Abstract
Purpose: To characterize HLA class I antigen expression in non-small cell lung cancer (NSCLC) lesions, and to assess the clinical significance of these molecules’ downregulation. Methods: One hundred and ninety primary formalin fixed, paraffin embedded NSCLC lesions were stained with HLA class I heavy chain-specific mAb HC-10. Results were scored as percentage of stained tumor cells and categorized into three groups: 0–24% (negative), 25–75% (heterogeneous) and >75% (positive). HLA class I antigen expression was correlated with clinical and pathologic predictors of time to progression and survival and analyzed using the chi-square test. Association between HLA class I antigen expression and survival was assessed using Cox regression models, while controlling for confounders. Results: HLA class I antigen expression was negative, heterogeneous and positive in 153, 25 and 12 primary NSCLC lesions, respectively. Independent variables significantly associated with survival included tumor stage, PS and weight loss. The median survival times were 40.6, 44.0 and 17.9 months for patients with a HLA class I antigen expression scored as negative, heterogeneous and positive, respectively. Conclusion: HLA class I antigen defects were found with high frequency (93.6%) in NSCLC lesions. HLA class I antigen downregulation was associated with improved survival, although this association was not statistically significant. These results, which parallel similar findings in uveal melanoma and in breast carcinoma, raise the possibility that NK cells may play a role in the control of NSCLC tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been known for some time that in humans as in other animal species malignant transformation of cells is frequently associated with defects in HLA class I antigen expression [3]. In spite of the potential role of these abnormalities in the clinical course of the disease, this topic has been neglected for a number of years because the validity of the immunosurveillance theory in tumor control has been called into question by results of experiments in animal model systems [22, 23]. Also, the lack of antibodies that detect HLA antigens in formalin fixed, paraffin embedded tissue sections has imposed restrictions on the analysis of HLA antigen expression in malignant lesions. Interest in this topic has been rekindled in recent years (1) by a renewed appreciation of the role of immune surveillance in the prevention and control of tumor growth [2] and (2) by the realization that HLA class I antigen defects in malignant lesions may have a negative impact on the outcome of T cell-based immunotherapy [13]. The latter is being applied with increasing frequency for the treatment of malignant diseases [16]. Furthermore from a practical viewpoint, the identification of monoclonal antibodies (mAb) that detect HLA class I antigens in formalin fixed, paraffin embedded tissue sections (FFPE) [10, 21, 24] has facilitated the utilization of archived material readily available in departments of pathology. This in turn has facilitated retrospective studies to assess the clinical significance of HLA class I defects in malignant lesions.
In recent years there has been a resurgence of interest in T cell-based immunotherapy for solid tumors such as NSCLC, the 5-year survival rate of which remains among the lowest of all major human cancers [4], despite advances in surgical, radiation and medical treatments [17]. However, there is a paucity of data regarding HLA class I antigen expression in NSCLC lesions. The scarcity of this information has a negative impact on the design of immunotherapeutic strategies in this disease. Therefore in the present study, we have analyzed HLA class I antigen expression in NSCLC surgical specimens from patients with well characterized clinical histories and have correlated the results of immunohistochemical (IHC) staining of the lesions with their histopathological characteristics and with the clinical course of the disease.
Patients and methods
Study population
This study is a retrospective analysis of patients diagnosed with NSCLC at Roswell Park Cancer Institute between 1996 and 1999. This study included patients with primary NSCLC, who had received surgery as initial treatment modality, had adequate archival tissue for analysis and had complete clinicopathologic data. One hundred and ninety patients met these criteria. Patients were followed through October 1, 2004. Data collected included age, gender, smoking history, performance status (PS) using the Eastern Cooperative Oncology Group scale, date of initial diagnosis, histological diagnosis, grade of tumor differentiation, pathologic tumor stage, adjuvant therapy, time to progression, and date of death from NSCLC or last follow-up. Histological diagnosis and grade of differentiation were determined in accordance with the World Health Organization criteria for lung and pleural tumors [25]. Pathologic stage was based on the revised international system [15]. This study was performed following an Institutional Review Board approved protocol to investigate molecular markers relevant to lung cancer pathogenesis.
