Abstract
Purpose
To determine the immunomodulatory effects of in vivo COX-2 inhibition on leukocyte infiltration and function in patients with head and neck cancer.
Experimental design
Patients with squamous cell carcinoma of the head and neck preoperatively received a specific COX-2 inhibitor (rofecoxib, 25 mg daily) orally for 3 weeks. Serum and tumor specimens were collected at the start of COX-2 inhibition (day 0) and again on the day of surgery (day 21). Adhesion to peripheral blood monocytes to ICAM-1 was examined. Percentages of tumor-infiltrating monocytes (CD68, CCR5) and lymphocytes (CCR5, CD4, CD8 and CD25) were determined by immunohistochemistry.
Results
Monocytes obtained from untreated cancer patients showed lower binding to ICAM-1 compared to monocytes of healthy donors but significantly regained adhesion affinity following incubation in sera of healthy donors. Conversely, sera of cancer patients inhibited adhesion of healthy donors’ monocytes. Tumor monocyte adhesion to ICAM-1 was increased (P < 0.001) after 21 days of COX-2 inhibition, and concomitant increases in tumor infiltrating monocytes (CD68+), lymphocytes (CD68− CCR5+, CD4+ and CD8+) and activated (CD25+) T cells were observed.
Conclusions
Short-term administration of a COX2 inhibitor restored monocyte binding to ICAM-1 and increased infiltration into the tumor of monocytes and Th1 and CD25+ activated lymphocytes. Thus, in vivo inhibition of the COX-2 pathway may be useful in potentiating specific active immunotherapy of cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclooxygenase-2 (COX-2) over expression in a variety of malignancies is central to the generation of tumor immune suppressor mechanisms. COX-2 catalyzes the first step in synthesis of eicosanoids from arachidonic acid and leads to an abundant production of prostaglandins (PGs), which have multiple and pleiotropic effects [1]. Prostaglandin E2 (PGE2) can modulate immune function through inhibiting dendritic cell differentiation, T-cell proliferation and suppressing the anti-tumor activity of natural killer cells and macrophages [2, 3]. Appropriately activated macrophages have tumoricidal activity through ICAM-1 mediated binding to tumor cells [4, 5]. However, tumor associated macrophages (TAMs) do not display tumoricidal activity and their function is subverted to an immunosuppressive role through the decreased secretion of IL-12 and increased secretion of PGE2, TGF-β and IL-10 [6–9]. Consequently, TAMs suppress the proliferation and effector functions of immune cells and contribute greatly to tumor non-responsiveness. Since the rate-limiting step for PGE2 production is the activity of COX enzyme, clearly the use of COX inhibitors as immunomodulating agents is an attractive approach to increase the efficacy of immune mediated therapeutic strategies.
For immunotherapeutic approaches to be effective, sufficient numbers of immune cells must be able to traffic to and infiltrate the tumor stroma and become activated through the presentation of tumor-associated antigens (TAAs) by antigen-presenting cells such as macrophages, fibroblasts, B cells or dendritic cells. Dubinett and colleagues reported that pharmacological inhibition of COX-2 in a mouse Lewis lung carcinoma model resulted in increased lymphocyte infiltration into tumors with a significant reduction in tumorigenesis [10]. COX-2 inhibition was accompanied by a significant decrease in IL-10 and a concomitant restoration of IL-12 by antigen-presenting cells (APCs) [10]. The same group recently showed that the combination of COX inhibition with vaccination strategies can serve to enhance the generation of antitumor immunity and this effect was abrogated following neutralization of IFN-γ [11]. This suggests that COX-2 inhibition has an immunomodulating role that can be used as a strategy to enhance immunotherapeutics. While rodent models are indispensable tools for understanding carcinogenesis and to obtaining preliminary results of potential efficacy, it has always been a challenge to extrapolate animal data to the clinical setting. This is particularly so with drugs, which block COX activity but may have other effects in addition to COX inhibition [12–17].
This study was performed to determine the immunomodulating role of COX-2 inhibition in the clinical setting. Given the central role of TAMs in mediating tumor immunosuppression [18], the effect of macrophage function was examined in patients with head and neck squamous cell carcinoma (HNSCC), a tumor, which is particularly poorly immunogenic and strongly immunosuppressive. We report that monocytes derived from patients have a functional defect in ICAM-1 binding, which is restored following in vivo COX-2 inhibition. Also, we observed that the tumors of treated patients were infiltrated by higher numbers of immune effector cells including Th1 and CD25+ lymphocytes. The restoration of ICAM-1 binding following COX-2 inhibition represents a critical step in the restoration of monocyte/macrophage tumoricidal activity. Combined with our observation of increased leukocyte infiltration into tumors, our findings suggest that inhibition of COX-2 in cancer patients can serve to enhance the generation of antitumor immunity.
