Abstract
HER-2/neu is an immunogenic protein eliciting both humoral and cellular immune responses in patients with HER-2/neu-positive (+) tumors. Preexisting cytotoxic T lymphocyte (CTL) immunity to HER-2/neu has so far been mainly evaluated in terms of detection of CTL precursor (CTLp) frequencies to the immunogenic HLA-A2–binding nona-peptide 369-377 (HER-2(9369)). In the present study, we examined patients with HER-2/neu + breast, ovarian, lung, colorectal, and prostate cancers for preexisting CTL immunity to four recently described HER-2/neu–derived and HLA-A2–restricted "cytotoxic" peptides and to a novel one spanning amino acids 777–785 also with HLA-A2–binding motif. We utilized enzyme-linked immunosorbent spot (ELISpot) assay, which allows a quantitative and functional assessment of T cells directed against specific peptides after only brief in vitro incubation. CTL reactivity was determined with an interferon γ (IFN-γ) ELISpot assay detecting T cells at the single cell level secreting IFN-γ. CTLp were defined as peptide-specific precursors per 106 peripheral blood mononuclear cells (PBMCs). Patients' PBMCs with increased CTLp were also tested against autologous tumor targets and peptide-pulsed dendritic cells (DCs) in cytotoxicity assays. We also studied patients with HER-2/neu-negative (-) tumors and healthy individuals. Of the HER-2/neu+ patients examined, 31% had increased CTLp to HER-2(9952), 19% to HER-2(9665), 16% to HER-2(9689), and 12.5% HER-2(9435), whereas only 2 of 32 patients (6%) responded to HER-2(9777). The CTLp recognizing HER-2(9952) were extremely high in two patients with breast cancer, one with lung cancer, and one with prostate cancer. None of the HER-2/neu- patients or healthy donors exhibited increased CTLp to any of these peptides. Besides IFN-γ production, preexisting CTL immunity to all five HER-2/neu peptides was also shown in cytotoxicity assays where patients' PBMCs with increased CTLp specifically lysed autologous tumor targets and autologous peptide-pulsed DCs. Our results demonstrate for the first time that (1) preexisting immunity to peptides HER-2(9435), HER-2(9952), HER-2(9689), HER-2(9665), and HER-2(9777) is present in patients with HER-2/neu + tumors of distinct histology, (2) HER-2(9777) is a naturally processed peptide expressed on the surface of HER-2/neu + tumors, as are the other four peptides, and (3) HER-2/neu + prostate tumor cells can be recognized and lysed by autologous HER-2 peptide-specific CTL. Our findings broaden the potential application of HER-2/neu-based immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The HER-2/neu gene encodes a 185-kDa transmembrane glycoprotein with tyrosine-specific kinase activity that has a similarity in structure and sequence to the epidermal growth factor receptor [1, 9]. It has been reported to be overexpressed in a large proportion of aggresive breast and ovarian tumors and in other cancers of epithelial origin [27, 38, 40, 41, 48]. The HER-2/neu protein appears to be an ideal tumor-associated antigen (TAA) for immunotherapy, because cytotoxic T-lymphocyte (CTL) responses specific for major histocompatibility complex (MHC) class I epitopes have been observed in some cancer patients [12, 16, 26, 49]. Furthermore, there is already evidence of the existence of MHC class II T-lymphocyte responses to HER-2/neu: first, some patients with HER-2/neu + colorectal [47], breast [11, 13], and prostate cancer [28] produce IgG antibodies against HER-2/neu, suggesting that this protein triggers CD4+ T-lymphocyte responses which are essential for IgG class switching [11], and, second, CD4+ T-lymphocytes have been demonstrated to proliferate and produce IFN-γ in an MHC class II–restricted fashion in response to stimulation with HER-2/neu–derived synthetic peptides [31, 43]. Finally, tumor-reactive CTL responses have been induced in vitro using various recently identified MHC class I–binding synthetic peptides derived from the HER-2/neu sequence [5, 6, 16, 24, 37]. Regarding MHC class I–restricted cytolytic responses, it is of particular interest that peptide-specific CTLs will be able to recognize HER-2/neu + tumor targets presenting in the context of the appropriate MHC class I allele the naturally processed relevant peptide [5, 23, 37]. The identification of such MHC class I–restricted HER-2/neu–specific CTL peptides will allow the selection of the epitopes with the highest potential for vaccination. Such epitopes must be highly immunogenic and must be able to recruit a wide spectrum of functional CTLs, capable of generating effective antitumor responses.
