles River (Kanagawa, Japan). Mice were housed under temperature- and humidity-controlled (24 ± 2°C, 55 ± 10%), specific pathogen free conditions with lighting cycle from 7:00 a.m. to 7:00 p.m., and had free access to autoclaved food and sterilized tap water. All experimental procedures were approved by the Committee of Ethics on Animal Experiments of the Biomedical Research Laboratories, Kureha Corporation (Tokyo, Japan), and conducted in accordance with the guidelines of the institute.
Primary CD4+ T cell isolation and culture
CD4+ T cells were prepared from MLNs of naive BALB/c mice in all experiments. Pooled MLNs from at least two mice matched for age were minced over a 100 μm cell strainer (BD Falcon, Tokyo, Japan), and washed. MLN CD4+ T cells were then prepared using a mouse CD4+ T cell isolation kit and an AutoMACS separator (both from Miltenyi Biotec, Tokyo, Japan). Following these procedures, cell yields of over 99% viable cells that expressed CD3 were routinely obtained as determined by flow cytometry (FACSCalibur; Becton Dickinson, Tokyo, Japan) staining with APC-labeled anti-CD3e monoclonal antibody (clone 145-2C11; Fig. 1a). The purity of CD4+ or CD8+ cells in separated CD3+ cells was also assessed by flow cytometry staining with FITC-labeled anti-CD4 monoclonal antibody (clone RM4-5), PE-labeled anti-CD8a monoclonal antibody (clone 53-6.7), and approximately 96% of cells were CD4 positive (Fig. 1b). In these flow cytometric analyses, dead cells were excluded by staining with 7AAD dye, and all antibodies and reagents were purchased from BD Pharmingen (Tokyo, Japan). The primary CD4+ T cells were cultured in RPMI 1640 medium (Sigma, St Louis, MO) supplemented with 10% heat-inactivated fetal calf serum (MP Biomedicals, Aurora, OH), 50 μg/mL gentamicin (Sigma). In all cases, cells were cultured at 37°C in a 5% CO2 humidified incubator.
Stimulation of T cells
The MLN CD4+ T cells were TCR stimulated with anti-CD3e monoclonal antibody (10 μg/mL, clone 145-2C11, sodium azide-free and low endotoxin grade; BD Pharmingen) immobilized to culture vessels, which could induce IL-2 production and intracellular signaling activation in the absence of antigen-presenting cells [21]. PSK (Kureha Corp., Tokyo, Japan) at various concentrations was added at the same time of TCR stimulation unless otherwise indicated. In some experiments, cells were stimulated with phorbol 12-myristate 13-acetate (PMA) plus ionomycin (both from Sigma).
Cytokine ELISA
Cells (1 × 106 mL−1) were cultured with PSK in the presence or absence of TCR stimulation as described above for 8, 24 or 48 h at 37°C in 96-well culture plates. IL-2, interferon-gamma (IFN-g) and IL-4 concentrations in the culture supernatants were determined using commercially available ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The detection thresholds of IL-2, IFN-g and IL-4 are 15.6, 9.4 and 7.8 pg/mL, respectively. In some experiments, anti-mouse IL-2 monoclonal antibody (clone JES6-1A12, 20 μg/mL) or control rat IgG2a monoclonal antibody (clone 54447; both from R&D Systems) was added at the start of cultures to neutralize the bioactivity of IL-2 in culture medium. To compare the effect of PSK with Toll-like receptor (TLR) ligands, TLR2 ligand Pam3Cys-Ser-(Lys)4 hydrochloride (Pam3CSK4; Calbiochem, San Diego, CA) or TLR4 ligand ultra-pure lipopolysaccharide (LPS, lipoprotein-free grade, from E. coli 0111:B4; InvivoGen, San Diego, CA) was added to cultures instead of PSK. In addition, anti-mouse TLR2 monoclonal antibody (clone T2.5, 10 μg/mL; Hycult Biotechnology, Uden, Netherlands) or control mouse IgG1 monoclonal antibody (clone 107.3; BD Pharmingen) was added at the start of cultures.
Quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
TCR stimulated cells were cultured with PSK (100 μg/mL) for 2, 5 or 8 h at 37°C. Total RNA was extracted using a RNeasy mini kit (Qiagen, Tokyo, Japan) according to the manufacturer’s instructions. Hundred nanograms of total RNA in a 20 μL reaction volume was reverse transcribed with oligo dT and random hexamer primers using a PrimeScript RT reagent kit (Takara Bio, Shiga, Japan). Real-time RT-PCR was then performed with twofold-diluted cDNA using a QuantiTect SYBR Green PCR kit (Qiagen) and primer pairs for mouse IL-2 and GAPDH (QuantiTect Primer Assay; Qiagen). Serial dilutions of known amounts of cDNA from anti-CD3e-stimulated MLN CD4+ T cells were used to generate a standard curve to determine the relative quantity of mRNA expression in each sample at a given fluorescence intensity after product amplification. Values for IL-2 mRNA were normalized to GAPDH mRNA expression. Analyses were conducted with a LightCycler Instrument (Roche Diagnostics, Tokyo, Japan) and performed in duplicate.
Transient transfection and luciferase assay
The luciferase reporter plasmids for nuclear factor of activated T cells (NF-AT), activator protein 1 (AP1) and cAMP-response element (CRE) were purchased from Clontech (Mountain View, CA). Primary MLN cells were treated with PMA (2 ng/mL) and ionomycin (1 μg/mL) for 2 days, and then washed and treated with recombinant mouse IL-2 (20 ng/mL, R&D systems) for an additional 1 day. T cell blasts (5 × 106 cells) were harvested and resuspended in serum-free RPMI 1640 medium and co-transfected with both luciferase reporter gene plasmid and control plasmid (phRL-TK; Promega, Madison, WI) by electroporation (800 μF, 250 V) using a Cell Porator (Biometra, Goettingen, Germany). The electroporated cells were resuspended in complete medium containing serum and cultured for 3 h. The cells were then stimulated with immobilized anti-CD3e monoclonal antibody as described above in the presence or absence of PSK (100 μg/mL). After 6 h of culture, cells were washed with ice-cold phosphate-buffered saline and cellular luciferase activity was measured using a dual luciferase reporter assay system (Promega) with a Lumat LB9501 luminometer (Berthold Technologies, Bad Wildbad, Germany). Data are presented as the ratio of Photinus pyralis luciferase activity/Renilla reniformis luciferase activity.
Immunoblot analysis
Cells were treated with PSK (100 μg/mL) for 1 h prior to TCR stimulation, and were then stimulated with immobilized anti-CD3e monoclonal antibody without removal of PSK. Cells were lysed in RIPA lysis buffer containing 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, 0.1% SDS, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 mM sodium orthovanadate and 1 mM sodium fluoride, at various time points as indicated in the figures. After incubation for 30 min on ice, the lysates were centrifuged for 10 min at 15,000 g and the supernatants were collected. Protein concentrations were determined using a Dc protein assay kit (Bio-Rad, Tokyo, Japan) based on Lowry’s method [14] with bovine serum albumin as standard. Lysates (2–5 μg) were mixed with 4× reducing sample buffer (200 mM Tris–HCl, pH 6.8, 8% SDS, 40% glycerol, 0.4% bromophenol blue, 4% 2-mercaptoethanol), heated for 5 min at 95°C and separated by SDS-PAGE. After transferring the samples onto a polyvinylidene difluoride membrane (GE Healthcare Bioscience, Tokyo, Japan), the membranes were blocked and treated with one of following primary antibodies: rabbit anti-phosphorylated (Thr202/Tyr204) extracellular signal-regulated kinase (ERK) 1/2 monoclonal antibody, rabbit anti-ERK 1/2 monoclonal antibody, rabbit anti-phosphorylated (Tyr191) linker for activation of T cells (LAT) polyclonal antibody or rabbit anti-LAT polyclonal antibody (all from Cell Signaling Technology, Danvers, MA). After treatment with horseradish peroxidase-conjugated secondary antibody (GE Healthcare Bioscience), signals were visualized using an ECL plus detection kit and Hyperfilm ECL (GE Healthcare Bioscience).