Materials and methods
Treatment of HNSCC patients by the oral intake of a selective COX-2 inhibitor
A pilot clinical trial was designed in which 21out of 24 eligible HNSCC patients were enrolled and randomly assigned to either the COX-2 inhibition group or the untreated control group (Table 1). The study protocol was approved by the institutional review board, and written informed consent was obtained from all patients. Patients were considered eligible if they had potentially curable disease and if their clinical stages were >cT1NX or cTX N+. Pretreatment evaluation included a complete history and physical examination, routine laboratory evaluation and chest computer tomography (CT). Clinical T/N stages were determined by panendoscopy and CT scan. Patients were considered ineligible for the study if they had received prior chemotherapy or radiotherapy, had unstable cardiovascular disease, a history of previous heart attacks or strokes, or had a Karnofsky performance status of less than 60%.
Nine HNSCC patients received rofecoxib (25 mg daily) orally for three weeks preoperatively. The control group comprised 12 patients, which were left untreated previous to surgery. Serum as well as tumor specimens were collected at time of diagnosis (day 0 = start of rofecoxib intake) and surgery (day 21 = end of COX-2 inhibition).
Isolation of monocytes
Peripheral blood mononuclear cells (PBMC) were obtained from patients enrolled in the clinical trial, from additional untreated patients with histologically proven squamous cell carcinoma of the head and neck (n = 24) and from age-matched healthy volunteers (n = 24). PBMC were separated by F/H gradient centrifugation. Monocytes were enriched by adhesion to plastic surfaces for 2 h at 37°C in RPMI and removal of non-adherent cells by washing with PBS. Adherent cells yielded approximately 60–70% CD14+ monocytes as confirmed by flow cytometry.
Adhesion assay
Adhesion of monocytes to ICAM-1 was examined as previously described [19]. Briefly, monocytes from tumor patients and healthy controls were isolated by F/H gradient centrifugation and enriched by plastic adherence for 2 h at 37°C in RPMI/10% FCS. Ninety-six-well plates (Falcon, Franklin Lakes, NJ) were coated for 1.5 h with a human IgG-specific antibody (5 μg/ml; Dianova, Hamburg, Germany) in 50 mM Tris–Cl, pH 9.4. After washing, plates were incubated for 4 h at room temperature with the supernatant from HEK293 cells that have been transfected to produce a human-IgG1/ICAM-1 fusion protein (a gift of Dr. Kolanus, Munich, Germany). Next, unbound protein was removed by washing. In order to quantify specific adhesion of monocytes to ICAM-1, cells were pre-incubated in either autologous or allogeneic sera (5% in RPMI) for 24 h at 37°C and 2 × 104 monocytes were then transferred to ICAM-1-coated cell culture plates and incubated for another 45 min at 37°C. After two final washings, adherent cells were trypsinized and counted by light microscopy. Sera were obtained from the supernatant of the F/H gradient and either used freshly or cryopreserved at −80°C.
Immunohistochemistry
Antibodies used for immunohistochemistry were as follows: mouse monoclonal antibodies (mAbs) against CD4, CD8, CD25, CD68, FoxP3 (Dako, Glostrup, Denmark) and CCR5 (BD Biosciences, Heidelberg, Germany). All mAbs were titered on sections of human tonsils to determine the optimal staining dilutions. The ABC (avidin-biotin complex)-method was used for staining. Frozen sections (4 μm thick) were prepared on a cryostat at −25°C and mounted onto superfrost plus slides (Menzel, Braunschweig, Germany). Following fixation in acetone, the endogenous peroxidase activity was suppressed by treating sections in 0,3% hydrogen peroxide in phosphate-buffered saline (PBS), followed by incubation with primary antibodies. After several washing steps, the sections were treated with a biotinylated rabbit anti-mouse IgG secondary antibody and the avidin–biotin peroxidase complex (Vectastain, Burlingame, CA, USA). The respective antigens were visualized by means of the peroxidase reaction with 0.01% 3-amino-9-ethylcarbazole (AEC) as chromogen (Sigma, St. Louis, USA). After counter staining with Mayer’s hematoxylin, slides were cover-slipped with Kaiser’s glycerol gelatine (Merck, Darmstadt, Germany). In addition, control sections were stained using mouse non-immune serum instead of the specific antibodies (negative control). As positive control, sections of human tonsils were stained in parallel with tumor sections.