HER-2/neu epitopes spanning amino acids 435–443 (HER-2(9435)), 665–673 (HER-2(9665)), 689–697 (HER-2(9689)), and 952–960 (HER-2(9952)) have been recently demonstrated to bind with high or intermediate affinities to human leukocyte antigen (HLA)-A2.1 molecules and to elicit CTLs from tumor-associated lymphocytes of patients with ovarian cancer [5, 37]. Such CTLs specifically killed peptide-sensitized target cells in an HLA-A2–restricted manner and, most importantly, a HER-2/neu–transfected cell line and HLA-A2+, HER-2/neu + autologous tumor cells [37]. The HER-2(9689) epitope was also found to be immunodominant in gastric cancer–specific CTLs [23]. Most recently [5], we have found that, besides classical CTLs, these HER-2/neu–derived peptides can elicit NKT (natural killer T lymphocyte) cells specifically recognizing their autologous HLA-A2+, HER-2/neu + ovarian tumors. The generation of both T and NKT cytotoxic effectors specific for HER-2/neu peptide epitopes may provide an effective means toward HER-2/neu expressing tumor cell destruction in vivo during cellular adoptive immunotherapy. In addition, immunizing patients harboring HER-2/neu + tumors with peptide-based vaccines may also generate in vivo CTL immunity. It is therefore important to know if and to which levels CTL precursor (CTLp) frequencies specific for these HER-2/neu peptides exist in nonimmunized patients. Quantification of HER-2/neu peptide-specific CTLp in the peripheral blood of patients with HER-2/neu + tumors will be indicative of the immunodominance of the respective HER-2/neu epitopes in vivo. This could possibly assist in the selection of the appropriate peptides to be included in the vaccination protocol.
In the present study, we quantified CTLp specific for HER-2(9435), HER-2(9665), HER-2(9689),and HER-2(9952) peptides by utilizing an interferon γ (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) assay. In addition, CTLp recognizing a novel HER-2/neu peptide (HER-2(9777)) that also contains HLA-A2–binding motifs were estimated. All estimations were performed using peripheral blood mononuclear cells (PBMCs) from patients with HER-2/neu + or HER-2/neu - tumors of distinct histology and from healthy donors. PBMCs from patients with increased CTLp demonstrated specific lysis of autologous tumor cells suggesting that these peptides are naturally processed and expressed by HER-2/neu + carcinomas. Our data are relevant to the use of the above HER-2/neu–derived peptides in vaccination studies with patients harboring HER-2/neu + carcinomas.
Materials and methods
Patients
HLA-A2.1 patients with histologically confirmed breast, ovarian, lung, colorectal, and prostate carcinomas were included in this study (Tables 1 and 2). Tumor cells were isolated from pleural or ascitic fluids collected during routine aspirations and from surgically excised tumor specimens. PBMCs were collected from peripheral blood samples. Patients had not received any antineoplastic therapy during at least 4 months preceding the onset of the study. TNM classification was used for patients' tumor staging. Biologic material was provided by the Breast Cancer Clinic of Saint Savas Cancer Hospital and the Department of Pathophysiology, Laikon General Hospital, under the Institutional Review Board of both institutions. All volunteers provided informed consent before entering these studies.
Isolation of tumor cells
Tumor cells were isolated either from malignant pleural or peritoneal fluids (breast, ovarian, colorectal, prostate, and lung cancer) or from surgically excised solid tumor specimens (breast cancer). Isolation of tumor cells from malignant effusions was performed as described [3]. Briefly, fluids were spun at 400 g for 5 min to sediment cells, that were further placed on top of 75% Ficoll Separation Solution (Biochrom, Berlin, Germany) gradient, overlaid on 100% Ficoll Separation Solution, and spun at 700 g for 25 min. Tumor cells were collected from the top of 75% Ficoll Separation Solution and cryopreserved in liquid nitrogen until use. Tumor cell isolation from solid tumor samples collected aseptically at the time of operation was performed as described [3]. Briefly, necrotic areas or any fat surrounding the tumor was carefully removed before the preparation of cells. Single cell suspensions were prepared mechanically using a scalpel and needle to tease apart the sample and release the cells into suspension. Suspensions of recovered cells were washed in RPMI-1640 medium (Life Technologies, Gaithersburg, MD), passed through a sterile nylon mesh, washed, and resuspended in RPMI-1640 medium. Cell viability exceeded 80%. Tumor cells were cryopreserved in liquid nitrogen until use. At that time, cells were carefully thawed, slowly diluted in RPMI-1640 medium, and washed. Tumor cells were incubated in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2-mM l-glutamine and 50 μg/ml gentamicin (all purchased from Life Technologies).
