Abstract
Members of the inhibitor of apoptosis protein (IAP) family including survivin, are expressed in many tumors. However, age-related changes in their expression in cancer have not been clarified. Thus, we investigated the expression of mRNA-coding for IAP family proteins in colon cancer samples from young (<70 years of age) and elderly (>70 years) patients by real-time quantitative RT-PCR. Samples were collected from cases with well-differentiated adenocarcinoma or moderately differentiated adenocarcinoma and their adjacent normal epithelial tissue. Well-differentiated adenocarcinoma tended to express higher levels of survivin than normal mucosa, and expression in moderately differentiated adenocarcinoma was significantly greater than in normal mucosa in samples from both groups of patients (p<0.05, respectively). When samples were compared between the different age groups, the normal mucosa exhibited similar levels of survivin expression. However, samples from older patients showed a significantly higher level of expression than those from younger patients in well and moderately differentiated adenocarcinomas (p<0.05, respectively). In contrast, the levels of expression of cIAP1, cIAP2, and NAIP in the cancerous tissues were lower than those found in normal mucosa regardless of age. As for age-related changes, the expression of cIAP2 in normal mucosa and moderately differentiated adenocarcinoma was stronger in the elderly group than the young group (p<0.05, respectively), and NAIP expression in well-differentiated adenocarcinoma was higher in the young group than the elderly group (p<0.05). XIAP expression was similar in normal and cancerous tissues in both the young and elderly groups. These results suggest that the expression of IAP family proteins, especially survivin, is associated with the age-related biological characteristics of colon cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The regulation of apoptotic cell death has a profound effect on the pathogenesis and progression of colorectal malignancies. The inhibitor of apoptosis protein (IAP) family, including survivin, blocks apoptosis induced by a variety of triggers [1, 2], although the exact biochemical mechanism by which members of the IAP family suppress apoptosis is under debate. Survivin directly binds to and inhibits caspase-3 and caspase-7, which act as terminal effectors in apoptotic protease cascades [2, 3]. The expression of survivin is ubiquitous in fetal tissues, but is restricted during development, and is negligible in the majority of terminally differentiated adult tissues [4, 5]. However, analysis of the differences in gene expression between normal and tumor cells revealed that survivin was one of the proteins most consistently overexpressed in tumor cells relative to normal tissue [6]. In fact, survivin is prominently expressed in transformed cell lines and in many human cancers including colorectal cancer [7].
Survivin is usually found in the cytoplasm of tumor cells and is therefore widely regarded as a cytoplasmic protein [5, 8]. However, several studies showed a nuclear accumulation of survivin in gastric cancer cells [9] and lung cancer cells [10]. In colon cancer, Kawasaki et al. [7] revealed the cytoplasmic expression of survivin using immunohistochemistry. We recently reported that ALL cells principally exhibit nuclear localization of survivin, while CLL cells exhibit cytoplasmic distribution [11]. We suggested that the splice patterns of this molecule might affect its subcellular localization, although the mechanisms controlling the splicing process remained unresolved. Thus, the significance of its nuclear-cytoplasmic localization in tumor cells remains controversial.
In the present study, we collected colon cancer tissues from young and elderly patients to focus on the contribution of IAPs to the progression of colon cancer in association with aging. The expression of survivin was higher in cancerous tissue compared with adjacent normal mucosa. Immunostaining revealed that survivin was primarily localized in the cytoplasm. The expression of other IAPs, including cIAP1, cIAP2, NAIP and XIAP, all of which suppressed apoptosis by inhibiting caspase and procaspase [12–15], was also demonstrated in these samples. The IAPs are characterized by one or more 70–80 amino acid baculoviral IAP repeat (BIR) domains. They function as an endogenous caspase inhibitor, as well as participate in cell cycle regulation and in the modulation of receptor-mediated signal transduction [16]. The significances of IAP family proteins with regard to the aging effect as well as the biological characteristics of colon cancer are discussed.