Double staining experiments to discriminate between immune cells were performed with CCR5 using the ABC-complex method (red staining) as described above and the alkalic phosphatase-antialkalic phosphatase (APAAP) method (blue staining) for anti-CD68 (KP1) antibody. The ABC-method was carried out as described in the previous subsection. For APAAP staining a rabbit anti mouse immunoglobulin (Dako, Glostrup, Denmark) was used as secondary antibody. 0.05 M Tris-buffered saline solution pH 7.6 was taken instead of PBS as washing solution. APAAP-complex (Dako, Glostrup, Denmark) was added thereafter and detected with Fast Blue BB salt (Sigma-Aldrich, Taufkirchen, Germany) as staining substrate. Optionally, Gills Hematoxylin (grey) was used for counterstaining.
Quantification of cellular infiltrates was performed following staining with specific antibodies. Sections were examined under a ×40 objective by light microscopy, and the numbers of total as well as positively stained cells were counted separately in five random microscopic fields for each coded specimen. The frequency of positively stained monocytes or lymphocytes was calculated as percentage of total cell number for every specimen. The mean percentage of positive cells for a given marker was then calculated for all patients. To avoid bias, two different investigators unaware of the specimen origin independently counted the numbers of positive cells.
Statistical analysis
All values are presented as mean ± SD. Comparisons between two groups were performed using Student’s t-test for paired or grouped data. Findings for P < 0.05 were considered significant.
Results
Sera from tumor patients inhibit adhesion of monocytes to ICAM-1
We demonstrated recently that the incubation of monocytes in conditioned sera from carcinoma cell lines down-regulated the β2-integrin CD11b/CD18 (Mac-1) in vitro [19]. In the present study, however, examination of cell surface Mac-1 expression on monocytes from healthy donors and tumor patients revealed no significant difference directly after isolation via venipuncture (P = 0.2; data not shown). Expression levels of integrins do not necessarily indicate their functional status since conformational changes can account for up-regulated affinity of integrins in response to stimulation [20]. Therefore, we examined whether monocytes from both groups differed in their capacity to adhere to ICAM-1, which is the main ligand for CD11b/CD18. Cell culture dishes were coated with a recombinant ICAM-1/Fc protein, monocytes derived from patients with carcinomas or from healthy donors were incubated for 24 h in autologous or allogeneic sera and subsequently transferred to ICAM-1-coated cell culture dishes, where they were allowed to specifically adhere to ICAM-1 for another 45 min. Non-adherent cells were then removed by washing and adherent cells were counted by light microscopy. It became clear that monocytes from healthy donors adhered significantly better than cells from previously untreated patients. More importantly, cells from cancer patients significantly gained adhesive affinity after incubation in allogeneic sera from healthy donors (n = 24; P < 0.001), whereas monocytes from healthy donors showed a significant reduction in their adhesive potential (n = 24; P = 0.01) upon incubation in allogeneic sera from tumor patients (Fig. 1). All sera were tested individually.
Monocyte adherence to ICAM-1 coated surfaces. Monocytes from tumor patients (pM) had a significantly reduced ability to bind to ICAM-1 when incubated in allogeneic patient sera (pS) compared to incubation in allogeneic healthy sera (hS). Conversely, incubation of healthy monocytes (hM) with allogeneic patient sera significantly decreased ICAM-1 binding relative to healthy monocytes incubated in allogeneic sera from healthy individuals. Sera from patients and controls were tested individually. The data are mean numbers of adherent monocytes per well ±SD obtained from experiments with monocytes of 24 patients and 24 normal donors
There was no significant difference in ICAM-1 binding between monocytes obtained from tumor patients incubated in allogeneic healthy sera and monocytes obtained from healthy volunteers incubated in allogeneic healthy sera. In addition, we noticed that sera from patients with advanced disease (T3 and T4) were more suppressive than sera from early stage patients. However, these results did not reach statistical significance (data not shown).