Isolation of PBMCs
PBMCs were isolated by density gradient centrifugation using Ficoll Separating Solution (Biochrom). Cells were washed twice with phosphate-buffered saline (PBS) and used immediately or kept frozen until use.
Peptide synthesis
All peptides used in this study [HER-2 (435–443) (ILHNGAYSL), HER-2 (665–673) (VVLGVVFGI), HER-2 (689–697) (RLLQETELV), HER-2 (777–785) (GSPYVSRLL), HER-2 (952–960) (YMIMVKCWM) as well as control HLA-A2.1–binding peptide gp100 (154–162) (KTWGQYWQV)] were synthesized by the solid phase method with an Ecosyn P peptide synthesizer (Eppendorf-Biotronik, Hamburg, Germany) using the Fmoc strategy and a 4-carboxybenzyl alcohol resin. Purification was performed by high-performance liquid chromatography. Purity was >95%. Quantitative and qualitative determination were controlled by amino acid analysis and matrix-assisted laser desorption mass spectrophotometry (Kratos Kompact Maldi II, Kratos Analytical, Manchester, United Kingdom). Peptides were lyophilized, dissolved in PBS, aliquoted at 2 mg/ml, and stored frozen at −20°C until use.
These peptides bind to HLA-A2.1 allele with different binding scores. A binding score is given in arbitrary units, and the intensity of binding is determined by the number of main, secondary, and auxiliary anchor residues for HLA-A2.1 [36]. Peptides with a binding score >20 are considered as high binders and those with a binding score <20 and >10 as intermediate binders. Accordingly, HER-2(9435), HER-2(9665), and HER-2(9689) are high binders (binding scores 27, 22, and 24, respectively), and HER-2(9952) and HER-2(9777) are intermediate binders (binding scores 16 and 14, respectively). Peptide gp100(9154) also binds with intermediate affinity to HLA-A2.1 (binding score 18) [36].
Monoclonal Abs and immunophenotyping
Expression of HER-2/neu on patients' tumor cells was determined using the PE-conjugated anti–HER-2/neu monoclonal antibody (mAb) (clone Neu 24.7), which recognizes the extracellular domain of HER-2/neu (Becton Dickinson, Mountain View, CA). For DC-typing anti-CD83 conjugated with phycoeryrthin (PE) mAb was obtained from Caltag Laboratories (Burlingame, CA). All other mAb including anti-CD16, anti-CD20, anti-CD40, and anti-CD80 conjugated with fluorescein isothiocyanate (FITC); anti-CD3, anti-CD14, anti-CD86, and anti-DR conjugated with PE were purchased from PharMingen (San Diego, CA). Cells to be immunostained were washed twice with ice-cold PBS supplemented with 1% bovine serum albumin (BSA) followed by incubation with saturating concentrations of the appropriate mAb for 20 min at room temperature. Thereafter, cells were washed twice in ice-cold PBS/1% BSA and fixed with 1% paraformaldehyde in PBS. Expression of HLA-A2.1 subtype was determined by indirect immunofluorescence using the BB7.2 mAb kindly provided by Professor H.-G. Rammensee (Department of Immunology, University of Tubingen, Tubingen, Germany) and FITC-conjugated rabbit antimouse Ig (DAKO, Glostrup, Denmark) as described [4]. Samples were analyzed using FACSCalibur (Becton Dickinson) and CellQuest analysis software.