Histological examination
Hematoxylin and eosin-stained slides of NSCLC tumors were reviewed for confirmation of the histological diagnosis and the adequacy of specimens for IHC analysis. All carcinoma lesions were reviewed and classified according to the histological classification of lung tumors of the World Health Organization [25]. The diagnosis of 181 lesions was established by examination of conventional hematoxylin and eosin stained slides. The diagnosis of six spindle cell/sarcomatous squamous cell carcinoma lesions and of three undifferentiated carcinoma lesions was confirmed by using ancillary techniques including electron microscopy, IHC, or mucin and PAS stains. Neutral buffered formalin fixed (10% vol/formalin in water; pH, 7.4) and paraffin-embedded tissue blocks containing NSCLC and normal lung tissue were retrieved from the tissue procurement facility, Department of Pathology. Areas of tumor and normal tissue elements were identified and marked by an investigator (DFT) for IHC.
Antibodies
The HLA class I heavy chain-specific mAb HC-10, which recognizes a determinant expressed on all β2m-free HLA-B and C heavy chains and on β2m-free HLA-A10, -A28, -A29, -A30, -A31, -A32 and -A33 heavy chains was developed and characterized as described [18, 21]. Biotynilated anti-mouse IgG xenoantibodies were purchased from Vector Laboratories, Burlingame, CA.
Immunohistochemistry
Five micrometer thick sections of formalin fixed, paraffin embedded specimens were deparaffinized in xylene and rehydrated through a graded series of ethanol into PBS. High temperature antigen retrieval was carried out by microwaving slides in 0.01 M citrate buffer (Antigen Unmasking Solution, Vector Laboratories). The solution was brought to a boil, boiled for 10 min and then cooled for 20 min. Slides were transferred to PBS. Following blocking for endogenous peroxidase, tissue sections were blocked with 0.03% casein in PBS with 0.05% Tween 20 (Sigma, St. Louis, MO). Tissue sections were incubated at room temperature for 1 h with an optimal amount of mAb HC-10 washed in PBS, labeled with a biotinylated secondary antibody (Vector Laboratories) and then labeled with the ABC Vectastain Elite kit (Vector Laboratories). Bound antibody was visualized with 3,3′-diaminobenzidine (DAB). Negative control sections were immunostained under the same conditions substituting mouse IgG for primary antibodies. The IHC slides were independently reviewed by two investigators (DFT, LM). Results were scored as negative, heterogeneous and positive when the percentage of stained tumor cells was 0–24, 25–75 and >75, respectively [8].
Statistical analysis
The χ2 test was used to assess the significance of the association of HLA class I antigen expression with clinicopathological parameters. Follow-up time was calculated using the potential follow-up method. Overall patient survival was calculated from the date of diagnosis to the date of last follow-up (censored) or date of patient death (event). Differences in survival times between patient subgroups were analyzed using the log-rank statistic test. Survival probabilities were calculated using the Kaplan-Meier method. Cox proportional hazards regression analysis was used to measure the association of clinicopathologic variables with overall survival. Statistical significance for model parameters was based on the likelihood ratio test. In all tests, statistical significance was set at 5%.
Results
Patients’ clinical and pathological characteristics
The clinicopathologic features of the patients evaluated are summarized in Table 1. The median patient age was 75.2 years (range 37.5–88.3 years). One hundred and five patients were male, 85 female. Smoking history was noted in 172 patients. PS information was available in all but four patients. One hundred and twenty-two patients had a PS 0, 58 had a PS 1, five had a PS 2 and one patient had a PS 3. Information pertaining to weight gain or loss was available only in 83 patients; 14 of them had weight loss. Eighty-eight patients had stage I disease, 30 had stage II, 40 had stage III and 32 had stage IV. Stage IV disease was noted at the time of surgery. The primary tumor type was adenocarcinoma in 109 patients, and squamous cell carcinoma in 64 patients. Seventy-seven tumors were well to moderately differentiated, and 113 tumors were poorly differentiated or undifferentiated. Fifty-two patients received either neoadjuvant or adjuvant chemotherapy and/or radiation. Time to progression data was available in 71 patients.