Statistical analysis
For comparisons of the means between two groups, statistical analysis was performed by two-tailed unpaired t test after confirming equality between the variances of the groups. If the variances were unequal, Mann–Whitney U tests were performed. Comparisons of the means among more than three groups were done by one-way factorial analysis of variance (ANOVA) followed by Bonfferoni/Dunn post hoc test. In the time course experiments, statistical analysis was performed by two-way ANOVA with repeated measures to evaluate overall treatment effect and treatment–time interaction. P values of less than 0.05 were considered to be significant. Statistical analysis was performed using Statview 4.11 software (Abacus concepts).
Results
PSK enhanced cytokine production from MLN CD4+ T cells stimulated with anti-CD3e antibody but not PMA/ionomycin
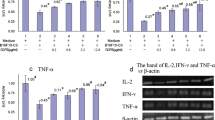
In unstimulated MLN CD4+ T cells cultured in the absence and presence of PSK (100 μg/mL), IL-2, IFN-g and IL-4 were not detected at least 48 h of culture (data not shown). Contrary to this, in the presence of TCR stimulation with immobilized anti-CD3e antibody, two-way repeated measures ANOVA indicated PSK significantly augmented IL-2 production in treatment–time interaction at 30 μg/mL (P < 0.0001) and 100 μg/mL (P < 0.0001) and in overall treatment effect at 30 μg/mL (P = 0.0005) and 100 μg/mL (P < 0.0001); furthermore, significant treatment–time interaction (P = 0.0009) and overall treatment effect (P = 0.0022) were observed between 30 and 100 μg/mL (Fig. 2a). In addition, two-way repeated measures ANOVA indicated PSK also augmented IFN-g and IL-4 production in treatment–time interaction at 30 μg/mL (IFN-g, P = 0.0052) and 100 μg/mL (IFN-g, P = 0.0005; IL-4, P = 0.0355) and in overall treatment effect at 30 μg/mL (IFN-g, P = 0.0014; IL-4, P = 0.0034) and 100 μg/mL (IFN-g, P = 0.0071; IL-4, P = 0.0026); however, significant effect was not observed between 30 and 100 μg/mL (Fig. 2b, c). Furthermore, the TCR stimulation-induced IFN-g and IL-4 production was almost completely inhibited by anti-IL-2 neutralizing antibody (Fig. 3).
Effect of PSK on the cytokine production from MLN CD4+ T cells under TCR stimulation. Cells were stimulated with immobilized anti-CD3e antibody (10 μg/mL) in the presence or absence of PSK (30 or 100 μg/mL) for 8, 24 or 48 h. IL-2 (a), IFN-g (b) and IL-4 (c) concentration in culture supernatants was measured by ELISA. Data are expressed as mean ± SD of triplicate cultures. Results are representative of three independent experiments
Effect of IL-2 neutralization on the TCR stimulation-induced IFN-g and IL-4 production. TCR-stimulated cells were treated with medium alone or PSK (30 or 100 μg/mL) and cultured in the presence of either isotype-matched control rat IgG2a (open column) or anti-IL-2 neutralizing antibody (closed column) for 48 h. IFN-g (a) and IL-4 (b) concentration in culture supernatants was measured by ELISA. Data are expressed as mean ± SD of triplicate cultures. nd, not detected. Results are representative of three independent experiments
On the other hand, when the cells were stimulated with PMA plus ionomycin commonly used for pharmacological stimulation of T cells, PSK showed no effect on IL-2 production (Fig. 4).