Generation of DCs
DCs were generated from CD14+ monocyte precursors purified from PBMCs freshly isolated from 20-ml peripheral blood by positive immunoselection using an anti-CD14 mAb coupled onto magnetic microbeads (Miltenyi Biotech, Auburn, CA) under the manufacturer's protocol. Monocyte differentiation in DCs was performed as described [31]. In brief, the CD14+ cells were cultured in 2-ml X-VIVO 15 medium (Life Technologies) supplemented with 1% autologous heat inactivated plasma, 1,000 IU/ml interleukin 4 (IL-4) (R&D Systems, Europe) and 1,000 IU/ml granulocyte-macrophage cell stimulating factor (GM-CSF) (Immunex, Seattle, WA). Fresh medium (2 ml) with cytokines was added on days 2 and 4. Tumor necrosis factor α (R&D Systems) was added at 10 ng/ml on day 6. DCs were harvested on day 7 and used as antigen-presenting cells (APCs) or cryopreserved for later use. The percentage of mature DCs recorded was >50%, based on the expression of a CD3-, CD14-, CD16-, CD20-, CD40+, CD80+, CD83+, CD86+, and HLA-DR+ phenotype analyzed by flow cytometry. DCs were used as APCs pretreated with 100 μg/ml mitomycin C (Kyowa, Tokyo, Japan) for 45 min at 37°C. Following an extensive wash in Hank's balanced salt solution (Life Technologies), DCs were pulsed with 50 μg/ml of the peptide for 4 h at 37°C.
ELISpot assay
The ELISpot assay was used to determine CTLp specific to HER-2/neu peptides and to tetanus toxoid (TT). On day 0, PBMCs from every individual were plated at 500,000/well in quadruplicates in 96-well flat-bottom plates. Peptide-pulsed autologous DCs (50 μg/ml) were added to PBMCs at a cell ratio of 1:10 in a total volume of 200 μl/well X-VIVO 15 medium supplemented with 1% autologous heat inactivated plasma, 10 ng/ml interleukin 7 (IL-7) and 100 pg/ml interleukin 12 (IL-12) (both purchased from R&D Systems). Control cultures contained PBMCs stimulated with unpulsed DCs or DCs pulsed with soluble TT (Ladecle Laboratories, Pearl River, NY) at 0.1 flocculation units (LfU)/ml. Cultures were incubated at 37°C in a CO2 incubator. On day 3, 100 μl of the culture supernatant was decanted and replaced by an equal volume of fresh medium supplemented with 20 ng/ml IL-7 and 200 pg/ml IL-12. Growing microcultures were restimulated on day 7 with DC pulsed with the same concentration of the respective peptide. Twenty-four hours later, IFN-γ production was estimated using the Biosource IFN-γ ELISpot assay kit (Biosource International, Camarillo, CA) under the manufacturer's protocol. Spots were counted under stereomicroscope (Zeiss, Germany) using Image ProPlus software (Digital Image Analysis). Specific spots were calculated by subtracting the mean number of spots obtained from the control cultures (i.e., with unpulsed DCs) plus 2 SD, from the mean number obtained in the experimental cultures (with peptide-pulsed DCs or TT-pulsed DCs). Peptide-specific CTLp were also enumerated from PBMCs from patients with HER-2/neu - tumors and healthy individuals. We considered responders those HER-2/neu + patients whose individual CTLp frequencies to HER-2/neu peptides were higher compared with the highest CTLp frequencies to the same peptide observed among HER-2/neu - patients and healthy individuals plus 2 SD (see also "Results"). PBMC cultures from responders with high CTLp were also tested in the cytotoxicity assay.
Cytotoxicity assay
Cytotoxic activity of PBMC effectors was determined in a standard 4-h 51Cr-release assay against various targets including autologous tumor cells and autologous DCs peptide-pulsed or unpulsed, as previously described [32]. In brief, target cells were labeled with 100-μCi sodium [51Cr] chromate (Radiochemical Centre, Amersham, UK) per 106 target cells for 1 h. Effector cells (E) were incubated with target cells (T) at the indicated ratios. Spontaneous 51Cr release was measured by incubating target cells in the absence of effector cells. Maximum 51Cr release was determined by adding 1% Triton X-100 (Sigma, St. Louis, MO). Spontaneous lysis did not exceed 10% of the maximum release. The amount of 51Cr released was measured in a γ-counter (Packard, Downers Grove, IL) and the percent lysis was calculated as follows: % specific lysis = (experimental 51Cr release – spontaneous 51Cr release) / (maximum 51Cr release – spontaneous 51Cr release) x 100. Cytotoxicity values were considered to indicate significant recognition of a target when the differences between mean values (from triplicate cultures) for percentage lysis of the particular target (i.e., autologous tumor targets or DCs pulsed with the relevant HER-2/neu peptide) and unloaded DCs or DCs pulsed with irrelevant control peptide were ≥10% at an E/T ratio of 50:1 and 25:1 [19]. Statistical significance was at p<0.05.