Taken together, these results demonstrate that tumor-derived factors present in the sera from HNSCC patients account for a reduced adhesion to ICAM-1 of monocytes from tumor patients. This phenomenon may be a common feature of immune suppression characteristic to tumor patients. We have demonstrated recently that conditioned supernatants derived from established cancer cell lines have similar effects on monocytes and that adhesion was restored when the supernatants were generated in the presence of COX-inhibitors [19].
Cyclooxygenase-inhibition restores monocyte function
To determine whether inhibition of COX-2 restores monocyte adhesion, a clinical study with cancer patients suffering from histologically proven HNSCC was initiated. Patients enrolled in this study (n = 9) received the COX-2-inhibiting drug prior to surgery for a period of 3 weeks. Cancer patients of the control group (n = 12) were left untreated. Monocytes from patients were taken at day 0 and 21 and adhesion to ICAM-1 was investigated. These experiments revealed that the adhesive potential of monocytes in the untreated control group tended to decline while selective COX-2 inhibition significantly increased monocyte adhesion (P = 0.001; Fig. 2). The difference between both groups was highly significant (P = 0.001) at the end of treatment.
In vivo COX-2 inhibition enhances monocyte adherence to ICAM-1. Monocytes were obtained from peripheral blood of the patients enrolled in the clinical trial on day 0 and 21. As compared to pretreatment value (=untreated, day 0) COX-2-inhibition significantly increased the adhesion capacity of monocytes to ICAM-1 (=COX-2 inhib. day 21; P < 0.001) whereas adhesion capacity declined further in the untreated control group (=control day 21). The data are mean numbers of adherent monocytes per well ±SD. The difference between both groups was significant (P < 0.001) at the end of treatment
COX-2 inhibition improves the cellular tumor infiltrate
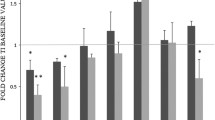
To infiltrate tumors, monocytes must first attach to the vessel wall by binding to adhesion molecules such as ICAM-1 in order to be recruited to the subendothelial space and migrate to the tumor site. Having demonstrated that COX-2 inhibition improved the adhesion capacity of peripheral monocytes/macrophages, we wanted to determine whether this improvement could result in an increase in immune effector cells infiltrating the tumor and its environment. To this end, we performed immunohistochemistry on tumor biopsies taken from study patients on day 0 and 21. These investigations revealed a significant increase in CD68+ monocytes/macrophages infiltrating the tumors of patients who were treated with the COX-2 inhibitor (Fig. 3). Moreover, we observed an increase of CCR5+CD68− cells, most probably Th1 T helper cells, which are the only class of immune cells that express CCR5 besides monocytes [15, 21]. These results are in line with a significant increase in the number of both CD4+ and CD8+ tumor-infiltrating T cells in patients after 3 weeks of COX-2 inhibition compared to untreated control patients (Fig. 4a, b). Also, more of these T cells revealed an activated phenotype as demonstrated by the expression of the high-affinity IL-2 receptor, CD25 (Fig. 4c). Immunohistochemistry with an antibody against FoxP3, an transcription factor that is specifically expressed in immunosuppressive regulatory T cells (Tregs) revealed no significant changes in the number of tumor-infiltrating Tregs within tree weeks of COX-inhibition (Fig. 4d).
Immunohistochemistry for tumor infiltrating mononuclear cells before and after treatment of patients with rofecoxib. Double-staining for macrophages (CD68, blue) and CCR5 (red; expressed on macrophages [44] and Th1 T cells) suggests enrichment in CD68+ and CCR5+CD68− immune cells in a representative tumor specimen collected after COX-2 inhibition (b) relative to the same tumor biopsied before therapy with rofecoxib (a). A significant increase in the percentages of CD68+ (c) and CCR5+ (d) immune cells infiltrating the tumor after treatment was demonstrated by quantitative microscopic analysis of mononuclear cell infiltrates. Magnification in a and b is 40×
In vivo Cox-2 inhibition leads to enrichment in CD25+ lymphocytes in the tumor. Pre-operative therapy with the COX-2 inhibitor for 3 weeks led to a significant increase in CD4+ (a) and CD8+ (b) tumor infiltrating T lymphocytes (TILs). Additionally, in the patients receiving the inhibitor, a significantly higher percentage of TILs expressed the activation marker CD25 (c). In contrast, COX-2 inhibition had no significant impact on the number of tumor-infiltrating FoxP3± regulatory T cells (d)
Discussion
In this report we show that short-term administration of a specific COX-2 inhibitor to patients with HNSCC restores monocyte adhesion to ICAM-1 and enhances infiltration of monocytes and both CD4+ and CD8+ T cells into the tumor microenvironment. The CD4+ cells are predominantly Th1 cells, as determined by the increase in infiltration of cells, which are CCR5+CD68−. Increased infiltration of lymphocytes has also been demonstrated in a LLC mouse model of lung cancer following abrogation of COX-2 expression [10], with a restoration in the balance of Th1 lymphocytes. The expression of Th1 cytokines has been suggested by a number of studies to be associated with favorable clinical outcomes, while the Th2 cytokines are associated with unfavorable prognosis [22–27]. Our findings provide evidence that in cancer patients, one mechanism by which COX-2 inhibitors exert their antineoplastic effect is through increased infiltration of Th1 helper cells.