Effect of PSK on the PMA/ionomycin-induced IL-2 production. Cells were stimulated with PMA/ionomycin in the presence or absence of PSK (100 μg/mL) for 24 h. IL-2 concentration in culture supernatants was measured by ELISA. Data are expressed as mean ± SD of triplicate cultures. ns, not significant (unpaired t test). Results are representative of three independent experiments
PSK enhanced TCR stimulation-induced IL-2 production via TLR2/4-independent mechanism
The TLR2 ligand Pam3CSK4 significantly augmented TCR stimulation-induced IL-2 production from MLN CD4+ T cells similarly to PSK, whereas the TLR4 ligand ultra-pure LPS had no effect on IL-2 production (Fig. 5a). Furthermore, Pam3CSK4-induced augmented IL-2 production was completely inhibited by anti-TLR2 antibody; however, this antibody had no influence on the effect of PSK (Fig. 5b).
Comparison of the effect of PSK with TLR2 and TLR4 ligands on TCR stimulation-induced IL-2 production. a Cells were stimulated with immobilized anti-CD3e antibody (10 μg/mL) in the presence of PSK (100 μg/mL) or Pam3CSK4 (2 μg/mL) or ultra-pure LPS (1 μg/mL) for 24 h. IL-2 concentration in culture supernatants was measured by ELISA. Data are expressed as mean ± SD of triplicate cultures. ns, not significant; ***P < 0.0001 compared with anti-CD3e alone (one-way ANOVA followed by Bonferroni/Dunn post hoc test). b Isotype-matched control mouse IgG1 (open column) or anti-TLR2 antibody (closed column) was added at the start of cultures. Data are expressed as mean ± SD of triplicate cultures. ns, not significant (unpaired t test); †P < 0.05 (Mann–Whitney U test). Results are representative of three independent experiments
PSK-induced augmentation of IL-2 production was reflected at mRNA level
As shown in Fig. 6, IL-2 mRNA expression was induced at least 2 h after TCR stimulation in MLN CD4+ T cells, and this increase reached maximum level at 5 h. In the presence of PSK, IL-2 mRNA induction was significantly enhanced approximately 2.0, 1.6 and 1.7 fold at 2, 5, 8 h after TCR stimulation, respectively (P = 0.0323 in overall treatment effect, two-way repeated measures ANOVA).
Effect of PSK on the TCR stimulation-induced IL-2 mRNA expression in MLN CD4+ T cells. Total RNA was prepared from the cells stimulated with immobilized anti-CD3e antibody (10 μg/mL) in the absence (open square) or presence (closed square) of PSK (100 μg/mL) for 2, 5 or 8 h. Relative IL-2 mRNA expression was determined by quantitative real-time RT-PCR as described in “Materials and methods”. GAPDH was used as internal standard. Data are expressed as mean ± SD of three independent experiments
PSK enhanced TCR stimulation-induced NF-AT and AP1 transcriptional activation
As shown in left columns of Fig. 7a, b, treatment with PSK alone had no effect on NF-AT and AP1 transcriptional activity similarly to the cytokine production. In contrast, transcriptional activation of NF-AT was strongly induced by TCR stimulation, and this increase was significantly augmented by PSK (Fig. 7a). Similarly, TCR stimulation-induced AP1 transcriptional activation was significantly augmented by PSK (Fig. 7b). By contrast, PSK had no effect on CRE transcriptional activity under both non-stimulated and TCR-stimulated conditions (data not shown).
Effect of PSK on the NF-AT and AP1 transcriptional activation induced by TCR stimulation. MLN T cell blasts were transiently co-transfected with pNFAT-Luc (a) or pAP1-Luc (b) reporter gene plasmid together with control phRL-TK plasmid as described in “Materials and methods”. The transfected cells were stimulated with immobilized anti-CD3e antibody (10 μg/mL) in the presence or absence of PSK (100 μg/mL) for 6 h. The cellular luciferase activity was measured by chemiluminescent assay, and expressed as the ratio of Photinus pyralis luciferase activity/Renilla reniformis luciferase activity. Data are expressed as mean ± SD of triplicate cultures. ns, not significant; *P < 0.05 (unpaired t test); †P < 0.05 (Mann–Whitney U test). Results are representative of three independent experiments
PSK enhanced activation of signaling cascade induced by TCR engagement
The phosphorylation of ERK1/2 (Fig. 8a) and LAT (Fig. 8b) was detected at least 15 min after TCR stimulation. Furthermore, PSK pretreatment augmented the phosphorylation from the early phase of TCR stimulation, and the augmentation continued even after 1 h of stimulation. In contrast, PSK exerted no effect in the absence of TCR stimulation.