Proliferation assay
Proliferative responses to phytohemagglutinin (PHA; Sigma) were performed as previously described [2]. Data are presented as stimulation index (i.e., counts per min [cpm]) from PBMC cultures with PHA divided by cpm from PBMC cultures without PHA.
Statistical analysis
Significant differences between mean CTLp for each HER-2/neu peptide and TT, as well as for the mean PHA stimulation index were assessed by applying Students' t-test. The same test was applied for assessing the significance of cytotoxic responses against autologous tumor targets and DCs pulsed with the relevant HER-2/neu peptide compared with those observed against unpulsed DCs or DCs pulsed with the control gp100(9154) peptide.
Results
Enumeration of HER-2/neu peptide–specific CTL precursors
HLA-A2.1 patients with HER-2/neu + (n=32) or HER-2/neu - (n=21) tumors were enrolled in this study (Tables 1 and 2). The median time from last chemotherapy was 6 months (range 4–11).
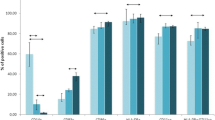
Patients were initially examined for immunocompetence by testing the capacity of their PBMCs to proliferate upon stimulation with PHA and by evaluating their precursor frequencies to whole TT using the IFN-γ ELISpot assay (Fig. 1). These values were compared with those of 15 healthy HLA-A2.1 volunteers. The mean PHA stimulation index of healthy donors was 94 (range 59–142) and of HER-2/neu + and HER-2/neu - patients was 90 (range 35–195) and 89 (range 49–135), respectively. The mean TT-specific CTLp, defined as TT-specific precursors / 106 PBMCs, of volunteer donors, was 570 (range 190–920) and of patients was 550 (range 210–1,000) for HER-2/neu + and 580 (range 230–990) for HER-2/neu -. The mean values for PHA and TT responses did not differ significantly from each other (p=0.1).
Assessment of immunocompetence of HER-2/neu + and HER-2/neu - patients. CTLp to TT were measured as IFN-γ ELISpot responses of total PBMCs to autologous TT-pulsed DCs and expressed as the number of spots (precursors) / 106 PBMCs. Proliferative responses to PHA were expressed as stimulation index (i.e., cpm with PHA divided by cpm without PHA). Each closed symbol represents one single individual tested (HER-2/neu + patients, n=32; HER-2/neu - patients, n=21; healthy donors, n=15). The solid lines indicate the mean CTLp frequencies or the mean stimulation index for the group
Generation of CTLp to the nonamers HER-2(9435), HER-2(9665), HER-2(9689), HER-2(9777), and HER-2(9952) was also evaluated in HER-2/neu + and HER-2/neu - patients and in healthy donors using the IFN-γ–based ELISpot assay, defined as peptide-specific CTLp / 106 (Fig. 2). Among the HER-2/neu + patients examined, responders were defined as those with CTLp higher than the highest CTLp detected in HER-2/neu - patients and healthy donors plus 2 SD. In these calculations, we considered the highest SD value from mean CTLp for each peptide from both groups (i.e., HER-2/neu - patients and healthy donors). For example, for HER-2(9435) the mean ± SD of CTLp for HER-2/neu - patients was 5.9±6.5 with highest CTLp 15/106 PBMCs and for healthy donors the mean CTLp ± SD was 6.0±7.2 with highest CTLp 12/106 PBMCs. For this particular peptide, responders were defined as those having CTLp >15+(2x7.2) or CTLp >29.4. The mean CTLp for the four HER-2/neu peptides, namely HER-2(9435), HER-2(9665), HER-2(9689), and HER-2(9952), was significantly higher in patients with HER-2/neu + tumors (17, 18, 14, and 40) than in HER-2/neu - patients (5.9, 7.6, 5.7, and 10.2) and healthy donors (5, 6.5, 5.3, and 6.5) (p<0.01) (Fig. 2). No statistically significant difference could be detected between the mean CTLp among HER-2/neu - patients and healthy donors. There were four responders to HER-2(9435) (patients with ovarian, [n=1], breast [n=1], prostate [n=1], and lung [n=1] cancer), six responders to HER-2(9665) (2 breast cancer, 2 ovarian cancer, 1 prostate cancer, and 1 lung cancer), five responders to HER-2(9689) (1 breast cancer, 2 ovarian cancer, and 2 colorectal cancer), and 10 responders to HER-2(9952) (3 breast cancer, 2 ovarian cancer, 1 prostate cancer, 2 colorectal cancer, and 2 lung cancer). Only four patients were defined as responders to more than one peptide (i.e., HER-2(9952) / HER-2(9435) and HER-2(9952) / HER-2(9665), both breast cancer [No. 20 and 27]; HER-2(9952) / HER-2(9689) and HER-2(9435) / HER-2(9665), both ovarian cancer [No. 6 and 29]).