Materials and methods
Patients
Fresh frozen samples from 32 patients with adenocarcinoma of the ascending, transverse, and descending colon were collected and prepared for mRNA analysis. Central portions of tumors that dominantly consisted of carcinoma cells were collected for analysis just after operation. At the same time, adjacent tissue samples from normal colonic mucosa were also collected. To eliminate the effects of factors other than the aging effect as much as possible, we collected well-differentiated or moderately differentiated adenocarcinoma with the same tumor histology from younger (<70 years old) and elderly (>70 years old) patients. The age distribution and gender of the patients were as follows: for well-differentiated adenocarcinoma from younger patients, the median of age was 58 years, the minimum was 38, and the maximum was 65, among six male and two female patients; for well-differentiated adenocarcinoma from elderly patients, the median of age was 81 years, the minimum was 75, and the maximum was 89, among five male and three female patients; for moderately differentiated adenocarcinoma from younger patients, the median of age was 56, the minimum was 45, and the maximum was 63, among six male and two female patients; and for moderately differentiated adenocarcinoma from elderly patients, the median of age was 81, the minimum was 77, and the maximum was 91, among three male and five female patients. Table 1 summarizes the Dukes’ classification of cases indicating that the stages of tumors were not significantly different between young and old patients. All patients did not receive radiotherapy or chemotherapy prior to operation. The procedures followed were in accordance with the ethical standards established by the ethics committee of Tokyo Medical and Dental University.
Preparation of RNA and quantitative assay for IAP family proteins using TaqMan RT-PCR
RNA was extracted from frozen colonic samples of normal mucosa and cancer tissue using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s directions. For quantitative RT-PCR, fluorescent hybridization probes and a TaqMan PCR Core Reagents Kit with AmpliTaq Gold (PerkinElmer Cetus, Norwalk, CT, USA) were used with an ABI Prism 7,900HT Sequence Detection System (PerkinElmer, Foster City, CA, USA). Oligonucleotides as specific primers and TaqMan probes for the IAP family and glutaraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA were synthesized at a commercial laboratory (PerkinElmer Cetus). The primers and TaqMan probes were as follows. The sequence of the forward primer for survivin mRNA was 5′-TGCCTGGCAGCCCTTTC-3′ and that of the reverse primer was 5′-CCTCCAAGAAGGGCCAGTTC-3′; the TaqMan probe was 5′-CAAGGACCACCGCATCTCTACATTC-3′. For cIAP1 mRNA, the forward primer was 5′-CAGCCTGAGCAGCTTGCAA-3′ and the reverse primer was 5′-CAAGCCACCATCACAACAAAA-3′; the TaqMan probe was 5′-TTTATTATGTGGGTCGCAATGATGATGTCAAA-3′. For cIAP2 mRNA, the forward primer was 5′-TCCGTCAAGTTCAAGCCAGTT-3′ and the reverse primer was 5′-TCTCCTGGGCTGTCTGATGTG-3′; the TaqMan probe was 5′-CCCTCATCTACTTGAACAGCTGCTAT-3′. The forward primer for NAIP mRNA was 5′-GCTTCACAGCGCATCGAA-3′ and the reverse primer was 5′-GCTGGGCGGATGCTTTC-3′; the TaqMan probe was 5′-CCATTTAAACCACAGCAGAGGCTTTAT-3′. The forward primer for XIAP mRNA was 5′-AGTGGTAGTCCTGTTTCAGCATCA-3′ and the reverse primer was 5′-CCGCACGGTATCTCCTTCA-3′; the TaqMan probe was 5′-CACTGGCACGAGCAGGGTTTCTTTATACTG-3′. Finally the forward primer for GAPDH mRNA was 5′-GAAGGTGAAGGTCGGAGT-3′ and the reverse primer was 5′-GAAGATGGTGATGGGATTTC-3′; the TaqMan probe was 5′-CAAGCTTCCCGTTCTCAGCC-3′. The conditions for the one-step RT-PCR were as follows: 30 min at 48°C (stage 1, reverse transcription), 10 minutes at 95°C (stage 2, RT inactivation and AmpliTaq Gold activation), and then 40 cycles of amplification for 15 s at 95°C and 1 min at 60°C (stage 3, PCR). The expression of survivin and other IAP family proteins was quantified according to a method described elsewhere [11]. Briefly, the intensity of the reaction was evaluated from the quantity of total RNA in Raji cells (ng) corresponding to the initial number of PCR cycles to reveal the linear increase in reaction intensity (threshold cycle) for each sample on a logarithmic standard curve. Data on the quantity of RNA (ng) for the IAP family were normalized using data for the GAPDH in each sample.