HLA class I antigen expression in normal lung
As an initial step, we examined HLA class I antigen expression in normal pulmonary tissue present in lung carcinoma tissue sections by staining formalin fixed, paraffin embedded (FFPE) tissue sections with mAb HC-10. All the non-neoplastic tissue elements including pneumocytes, bronchial mucosa, bronchial cartilage, submucosal glands of bronchus, stromal cells, and hilar lymph nodes were stained by mAb HC-10. However, the staining was restricted to a small percentage of cells. The stromal cells (endothelial cells and fibroblasts) showed a weaker staining. Most other tissues displayed patchy positivity, while hilar lymph nodes showed a diffuse staining pattern.
HLA class I antigen expression in NSCLC lesions
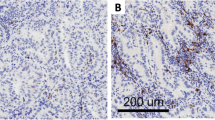
Overall, 153 tumors were classified as HLA class I antigen negative, i.e. less than 25% of tumor cells were stained by mAb HC-10 (example: Fig. 1a); 25 tumors were classified as heterogeneous, i.e. 25–75% of tumor cells were stained by mAb HC-10 (example: Fig. 1b, c) and 12 tumors were classified as positive, i.e. more than 75% of tumor cells were stained by mAb HC-10 (example: Fig. 1d). In the neoplastic sections, the intensity of staining varied from tumor to tumor and from one area to another within the same tumor. Heterogeneous staining was noted in all histological types of NSCLC. Some tumors showed focal patchy positive staining, and others displayed uniform staining pattern in tumor nests. Positive staining was observed in both the cell membrane and the submembranous cytoplasm region. Many of the negative cases also contained non-neoplastic lung tissue, which stained positively, serving as an internal control. No staining was detected in tissue sections incubated with irrelevant mouse IgG.
Patterns of HLA class I antigen expression in primary NSCLC lesions: a Moderately differentiated adenocarcinoma with negative staining by mAb HC-10 (bottom of photo), cells within an adjacent alveolar wall (top) are a positive control. b Adenocarcinoma with greater than 25% of tumor cells stained by mAb HC-10. c Poorly differentiated NSCLC with more than 50% of tumor cells stained. d A poorly differentiated carcinoma with more than 75% of tumor cells stained. All photographs were taken at magnification of ×400
HLA class I antigen expression in NSCLC lesions and clinicopathological parameters
HLA class I antigen expression in NSCLC lesions was not significantly associated with clinical parameters such as age, gender, smoking status, PS and weight loss as seen in Table 2. The frequency of HLA class I antigen loss was higher in adenocarcinomas (46.8%) than in squamous cell carcinomas (26%). However, HLA class I antigen expression was not significantly associated with histological grade or stage of tumor.
HLA class I antigen expression and clinical course of the disease
HLA class I antigen expression patterns did not correlate with histologic types of NSCLC or tumor grade (Table 2). Analysis of HLA class I antigen expression patterns suggests that patients whose tumors express higher levels of HLA class I antigens have a shorter time to progression as well as a shorter survival time than those patients whose tumors were classified as heterogeneous or negative (Table 3, Figs. 2, 3). Tables 4 and 5 show the relationship between clinicopathological variables and time to progression as well as survival. Univariate analysis indicated that there was a highly significant association of PS and tumor stage with survival (P<0.01). In the multivariate model, tumor stage and PS both remained significantly related to survival (P<0.01). Patients with stage II–IV disease had about two and a half times the risk of death from disease when compared to the stage I patients (relative risk: 2.40, 95% CI: 1.38–4.17, P<0.01). On the other hand, HLA class I antigen expression was not significantly associated with survival (P=0.79). In a multivariate Cox regression model of time to progression, patients with later stage of disease and older age at diagnosis were at increased risk of progression (P<0.05). No statistically significant association was observed between time to progression and HLA class I antigen expression in either univariate or multivariate Cox regression models.