Effect of PSK on the phosphorylation of ERK1/2 and LAT induced by TCR stimulation. Cells were preincubated for 1 h at 37°C in medium alone (lanes 1, 3, 5, 7) or in presence of 100 μg/mL PSK (lanes 2, 4, 6, 8). The cells were then stimulated with immobilized anti-CD3e antibody and incubated for the indicated periods at 37°C without removal of PSK. The total cell lysates were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes. a Immunoblot analysis was performed with anti-phosphorylated ERK 1/2 antibody (upper panel). The same membrane was stripped and reprobed with anti-ERK 1/2 antibody (lower panel) to confirm the total protein amount. b Immunoblot analysis was performed with anti-phosphorylated LAT antibody (upper panel). The same membrane was stripped and reprobed with anti-LAT antibody (lower panel). Results are representative of three independent experiments
Discussion
From the characteristic of PSK being an oral agent, it may be speculated that its mechanism of action is associated with the intestinal immune system. Our laboratory has previously demonstrated that orally administrated PSK suppresses tumor growth in a murine colorectal cancer model by inoculating syngeneic Colon 26 tumor into the subserosal space of the cecum, and concluded that PSK acts by augmenting anti-tumor CD4+ T cell response in MLNs [7]. Furthermore, in vitro studies have shown that PSK enhances the proliferation and cytotoxic activity of mouse T cells [8] and human lymphocytes [18]. These former studies suggest that the effect of PSK is associated with a direct action on CD4+ T cells in the GALT. In the present study, we examined the detailed mechanism of immunopotentiating effect of PSK using CD4+ T cells isolated from the MLNs of naive BALB/c mice.
First, we investigated the effect of PSK on the secretion of cytokines which produced by the activated CD4+ T cells. In non-stimulated MLN CD4+ T cells, PSK had no effect on IL-2, IFN-g and IL-4 production. However, when the cells were TCR stimulated with immobilized anti-CD3e antibody, PSK augmented IL-2 production in a concentration-dependent manner, in parallel with upregulation of IL-2 mRNA expression. These results indicate that PSK augments TCR stimulation-induced IL-2 synthesis of MLN CD4+ T cells. In addition, PSK also augmented TCR stimulation-induced IFN-g and IL-4 production, and besides, IL-2 neutralization almost completely inhibited these cytokines production. These results suggest that PSK augments TCR stimulation-induced IFN-g and IL-4 production mainly via augmentation of IL-2 production. On the other hand, PSK slightly augmented of IFN-g and IL-4 production even under IL-2-neutralized condition. In this study, the separated MLN CD4+ T cells were consisted with mainly naive phenotype (∼80% as determined by CD62L expression, data not shown) but contained a considerable number of memory cells. Besides, it has been reported that naive CD4+ T cells scarcely produce IFN-g and are not induce IL-4 mRNA at least 24 h after TCR stimulation, whereas a large amount of IL-2 is produced by naive CD4+ T cells [2, 3, 23]. Therefore, although further investigations using in vitro-generated or cloned Th1 and Th2 are required, these results indicate that PSK may augment TCR stimulation-induced IFN-g and IL-4 expression of memory CD4+ T cells as well as IL-2 production from naive CD4+ T cells.
We next examined the possibility of contribution of TLR, which has been reported to be expressed in T cells and function as a co-stimulatory receptor [12], and endotoxin contamination on the PSK-induced augmented IL-2 production. We found that lipoprotein-free LPS, which acts TLR4 but not TLR2, had no effect on TCR stimulation-induced IL-2 production, whereas Pam3CSK4, which acts TLR2, augmented IL-2 production and this effect was completely inhibited by anti-TLR2 antibody. However, this antibody had no influence on the PSK-induced augmented IL-2 production. These results indicate that TLR2 and TLR4 are not concerned in the effect of PSK, and the possibility of endotoxin contamination is excluded in this condition. In addition, since PSK has been reported to neutralize transforming growth factor-beta by direct binding [15], it is possible that the augmented IL-2 production may be associated with the neutralizing effect of PSK. We therefore examined the effect of anti-transforming growth factor-beta 1, 2, 3 neutralizing monoclonal antibody on IL-2 production by TCR stimulation, but no effect was observed (data not shown).