Immunohistochemistry for survivin
Tissue sections (4-μm thick) of formalin-fixed, paraffin-embedded samples from the same cases used in the RT-PCR study were sectioned and placed on slides covered with adhesive. Sections were deparaffinized, and endogenous peroxidase was quenched with 1.5% hydrogen peroxide in methanol for 10 min. Primary antibody was applied to identify survivin using polyclonal rabbit antibody against human survivin (SURV 11-A; Alpha Diagnostic International, San Antonio, TX, USA). All sections were developed using biotin-conjugated secondary antibodies against rabbit IgG or mouse IgG followed by a sensitive peroxidase-conjugated streptavidin system (DAKO) with DAB as the chromogen. Negative control staining was performed using rabbit or mouse immunoglobulin of irrelevant specificity, which was substituted for the primary antibody.
Statistical analysis
Statistically significant differences for the quantitative analysis were determined using the Mann–Whitney U-test.
Results
Age-related changes in the expression of IAP family protein mRNA in normal colonic mucosa
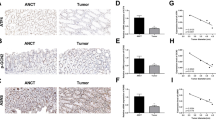
To quantitate age-related changes in mRNA expression of the IAP family in normal colonic mucosa, real-time quantitative RT-PCR was performed using samples from the normal mucosa of different age groups. As shown in Fig. 1A, B, D, and E, the expression levels of survivin, cIAP1, NAIP, and XIAP were similar in young and elderly individuals. However, cIAP2 expression of the normal mucosa was significantly higher in elderly individuals than young individuals (Fig. 1C; p<0.05).
Real-time quantitative RT-PCR analysis of IAP family proteins, A survivin, B cIAP1, C cIAP2, D NAIP, and E XIAP, using colonic samples from normal mucosa (Normal), well-differentiated adenocarcinoma (Well), and moderately differentiated adenocarcinoma (Mod.) of young and elderly patients. The relative intensity was calculated as [intensity of reaction of the IAP family (total Raji RNA, ng)] / [intensity of reaction of GAPDH (total Raji RNA, ng)]. Differences were significant between the expression of survivin (A) in samples from well-differentiated adenocarcinoma of young and elderly patients (*p<0.05 by the Wilcoxon’s test) and samples from moderately differentiated adenocarcinoma of young and elderly patients (*p<0.05). The expression of survivin was also significantly different between normal mucosa and well-differentiated adenocarcinoma (a p<0.05), normal mucosa and moderately differentiated adenocarcinoma (b p<0.05), and well and moderately differentiated adenocarcinomas (c p<0.05) in the elderly group. Between young and elderly groups, differences were significant in the expression of cIAP2 in normal mucosa (*p<0.05) and moderately differentiated adenocarcinoma (* p<0.05), and the expression of NAIP in well-differentiated adenocarcinoma (*p<0.05). The expression of cIAP1, cIAP2, and NAIP in cancerous tissues tended to show a lower degree of expression compared with normal mucosa. Differences were significant for comparisons of the indicated groups (B cIAP1, d p<0.05, e p<0.01, f p<0.05, g p<0.05; C cIAP2, h p<0.01, i p<0.01, j p<0.05; D NAIP, k p<0.05, l p<0.05, m p<0.05)
Age-related changes in the expression of IAP family protein mRNA in well-differentiated adenarcinomas of the colon
To determine age-related changes in the expression of IAP family proteins in colon cancer, samples were collected from different age groups with similar histological features. First, well-differentiated adenocarcinomas from young and elderly patients were compared. As shown in Fig. 1A, the expression of survivin was significantly higher in elderly patients than in young patients (p<0.05). Conversely, NAIP expression was significantly higher in younger patients than in elderly patients (Fig. 1D; p<0.05). Expression levels of other IAPs including cIAP1, cIAP2, and XIAP were almost similar between young and elderly patients.