Our observation of increased infiltration of immune-competent cells is also in agreement with a recent study demonstrating that the over expression of COX-2 in tumors reduced the infiltration of CD8+ T cells in endometrial carcinoma and that increased intratumoral accumulation of CD8+ cells produced a survival advantage in these patients [28]. We observed that a significant number of infiltrating T cells are activated within the tumor. Since the number of tumor infiltrating lymphocytes [29–34] has been reported to be a significant determinant of outcome for a variety of cancer types, these data are consistent with an activated cellular immune response.
It became clear recently, that PGE2 induces FoxP3 gene expression [35] and that COX-inhibitors reduce its expression as well as the immunosuppressive activity of Tregs [36, 37] In our investigations, we did not observe significant changes in the number of intratumoral Tregs. This, however, may be due to the relatively short period of time (21 days) of COX-inhibition. However, a reduction of the immunosuppressive properties of intratumoral Tregs due to COX-inhibition can be assumed and is currently investigated in our group. Also, changes in the activity and number of intratumor Tregs upon a longer application of COX-inhibitors await further investigations. It is an interesting observation that, despite the fact that Tregs normally exhibit immunosuppressive functions, intratumoral Tregs are obviously not an independent prognostic factor for a negative clinical outcome [37–39] and may even be associated with improved survival [40].
We have previously demonstrated that monocytes from patients with HNSCC have down-regulated surface expression of CCR5 and that the expression levels of this chemokine receptor were restored following short-term administration of a selective COX-2 inhibitor [19, 41]. The observations reported here together with our previous results suggest a general mechanism of suppression of monocyte function. Furthermore, this suppression likely results from tumor-derived soluble factors in the sera of cancer patients. Such sera caused migration and adhesion deficiencies in monocytes obtained from healthy donors.
The binding of monocytes to ICAM-1 is important not only for cell adhesion and migration but also anti-tumor cytotoxicity. Monocytes represent the circulating macrophage population and expansion of these cells in tumor patients is associated with profound immunosuppression. These cells have been re-programmed by the tumor to promote tumor growth not only by failing to kill cancer cells upon tumor infiltration, but also induce T-cell tolerance and fail to mature into fully immunogenic APCs. We show here that restoration of ICAM-1 binding following in vivo COX-2 inhibition is associated with an increase in numbers of tumor infiltrating CD68± monocytes. The role of TAMs in tumor growth is complex and multifaceted. Although some investigators claim a role for TAMs in tumor progression, numerous studies have provided contradictory results (see [36] for review). Blocking cyclooxygenase activity may thus tip the macrophage balance towards anti-tumor activities as has been demonstrated recently in melanoma [42].
The antitumor effects of COX-2 inhibitors are mostly explained by their pro-apoptotic and anti-angiogenic effect. In contrast, nothing is known about immunomodulating effects of COX-2 inhibitors in HNC patients. Thus, the data presented here are the first to demonstrate the COX-2 inhibitor on immune cell migration and their other anti-tumor functions in the tumor microenvironment.
In summary, our data provide evidence for a reversal of tumor-induced immune suppression in patients with HNSCC following short-term oral administration of a COX-2 inhibitor. The mechanism by which this is achieved likely involves a complex interplay of biological factors, which result in restored ICAM-1 binding by monocytes and greater leukocyte infiltration and activation. Our data provide, at least in part, a rationale for the antineoplastic effects of COX-2 inhibitors in cancer patients and predict that inhibition of this pathway will prove to be a useful option in potentiating specific active immunotherapy to cancer in existing and future cancer vaccine strategies [43].