Survival curves of patients with NSCLC according to their HLA class I antigen expression in their primary tumors. Survival is expressed in months calculated from the time of diagnosis to the time of last follow-up/death. Patients were divided into three groups, according to the percentage of stained tumor cells in their lesions
Time to progression (TTP) curves of patients with NSCLC according to their HLA class-I antigen expression in their primary tumors. The TTP is expressed in months calculated from the time of diagnosis to the time of documented recurrence. Patients were divided into three groups, according to the percentage of stained tumor cells in their lesions
Discussion
Immunohistochemical staining of 190 formalin fixed, paraffin embedded NSCLC lesions with the HLA class I heavy chain-specific mAb HC10 has shown HLA class I antigen downregulation in about 90% of the lesions analyzed. The frequency of HLA class I antigen defects we have found is higher than that reported in the literature for NSCLC. Total HLA class I antigen loss was found in 27 and 38% of the lesions analyzed in the two previously published studies [9, 19]. Several factors may be responsible for the difference between the information in the literature and our own results. First, there are differences in the methodology and in the characteristics of the mAb used to detect HLA class I antigens. Essentially, all previous studies have been carried out using frozen tissue sections and the mAb W6/32 that recognizes a framework determinant shared by all HLA-A, B, and C alloantigens [1]. In contrast, we have used formalin fixed, paraffin embedded tissue sections and an mAb that recognizes a determinant expressed on most, but not all HLA class I allospecificities. It is known that the immunoperoxidase staining reaction with FFPE tissue sections is slightly less [8] sensitive than that with frozen tissue sections. Furthermore, although unlikely, we cannot exclude that some of the unstained lesions might have had HLA-B and C antigen loss or downregulation but still expressed HLA-A allospecificities not recognized by mAb HC-10, thereby appearing negative. At any rate, it is noteworthy that even if these methodological variables play a role in the frequency of HLA class I antigen defects we observed, they could account for only a limited portion of this difference and not for all of it. Additionally, differences between the results obtained in previous studies and in the current study may be related to differences between the patient populations studied.
An unanticipated finding of our study is the apparent association of HLA class I antigen downregulation or loss with a favorable clinical course of the disease, although this trend did not reach the level of statistical significance. Such an association is not unique to NSCLC, since it has already been described in uveal melanoma [6], breast carcinoma [12], and colon cancer [14]. The improved survival of patients with these cancers whose tumors have undergone HLA class I antigen downregulation reflects the role of NK cells in the control of tumor growth since HLA class I antigen downregulation increases target cell sensitivity to NK cell-mediated lysis [11]. Whether this mechanism plays a role also in NSCLC remains to be shown. Whatever the mechanism the association between HLA class I antigen downregulation and improved survival found in NSCLC, uveal melanoma, breast carcinoma and colon cancer is at variance with the association between HLA class I antigen downregulation and poor prognosis, which has been found in the majority of the malignant diseases analyzed [3, 5, 7].
Studies performed with NSCLC cell lines have shown that HLA class I antigen defects are caused by functional abnormalities of the antigen processing machinery. Importantly, incubation of these NSCLC cell lines with IFN-γ was able to restore HLA class I antigen expression [20]. If these same mechanisms underlie the HLA class I antigen downregulation that we have observed in NSCLC lesions, HLA class I antigen may be restored in NSCLC lesions by the administration of IFN-γ. If so, the efficacy of T cell-based immunotherapeutic strategies could be improved by the intralesional administration of IFN-γ to enhance the HLA class I antigen expression on tumor cells and their recognition by HLA class I antigen restricted, tumor antigen-specific cytotoxic T cells.
From a methodological viewpoint, our study supports the use of FFPE tissue sections for immunohistochemical analysis of HLA class I antigen expression in malignant lesions. In addition to the histological advantages of using FFPE tissue rather than frozen tissues, the use of FFPE tissue sections has enabled a retrospective study which has taken advantage of archived material with well kept histopathological and clinical records and a pathologist’s involvement in the study. However, this approach is limited by the small number of antibodies that react with formalin fixed tissue epitopes. As the range of specificities increases, we can envision that this approach would become more routine.