HER-2/neu peptide–specific CTLp in HLA-A2.1 patients with HER-2/neu + or HER-2/neu - tumors and healthy individuals. Above the dotted line are shown responders to each single peptide. The solid lines indicate the mean CTLp frequencies for the group. Note that in groups there were some individual CTLp frequencies which were significantly higher compared with the mean CTLp. However, these according to the definition used in "Results" do not belong to the group of "responders"
In contrast to the above four HER-2/neu–derived peptides, the mean CTLp to HER-2(9777) in patients with HER-2/neu + tumors did not statistically differ from those observed with HER-2/neu - patients and healthy donors (Fig. 2). In addition, only 2 of 32 patients were scored as responders to this peptide (both with breast cancer).
Increased CTL precursor frequencies correlate with autologous tumor-specific cytotoxicity
So far, peptide-specific CTLp were extrapolated from the number of ex vivo stimulated T cells producing IFN-γ / 106 PBMCs. We next sought to examine whether such CTLp express cytotoxicity against autologous DCs pulsed with the relevant HER-2/neu peptide as well as against their autologous tumor cells. To this end, cultures with increased CTLp to one single peptide were tested in cytotoxicity assays against the above targets and also against autologous DCs unpulsed or pulsed with an irrelavent HLA-A2.1 binding peptide, as controls. As shown in Fig. 3, PBMCs from a breast cancer patient (No. 17; Table 1) with 244/106 PBMC CTLp to HER-2(9952) could lyse to relatively low, though significant levels, autologous tumor cells and autologous DCs pulsed with the same peptide but not unpulsed DCs or DCs pulsed with gp100(9154) (p<0.05 compared with unpulsed DCs or gp100(9154)-pulsed DCs). This was also the case with an ovarian cancer patient (No. 22) with 84/106 PBMC CTLp to HER-2(9665), a patient with lung cancer (No. 24) with 90/106 CTLp to HER-2(9435), and a patient with prostate cancer (No. 16) with 69/106 PBMC CTLp to HER-2(9689). PBMCs from one patient (breast cancer, No. 19) responder to HER-2(9777) proved to be also cytotoxic against their autologous tumor targets and peptide-pulsed DCs (Fig. 3).
Cytotoxic responses of responder PBMCs (i.e., PBMCs from patients with HER-2/neu + tumors with high peptide-specific CTLp) against their autologous tumors and their DCs unpulsed or pulsed with the relevant HER-2/neu peptide or with the irrelevant HLA-A2.1-binding gp100(9154) peptide. Five representative cases (one for each peptide) out of 17 tested are shown
In all cases, effector PBMCs from a single patient with increased CTLp for one given HER-2/neu peptide (e.g., HER-2(9952)) could not lyse autologous DCs pulsed with any of the other four HER-2/neu peptides (data not shown). This suggested that lysis of the autologous tumor cells was mediated indeed by the relevant CTLs (in this case, the HER-2(9952)–specific ones) and not by CTLs specific to any of the other HER-2/neu peptides existing at low frequencies within this particular patient's PBMCs.