Thus, although specific COX-2 inhibitors may have cardiac side effects in long-term users, our finding provide a rationale that inhibition of cyclooxygenase-2 could be a promising strategy to prevent or possibly treat human head and neck cancers in high-risk patients. An understanding of the mechanisms whereby COX-2 inhibitors mediate their antineoplastic activity awaits further investigations.
References
FitzGerald GA, Patrono C (2001) The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 345:433-442
Goodwin JS, Ceuppens J (1983) Regulation of the immune response by prostaglandins. J Clin Immunol 3:295–315
Yang L, Yamagata N, Yadav R, Brandon S, Courtney RL, Morrow JD, Shyr Y, Boothby M, Joyce S, Carbone DP, Breyer RM (2003) Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest 111:727–735
Bernasconi S, Peri G, Sironi M, Mantovani A (1991) Involvement of leukocyte (beta 2) integrins (CD18/CD11) in human monocyte tumoricidal activity. Int J Cancer 49:267–273
Webb DS, Mostowski HS, Gerrard TL (1991) Cytokine-induced enhancement of ICAM-1 expression results in increased vulnerability of tumor cells to monocyte-mediated lysis. J Immunol 146:3682–3686
Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, Vago L, Nebuloni M, Mantovani A, Sica A (2006) A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood 107:2112–2122
Elgert KD, Alleva DG, Mullins DW (1998) Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol 64:275–290
Huang M, Stolina M, Sharma S, Mao JT, Zhu L, Miller PW, Wollman J, Herschman H, Dubinett SM (1998) Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res 58:1208–1216
Sica A, Saccani A, Bottazzi B, Bernasconi S, Allavena P, Gaetano B, Fei F, LaRosa G, Scotton C, Balkwill F, Mantovani A (2000) Defective expression of the monocyte chemotactic protein-1 receptor CCR2 in macrophages associated with human ovarian carcinoma. J Immunol 164:733–738
Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, Zhu L, Kronenberg M, Miller PW, Portanova J, Lee JC, Dubinett SM (2000) Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol 164:361–370
Sharma S, Stolina M, Yang SC, Baratelli F, Lin JF, Atianzar K, Luo J, Zhu L, Lin Y, Huang M, Dohadwala M, Batra RK, Dubinett SM (2003) Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin Cancer Res 9:961–968
Grilli M, Pizzi M, Memo M, Spano P (1996) Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappaB activation. Science 274:1383–1385
Kashfi K, Rigas B (2005) Non-COX-2 targets and cancer: expanding the molecular target repertoire of chemoprevention. Biochem Pharmacol 70:969–986
Shureiqi I, Chen D, Lee JJ, Yang P, Newman RA, Brenner DE, Lotan R, Fischer SM, Lippman SM (2000) 15-LOX-1: a novel molecular target of nonsteroidal anti-inflammatory drug-induced apoptosis in colorectal cancer cells. J Natl Cancer Inst 92:1136–1142
Yamamoto Y, Yin MJ, Lin KM, Gaynor RB (1999) Sulindac inhibits activation of the NF-kappaB pathway. J Biol Chem 274:27307–27314
Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B (2000) Role of BAX in the apoptotic response to anticancer agents. Science 290:989–992
Zhang Z, DuBois RN (2000) Par-4, a proapoptotic gene, is regulated by NSAIDs in human colon carcinoma cells. Gastroenterology 118:1012–1017
Mantovani A, Schioppa T, Biswas SK, Marchesi F, Allavena P, Sica A (2003) Tumor-associated macrophages and dendritic cells as prototypic type II polarized myeloid populations. Tumori 89:459–468
Zeidler R, Csanady M, Gires O, Lang S, Schmitt B, Wollenberg B (2000) Tumor-derived Prostaglandin E2 inhibits monocyte function by interfering with their chemotactic and adhesive potential. FASEB J 14:661–668
Hughes PE, Pfaff M (1998) Integrin affinity modulation. Trends Cell Biol 8:359–364
Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM (1998) CCR5 is characteristic of Th1 lymphocytes. Nature 391:344–345
De Vita F, Orditura M, Galizia G, Romano C, Lieto E, Iodice P, Tuccillo C, Catalano G (2000) Serum interleukin-10 is an independent prognostic factor in advanced solid tumors. Oncol Rep 7:357–361
Elsasser-Beile U, Kolble N, Grussenmeyer T, Schultze-Seemann W, Wetterauer U, Gallati H, Schulte Monting J, von Kleist S (1998) Th1 and Th2 cytokine response patterns in leukocyte cultures of patients with urinary bladder, renal cell and prostate carcinomas. Tumour Biol 19:470–476