References
Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A (1978) Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell 14:9–20
Dunn GP, Old LJ, Schreiber RD (2004) The three Es of cancer immunoediting. Annu Rev Immunol 22:329–360
Garrido F, Cabrera T, Concha A, Glew S, Ruiz-Cabello F, Stern PL (1993) Natural history of HLA expression during tumour development. Immunol Today 14:491–499
Greenlee RT, Hill-Harmon MB, Murray T, Thun M (2001) Cancer statistics, 2001. CA Cancer J Clin 51:15–36
Hicklin DJ, Marincola FM, Ferrone S (1999) HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol Med Today 5:178–186
Jager MJ, Hurks HM, Levitskaya J, Kiessling R (2002) HLA expression in uveal melanoma: there is no rule without some exception. Hum Immunol 63:444–451
Kageshita T, Hirai S, Ono T, Hicklin DJ, Ferrone S (1999) Down-regulation of HLA class I antigen-processing molecules in malignant melanoma: association with disease progression. Am J Pathol 154:745–754
Kageshita T, Hirai S, Ono T, Ferrone S (1997) Comparison of the reactivity of frozen and formalin fixed, paraffin-embedded sections of melanoma lesions with anti-HLA class I mAb. In: Charron D (ed) Genetic diversity of HLA. Functional and medical implications. Proceedings 12th international histocompatibility workshop and conference, vol II. EDK, Sevres, France, pp 737–739
Korkolopoulou P, Kaklamanis L, Pezzella F, Harris AL, Gatter KC (1996) Loss of antigen-presenting molecules (MHC class I and TAP-1) in lung cancer. Br J Cancer 73:148–153
Lampson LA, Fisher CA, Whelan JP (1983) Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol 130:2471–2478
Ljunggren HG, Karre K (1990) In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 11:237–244
Madjd Zahra SI, Pinder S, Ellis IO, Durrant LG (2005) Loss of MHC class I is an independent indicator of good prognosis in breast cancer. Int J Cancer (in press)
Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S (2000) Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol 74:181–273
Menon AG, Morreau H, Tollenaar RA, Alphenaar E, Van Puijenbroek M, Putter H, Janssen-Van Rhijn CM, Van De Velde CJ, Fleuren GJ, Kuppen PJ (2002) Down-regulation of HLA-A expression correlates with a better prognosis in colorectal cancer patients. Lab Invest 82:1725–1733
Mountain CF (1997) Revisions in the international system for staging lung cancer. Chest 111:1710–1717
Parmiani G, Castelli C, Dalerba P, Mortarini R, Rivoltini L, Marincola FM, Anichini A (2002) Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? J Natl Cancer Inst 94:805–818
Pass HI MJ, Johnson DH, Turisi AT, Minna JD (2000) Lung cancer: principles and practice. Lippincott, Williams and Wilkins, Philadelphia
Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F (2003) Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol 171:1918–1926
Redondo M, Ruiz-Cabello F, Concha A, Cabrera T, Perez-Ayala M, Oliva MR, Garrido F (1991) Altered HLA class I expression in non-small cell lung cancer is independent of c-myc activation. Cancer Res 51:2463–2468
Restifo NP, Esquivel F, Kawakami Y, Yewdell JW, Mule JJ, Rosenberg SA, Bennink JR (1993) Identification of human cancers deficient in antigen processing. J Exp Med 177:265–272
Stam NJ, Spits H, Ploegh HL (1986) Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol 137:2299–2306
Stutman O (1979) Chemical carcinogenesis in nude mice: comparison between nude mice from homozygous matings and heterozygous matings and effect of age and carcinogen dose. J Natl Cancer Inst 62:353–358
Stutman O (1974) Tumor development after 3-methylcholanthrene in immunologically deficient athymic-nude mice. Science 183:534–536
Temponi M, Kekish U, Hamby CV, Nielsen H, Marboe CC, Ferrone S (1993) Characterization of anti-HLA class II monoclonal antibody LGII-612.14 reacting with formalin fixed tissues. J Immunol Methods 161:239–256
Travis WD, Travis LB, Devesa SS (1995) Lung cancer. Cancer 75:191–202
Acknowledgements
We thank Joan Beck for her assistance in the preparation and processing of tumor procurement and Debbie Malik for her expert assistance in histology
Author information
Authors and Affiliations
Corresponding author
Additional information
N. Ramnath and D. Tan contributed equally to the paper
Rights and permissions
About this article
Cite this article
Ramnath, N., Tan, D., Li, Q. et al. Is downregulation of MHC class I antigen expression in human non-small cell lung cancer associated with prolonged survival?. Cancer Immunol Immunother 55, 891–899 (2006). https://doi.org/10.1007/s00262-005-0085-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-005-0085-7