Age-related changes in the expression of IAP family protein mRNA in moderately differentiated adenocarcinomas of the colon
Next, the expression levels of IAP family proteins in moderately differentiated adenocarcnomas were compared. As shown in Fig. 1A, the expression of survivin was significantly higher in elderly patients than young patients (p<0.05). Similarly, cIAP2 expression was significantly higher in elderly patients than younger patients (Fig. 1C; p<0.05). Other IAPs including cIAP1, NAIP, and XIAP did not exhibit significant difference between young and elderly patients.
Comparison of the expression of IAP family protein mRNA in normal and cancerous tissues in young and elderly patients
As shown in Fig. 1A, the cancer tissues tended to express higher levels of survivin than that found in normal mucosa. Significant differences were observed between normal mucosa and well-differentiated adenocarcinoma (p<0.05), normal mucosa and moderately differentiated adenocarcinoma (p<0.01), and well and moderately differentiated adenocarcinoma (p<0.05) in the elderly group. By contrast, the expression of cIAP1, cIAP2, and NAIP in cancerous tissues was lower than that found in normal mucosa. For cIAP1 expression (Fig. 1B), differences were significant between the normal mucosa and the well-differentiated adenocarcinoma (young group, p<0.05; elderly group, p<0.05) and normal mucosa and moderately differentiated adenocarcinoma (young group, p<0.01; elderly group, p<0.05). For the expression of cIAP2 (Fig. 1C), significant differences were observed between normal mucosa and well-differentiated adenocarcinoma (elderly group, p<0.01) and normal mucosa and moderately differentiated adenocarcinoma (young group, p<0.01; elderly group, p<0.05). The difference in the expression of NAIP (Fig. 1D was significant between normal mucosa and well-differentiated adenocarcinoma (young group, p<0.05; elderly group, p<0.05) and normal mucosa and moderately differentiated adenocarcinoma (young group, p<0.05). The expression of XIAP did not exhibit any specific tendency between normal and cancerous tissues, as well as between the young and elderly groups (Fig. 1E).
Immunohistochemical localization of survivin in colon cancers
To investigate the distribution of survivin, immunohistochemical staining was performed using normal colonic mucosa and cancerous tissues. As shown in Fig. 2A (young) and C (elderly), survivin was mainly detected in the bottom of the glands of normal mucosa, and subcellular localization was mainly cytoplasmic. The staining intensity of positive cells in normal mucosa varied between different samples. In contrast, the majority of samples from colon cancer showed a diffuse localization of survivin, although the staining intensity varied from case to case. At the cellular level, survivin signals in colon cancer cells were predominantly localized in the cytoplasm. The distribution of survivin was similar in well-differentiated and moderately differentiated adenocarcinomas as well as in cancers from young and elderly patients (Fig. 2B, D). Tissue sections that reacted with preimmune rabbit antibody with irrelevant specificity showed no significant staining for any of samples (not shown).