IL-2 mRNA expression is strictly controlled by various trans-acting factors including NF-AT and AP1 [10]. Therefore, based on our finding that PSK augmented IL-2 expression at mRNA level, we investigated the effect of PSK on the transcription factors associated with transcription of the IL-2 gene. Because of the difficulty to transfect primary T cells, primary T cell blasts were used in this assay. We found that TCR stimulation-induced transcriptional activation of NF-AT and AP-1 was significantly augmented in the presence of PSK. Again, PSK demonstrated no such effect in non-stimulated cells. These findings thus suggest that PSK does not directly activate non-stimulated T cells, but when the T cells are in an activated state by TCR stimulation, PSK augments the transcriptional activation of NF-AT and AP1. With regard to the effect of PSK on transcriptional factors, it has been reported that PSK directly acted on NKL human NK cell line and increased the binding activity of CRE and AP1 nuclear transcription factor [6]. Therefore, we further investigated the effect of PSK on the transcriptional activation of CRE, but no effect was observed. These results indicate that the involvement of CRE nuclear transcription factor is little in the PSK-induced augmentation of IL-2 mRNA expression in MLN T cells.
Following TCR engagement, intracellular multiple immunoreceptor tyrosine-based activation motifs (ITAM) are tyrosine phosphorylated by Src family kinases, and ZAP-70 tyrosine kinase associates with the phosphorylated ITAM and become activated [1, 25]. Activated ZAP-70 induces tyrosine phosphorylation of Linker protein LAT, causing membrane localization of guanine nucleotide exchange factors that has affinity with phosphorylated LAT [30]. Subsequently, the transcription factor AP1 is activated via the Ras–Raf–MEK–ERK cascade [5]. At the same time, phosphorylation of LAT activates phospholipase C-gamma 1, and leads to the production of DAG and inositol 1,4,5-trisphosphate (IP3) through the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2). DAG activates the Ras pathway via PKC, and IP3 acts on the calcium channel on the rough endoplasmic reticulum membrane to increase the intracellular calcium level, inducing calmodulin activation followed by calcineulin activation, causing migration of the transcription factor NF-AT into nuclear [13]. Therefore, we investigated the effect of PSK on the intracellular signaling cascade mediated by the CD3/TCR complex. As a result, addition of PSK augmented the TCR stimulation-induced phosphorylation of ERK, which is located downstream of the Ras cascade and is essential for IL-2 production. Furthermore, PSK also augmented the phosphorylation of linker protein LAT, which is located upstream of the Ras cascade and IP3–Ca2+ signaling. These results indicate that PSK acts on LAT or its upstream region during activation signaling mediated by the CD3/TCR complex. In addition, our finding that PSK had no effect on stimulation with PMA (a PKC activator) plus ionomycin (a Ca2+ ionophore) also supports the hypothesis that PSK acts upstream of the PKC and calcium signaling or via other pathways.
Many negative regulatory molecules are presented in TCR signaling [20], especially in upstream region of LAT phosphorylation. For example, Grb-associated binder 2 (Gab2) phosphorylated by ZAP-70 under TCR stimulation associates with Src homology domain 2 (SH2)-containing protein tyrosine phosphatase (SHP)-2. This association promotes recruitment of SHP-2 to the TCR signaling complex, and negatively regulating TCR signaling [26]. In addition, SHP-1 has been shown to function as a negative regulator of the TCR signaling threshold [29], and its functional ligand has been suggested to be CD22 [22]. The present study did not provide definitive evidence of an association between PSK and these regulatory molecules. However, based on the findings that PSK augmented TCR stimulation-induced intracellular signaling at early stage but had no effect under unstimulated and PMA/ionomycin-stimulated conditions, we speculate that PSK may augment TCR-stimulated signal activation via acting on these negative regulatory molecules.