Lauerova L, Dusek L, Simickova M, Kocak I, Vagundova M, Zaloudik J, Kovarik J (2002) Malignant melanoma associates with Th1/Th2 imbalance that coincides with disease progression and immunotherapy response. Neoplasma 49:159–166
Mori T, Takada R, Watanabe R, Okamoto S, Ikeda Y (2001) T-helper (Th)1/Th2 imbalance in patients with previously untreated B-cell diffuse large cell lymphoma. Cancer Immunol Immunother 50:566–568
Nakamura E, Megumi Y, Kobayashi T, Kamoto T, Ishitoya S, Terachi T, Tachibana M, Matsushiro H, Habuchi T, Kakehi Y, Ogawa O (2002) Genetic polymorphisms of the interleukin-4 receptor alpha gene are associated with an increasing risk and a poor prognosis of sporadic renal cell carcinoma in a Japanese population. Clin Cancer Res 8:2620–2625
Wittke F, Hoffmann R, Buer J, Dallmann I, Oevermann K, Sel S, Wandert T, Ganser A, Atzpodien J. (1999) Interleukin 10 (IL-10): an immunosuppressive factor and independent predictor in patients with metastatic renal cell carcinoma. Br J Cancer 79:1182–1184
Ohno Y, Ohno S, Suzuki N, Kamei T, Inagawa H, Soma G, Inoue M (2005) Role of cyclooxygenase-2 in immunomodulation and prognosis of endometrial carcinoma. Int J Cancer 114:696–701
Ansell SM, Stenson M, Habermann TM, Jelinek DF, Witzig TE (2001) CD4+ T-cell immune response to large B-cell non-Hodgkin’s lymphoma predicts patient outcome. J Clin Oncol 19:720–726
Marrogi AJ, Munshi A, Merogi AJ, Ohadike Y, El-Habashi A, Marrogi OL, Freeman SM (1997) Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int J Cancer 74:492–501
Mihm MC Jr, Clemente CG, Cascinelli N (1996) Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest 74:43–47
Morita M, Kuwano H, Araki K, Egashira A, Kawaguchi H, Saeki H, Kitamura K, Ohno S, Sugimachi K (2001) Prognostic significance of lymphocyte infiltration following preoperative chemoradiotherapy and hyperthermia for esophageal cancer. Int J Radiat Oncol Biol Phys 49:1259–1266
Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM (1997) Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 182:318–324
Schumacher K, Haensch W, Roefzaad C, Schlag PM (2001) Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res 61:3932–3936
Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc’h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM (2005) Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol 175:1483–1490
Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM (2006) FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol 177:246–254
Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RK, Dubinett SM (2005) Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res 65:5211–5220
Grabenbauer GG, Lahmer G, Distel L, Niedobitek G (2006) Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res 12:3355–3360
Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi MB, Harpole DHJ, Patz EFJ (2006) Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer 107:2866–2872
Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, Montserrat E, Campo E, Banham AH (2006) High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood 108:2957–2964
Lang S, Lauffer L, Clausen C, Lohr I, Schmitt B, Holzel D, Wollenberg B, Gires O, Kastenbauer E, Zeidler R (2003) Impaired monocyte function in cancer patients: restoration with a cyclooxygenase-2 inhibitor. FASEB J 17:286–288
Duff M, Stapleton PP, Mestre JR, Maddali S, Smyth GP, Yan Z, Freeman TA, Daly JM (2003) Cyclooxygenase-2 inhibition improves macrophage function in melanoma and increases the antineoplastic activity of interferon gamma. Ann Surg Oncol 10:305–313
Gilboa E (2004) The promise of cancer vaccines. Nat Rev Cancer 4:401–411
Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR (1996) Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661–666
Acknowledgments
We thank Baerbel Schmitt for excellent technical assistance and Dieter Hoelzel for help with statistics. Supported by the Deutsche Forschungsgemeinschaft (grant Ze419/7), the Rudolf-Bartling Stiftung, and the Dr. Sepp und Hanne Sturm-Stiftung.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lang, S., Tiwari, S., Andratschke, M. et al. Immune restoration in head and neck cancer patients after in vivo COX-2 inhibition. Cancer Immunol Immunother 56, 1645–1652 (2007). https://doi.org/10.1007/s00262-007-0312-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-007-0312-5