Immunohistochemical localization of survivin in the colon from normal mucosa (A) (×100), well-differentiated adenocarcinoma of a young patient (B) (×200), and normal mucosa (C) (×100), well-differentiated adenocarcinoma of a elderly patient (D) (×200). The expression of survivin was observed in a few scattered cells of the normal colonic epithelia, mainly at the bottom of the glands (A, C). The subcellular distribution was dominantly cytoplasmic. In contrast, survivin-positive cells were diffusely distributed in cancerous tissues, and staining was cytoplasmic at the cellular level (B, D)
Discussion
For the expression of survivin in colon cancer, a previous study revealed its overexpression in cancer tissue while normal colonic epithelia exhibited weak signals [7]. However, little is known about the potential roles of IAPs in the homeostasis of normal epithelia as well as the pathogenesis of colon cancers among different age groups. In the colon cancer samples in the present study, cancerous tissues had a tendency to exhibit higher levels of survivin but lower levels of cIAP1, cIAP2, and NAIP than the normal mucosa. Immunohistochemical staining revealed a high degree of survivin expression in many cancer cells in the majority of cases, although in normal mucosa, the number of positive cells was small. Thus, differences in the positive cell ratio influenced the intensity and overall expression of survivin mRNA. Kawasaki et al. [7] reported that the expression of survivin correlated with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. We also confirmed the progression-related overexpression of survivin by our findings that moderately differentiated adenocarcinomas expressed higher levels of survivin than well-differentiated adenocarcinomas. Well and moderately differentiated adenocarcinomas, cancerous tissues from elderly patients, demonstrated a significantly higher degree of survivin expression than those from young patients. It would be possible that chances for genetic mutations might increase associated with progression of cancer as well as aging and might result in dysregulation in controlling survivin expression. However, further studies are necessary to determine whether age-related overexpression and differentiation-related overexpression of survivin in colon cancers occurs through the same mechanisms.
In addition to its antiapoptotic function, survivin also plays a role in the regulation of cell cycle progression during mitosis [8]. The highly proliferative activity and low frequency of apoptosis in colon cancer cells is associated with the significant expression of survivin [7]. We recently reported that the proliferative activity and apoptotic frequency of cancer cells from colorectal cancers exhibited a positive correlation with age [17]. However, in the present study, survivin expression in colon cancers was significantly higher in elderly patients than in young patients. Thus, increased apoptosis in cancers of elderly patients is not attributable to a lack of antiapoptotic regulatory mechanisms by survivin. The manner of age-related changes in the expression of survivin might be associated with the proliferative activities of colon cancers rather than apoptosis. We can also postulate the other possibility that increased apoptosis in cancers of the elderly might be controlled by the caspase-independent mechanisms and, thus, survivin would be overexpressed as the feedback mechanisms of the cells.
Wild-type p53, and not mutant p53, represses survivin expression at the mRNA and protein level [18]. Modification of chromatin within the survivin promoter would explain the silencing of survivin gene transcription by p53 [19]. On the other hand, the overexpression of exogenous survivin protein rescues cells from p53-induced apoptosis in a dose-dependent manner, suggesting that the loss of survivin in part mediates the p53-dependent apoptotic pathway [19]. As there is a high frequency of p53 mutations in many solid cancers, p53 mutation may play a role in controlling the overexpression of survivin in colon cancer. Regarding the age-dependent changes in expression of tumor tissues, a microarray experiment revealed an activation of p53 and some of the genes controlled by p53 at more advanced age [20]. Thus, higher expression of survivin might be induced in response to the increased p53-dependent apoptosis in cancers of the elderly group. However, mutations/loss of p53 gene may occur more frequently with aging in certain settings [21] and, therefore, the p53-associated mechanisms controlling aging and carcinogenesis are complicated.
Using an in vitro cell culture system of colon carcinoma cell lines, Wang et al. [22] reported that the expression of cIAP2 was induced by PKC/NF-κB-dependent pathways. However, the present study revealed that cIAP2 expression was not elevated in colon cancers in vivo. Instead, cIAP1, cIAP2, and NAIP exhibited lower expression in cancerous tissues than in normal mucosa. Further studies are necessary to clarify the function of these molecules in the colonic mucosa as well as in carcinogenesis of the colon.
Signaling pathways involved in survival responses may attenuate the apoptosis response to the cytotoxic tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) in colon carcinoma cell lines. Some lines are sensitive, while others are resistant to TRAIL-induced apoptosis [23, 24]. The mechanisms of this resistance include blocking caspase processing by XIAP. However, in the present study, changes in XIAP expression were not notable among normal/cancerous tissues, well/moderately differentiated adenocarcinomas, and cancers of young/elderly patients. Thus, XIAP function in in vivo colon cancer might not be essential for the survival of cancer cells.