Collectively, the results of this study demonstrate that PSK augments TCR signaling in MLN CD4+ T cells. This study is the first to elucidate the detailed mechanism of action of PSK using primary cultured immune cells isolated from GALT, which is assumed to be the primary target organ of PSK, and provides important knowledge for the elucidation of the true target molecule(s) of PSK.
Abbreviations
- ANOVA:
-
Analysis of variance
- AP1:
-
Activator protein 1
- CRE:
-
cAMP-response element
- ERK:
-
Extracellular signal-regulated kinase
- GALT:
-
Gut-associated lymphoid tissue
- GAPDH:
-
Glyseraldehyde-3-phosphate dehydrogenase
- IFN-g:
-
Interferon-gamma
- IL:
-
Interleukin
- LAT:
-
Linker for activation of T cells
- LPS:
-
Lipopolysaccharide
- MLN:
-
Mesenteric lymph node
- NF-AT:
-
Nuclear factor of activated T cells
- Pam3CSK4:
-
Pam3Cys-Ser-(Lys)4
- PMA:
-
Phorbol 12-myristate 13-acetate
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- TCR:
-
T cell receptor
- TLR:
-
Toll-like receptor
References
Chu DH, Morita CT, Weiss A (1998) The Syk family of protein tyrosine kinases in T-cell activation and development. Immunol Rev 165:167–180
Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K (1995) Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med 182:1591–1596
Croft M, Swain SL (1995) Recently activated naive CD4 T cells can help resting B cells, and can produce sufficient autocrine IL-4 to drive differentiation to secretion of T helper 2-type cytokines. J Immunol 154:4269–4282
Fisher M, Yang LX (2002) Anticancer effects and mechanisms of polysaccharide-K (PSK): implications of cancer immunotherapy. Anticancer Res 22:1737–1754
Franklin RA, Tordai A, Patel H, Gardner AM, Johnson GL, Gelfand EW (1994) Ligation of the T cell receptor complex results in activation of the Ras/Raf-1/MEK/MAPK cascade in human T lymphocytes. J Clin Invest 93:2134–2140
García-Lora A, Pedrinaci S, Garrido F (2001) Protein-bound polysaccharide K and interleukin-2 regulate different nuclear transcription factors in the NKL human natural killer cell line. Cancer Immunol Immunother 50:191–8
Harada M, Matsunaga K, Oguchi Y, Iijima H, Tamada K, Abe K, Takenoyama M, Ito O, Kimura G, Nomoto K (1997) Oral administration of PSK can improve the impaired anti-tumor CD4+ T-cell response in gut-associated lymphoid tissue (GALT) of specific-pathogen-free mice. Int J Cancer 70:362–372
Hirai R, Oguchi Y, Sugita N, Matsunaga K, Nomoto K (1993) Enhancement of T-cell proliferation by PSK. Int J Immunopharmacol 15:745–750
Hirose K, Zachariae CO, Oppenheim JJ, Matsushima K (1990) Induction of gene expression and production of immunomodulating cytokines by PSK in human peripheral blood mononuclear cells. Lymphokine Res 9:475–483
Jain J, Loh C, Rao A (1995) Transcriptional regulation of the IL-2 gene. Curr Opin Immunol 7:333–342
Kanazawa M, Mori Y, Yoshihara K, Iwadate M, Suzuki S, Endoh Y, Ohki S, Takita K, Sekikawa K, Takenoshita S (2004) Effect of PSK on the maturation of dendritic cells derived from human peripheral blood monocytes. Immunol Lett 91:229–238
Komai-Koma M, Jones L, Ogg GS, Xu DM, Liew FY (2004) TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci USA 101:3029–3034
Lewis RS (2001) Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol 19:497–521
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Matsunaga K, Hosokawa A, Oohara M, Sugita N, Harada M, Nomoto K (1998) Direct action of a protein-bound polysaccharide, PSK, on transforming growth factor-beta. Immunopharmacology 40:219–230