In conclusion, we demonstrated the differentiation-related and age-related overexpression of survivin in colon cancer samples. Further studies are warranted to clarify the regulatory mechanisms of IAP expression in colon cancer in association with the apoptotic/proliferative signaling pathways.
References
LaCasse EC, Baird S, Korneluk RG et al (1998) The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene 17:3247–3259
Tamm I, Wang Y, Sausville E et al (1998) IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res 58:5315–5320
Shin S, Sung B-J, Cho Y-S et al (2001) An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry 40:1117–1123
Adida C, Crotty PL, McGrath J, Berrebi D et al (1998) Developmentally regulated expression of the novel cancer anti-apoptotic gene survivin in human and mouse differentiation. Am J Pathol 152:43–49
Ambrosini G, Adida C, Altieri DC (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3:917–921
Velculescu VE, Madden S, Zhang L et al (1999) Analysis of human transcriptomes. Nature Genet 23:387–388
Kawasaki H, Toyoda M, Shinohara H et al (2001) Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer 91:2026–2032
Gianani R, Jarboe E, Orlicky D et al (2001) Expression of survivin in normal, hyperplastic, and neoplastic colonic mucosa. Hum Pathol 32:119–125
Okada E, Murai Y, Matsui K et al (2001) Survivin expression in tumor cell nuclei is predictive of a favorable prognosis in gastric cancer patients. Cancer Lett 163:109–116
Rodriguez JA, Span SW, Ferreira CGM et al (2002) CRM1-mediated nuclear export determines the cytoplasmic localization of the antiapoptotic protein survivin. Exp Cell Res 275:44–53
Nakagawa Y, Yamaguchi S, Hasegawa M et al (2004) Differential expression of survivin in bone marrow cells from patients with acute lymphocytic leukemia and chronic lymphocytic leukemia. Leuk Res (in press)
Duckett CS, Nava VE, Gedrich RW et al (1996) A conserved family of cellular genes related to the baculovirus IAP gene and encoding apoptosis inhibitors. EMBO J 15:2685–2694
Liston P, Roy N, Tamai K et al (1996) Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature 379:349–353
Rothe M, Pan MG, Henzei WJ et al (1995) The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 83:1243–1152
Roy N, Dereraux QL, Takahashi R et al (1997) The cIAP-1 and cIAP-2 proteins are distinct inhibitors of specific caspases. EMBO J 16:6914–6925
Liston P, Fong WG, Korneluk RG (2003) The inhibitor of apoptosis: there is more to life than Bcl2. Oncogene 22:8568–8580
Tanaka K, Nagaoka S, Takemura T et al (2003) Incidence of apoptosis increases with age in colorectal cancer. Exp Gerontol 37:1469–1479
Hoffman WH, Biade S, Zilfou JT et al (2002) Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem 277:3247–3257
Mirza A, McGuirk M, Hockenberry TN et al (2002) Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene 21:2613–2622
Kirschner M, Pujol G, Radu A (2002) Oliginucleotide microarray data mining: search for age-dependent gene expression. Biochem Biophys Res Commun 298:772–778
Sharpless NE, DePinho RA (2004) Telomeres, stem cells, senescence, and cancer. J Clin Invest 113:160–168
Wang Q, Wang X, Evers BM (2003) Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. J Biol Chem 278:51091–51099
Deng Y, Lin Y, Wu X (2002) TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev 16:33–45
Tillman DM, Izeradjene K, Szucs KS et al (2003) Rottlerin sensitizes colon carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis via uncoupling of the mitochondria independent of protein kinase C. Cancer Res 63:5118–5125
Acknowledgements
This work was supported in part by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 14570180).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Endo, T., Abe, S., Seidlar, H.B.K. et al. Expression of IAP family proteins in colon cancers from patients with different age groups. Cancer Immunol Immunother 53, 770–776 (2004). https://doi.org/10.1007/s00262-004-0534-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-004-0534-8