Matsunaga K, Morita I, Oguchi Y, Fujii T, Yoshikumi C, Nomoto K (1987) The effect of a biological response modifier, PSK, on the intestinal immune system in tumor-bearing mice. Anticancer Res 7:509–512
Nakazato H, Koike A, Saji S, Ogawa N, Sakamoto J (1994) Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer. Study Group of Immunochemotherapy with PSK for Gastric Cancer. Lancet 343:1122–1126
Noguchi K, Tanimura H, Yamaue H, Tsunoda T, Iwahashi M, Tani M, Mizobata S, Hotta T, Arii K, Tamai M, Terasawa H (1995) Polysaccharide preparation PSK augments the proliferation and cytotoxicity of tumor-infiltrating lymphocytes in vitro. Anticancer Res 15:255–258
Ohwada S, Ikeya T, Yokomori T, Kusaba T, Roppongi T, Takahashi T, Nakamura S, Kakinuma S, Iwazaki S, Ishikawa H, Kawate S, Nakajima T, Morishita Y (2004) Adjuvant immunochemotherapy with oral Tegafur/Uracil plus PSK in patients with stage II or III colorectal cancer: a randomised controlled study. Br J Cancer 90:1003–1010
Saito T, Yamasaki S (2003) Negative feedback of T cell activation through inhibitory adapters and costimulatory receptors. Immunol Rev 192:143–160
Samelson LE, O’Shea JJ, Luong H, Ross P, Urdahl KB, Klausner RD, Bluestone J (1987) T cell antigen receptor phosphorylation induced by an anti-receptor antibody. J Immunol 139:2708–2714
Sathish JG, Walters J, Luo JC, Johnson KG, Leroy FG, Brennan P, Kim KP, Gygi SP, Neel BG, Matthews RJ (2004) CD22 is a functional ligand for SH2 domain-containing protein-tyrosine phosphatase-1 in primary T cells. J Biol Chem 279:47783–47791
Swain SL, Bradley LM, Croft M, Tonkonogy S, Atkins G, Weinberg AD, Duncan DD, Hedrick SM, Dutton RW, Huston G (1991) Helper T-cell subsets: phenotype, function and the role of lymphokines in regulating their development. Immunol Rev 123:115–144
Vanky F, Wang P, Klein E (1992) The polysaccharide K (PSK) potentiates in vitro activation of the cytotoxic function in human blood lymphocytes by autologous tumor cells. Cancer Immunol Immunother 35:193–198
Wange RL, Samelson LE (1996) Complex complexes: signaling at the TCR. Immunity 5:197–205
Yamasaki S, Nishida K, Hibi M, Sakuma M, Shiina R, Takeuchi A, Ohnishi H, Hirano T, Saito T (2001) Docking protein Gab2 is phosphorylated by ZAP-70 and negatively regulates T cell receptor signaling by recruitment of inhibitory molecules. J Biol Chem 276:45175–45183
Yefenof E, Gafanovitch I, Oron E, Bar M, Klein E (1995) Prophylactic intervention in radiation-leukemia-virus-induced murine lymphoma by the biological response modifier polysaccharide K. Cancer Immunol Immunother 41:389–396
Zhang H, Morisaki T, Matsunaga H, Sato N, Uchiyama A, Hashizume K, Nagumo F, Tadano J, Katano M (2000) Protein-bound polysaccharide PSK inhibits tumor invasiveness by down-regulation of TGF-beta1 and MMPs. Clin Exp Metastasis 18:343–352
Zhang J, Somani AK, Siminovitch KA (2000) Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signaling. Semin Immunol 12:361–378
Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE (2000) Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. J Biol Chem 275:23355–23361
Acknowledgments
The authors thank Takao Ando for his comment on the manuscript and Shunsuke Ito for his technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asai, H., Iijima, H., Matsunaga, K. et al. Protein-bound polysaccharide K augments IL-2 production from murine mesenteric lymph node CD4+ T cells by modulating T cell receptor signaling. Cancer Immunol Immunother 57, 1647–1655 (2008). https://doi.org/10.1007/s00262-008-0498-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0498-1