Discussion
In this study, we used the ELISpot assay to evaluate T-cell precursor frequencies in patients with HER-2/neu + or HER-2/neu - tumors of distinct histology and in healthy individuals. We have determined that patients whose tumors overexpressed HER-2/neu have increased frequencies of T-cell precursors specific for peptides HER-2(9952), HER-2(9453), HER-2(9665), and HER-2(9689). Since these nonamers have been demonstrated to be recognized by CTLs [5, 23, 37], it is reasonable to assume that the lymphocytes within the total PBMC population producing IFN-γ in response to these peptides in the ELISpot assay belong to this particular T-lymphocyte subset. Furthermore, our study supports the following points: It demonstrates for the first time that (1) besides ovarian [5, 37] and gastric [23] cancers, these HER-2/neu–derived peptides are immunogenic, eliciting CTL responses also in breast, colorectal, lung, and prostate HER-2/neu + cancers; (2) patients' CTLs recognize these HER-2/neu–encoded epitopes presented as naturally processed peptides on autologous tumor cells, and (3) HER-2(9777) represents a novel immunogenic epitope also naturally processed and recognized by CTLs on autologous tumor cells.
So far, preexisting immunity to HER-2/neu MHC class I–restricted peptides recognized by CTLs has been studied to some extent for HER-2(9369). CTLp to this peptide within patients' PBMCs could not be detected [14] or were detected at very low frequencies [15, 20]. Nevertheless, vaccination with HER-2(9369) or with longer HER-2/neu peptides encompassing HER-2(9369) increased the mean frequencies of CTLp specific to this peptide. CTLp frequencies to HER-2(9689) were also undetectable among nonimmunized HER-2/neu–overexpressing breast and ovarian cancer patients [20]. Postimmunization precursor frequencies in 4 of 15 patients, after vaccination with a "helper" 15-amino acid HER-2/neu vaccine (HER-2(15688)) which contained HER-2(9689), were increased to an average of 25 per 106 PBMCs. In the present study, we could detect high levels of preexisting immunity in 5 of 32 patients tested (15.6%) to the same peptide with CTLp ranging from 55–78/106 PBMCs. Since an IFN-γ ELISpot assay was used to determine precursor frequencies of HER-2(9689)–specific CTLs in both studies, we believe that technical differences in our and their protocols may account for such quantitative discrepancies. First of all, we must stress the fact that we estimated precursor frequencies from PBMCs and not isolated CD8+ T cells, as they also did. But since it is well established that HER-2(9689) is recognized by MHC class I–restricted CD8+ CTLs [5, 23, 25] (as is the case with peptides consisting of 8–10 amino acids) [5, 6, 16, 37], we can be sure that we measured CD8+ CTLp frequencies. Regarding culture conditions during the incubation period it is essential to note that we used autologous DCs (instead of PBMCs) as peptide-presenting cells in the presence of exogenously added IL-7 and IL-12 (instead of IL-2) both of which are known to support antigen-specific CD8+ T-cell responses [8, 44, 45]. In this way, we may have established a more sensitive culture system for the detection of preexisting CD8+ T cell–mediated responses. The sensitivity of our method may apply to the provision of an optimal culture environment (i.e., besides the exogenously added cytokines, also costimulation plus cytokine production by DCs) for stimulating CTLp with lower avidity T-cell receptors (TCRs) for the bimolecular complex of peptide and HLA-A2.1 and/or for reactivating CTLp being partially tolerated or suppressed during tumor progression as a mechanism of tumor escape from immunosurveillance [30].
HER-2(9952) proved to be the most immunodominant among those tested herein, existing at increased CTLp frequencies in 10 of 32 patients with HER-2/neu + tumors tested. In 4 of these 10 responders the CTLp were high, ranging from 103 to 244/106 PBMCs. In the same patients, preexisting immunity to HER-2(9665) and HER-2(9435) was at lower levels with six and four responders (12.5% and 18.75%), respectively, and with CTLp ranging from 64 to 84/106 PBMCs and from 57 to 90/106 PBMCs. Rongcun et al. [37] were the first to test the immunodominance of these HER-2/neu peptides in vitro. By testing CTL clones developed from patient-derived CTL lines stimulated solely with autologous tumor cells, they found one CTL clone to be specific for HER-2(9369), while two additional CTL clones from the same donor also recognized HER-2(9435) and HER-2(9689) epitopes. The HER-2(9689) epitope was also recently found to be immunodominant in gastric cancer–specific CTLs [23]. In our recent study [5], by stimulating patients' PBMCs with DCs pulsed with total peptide extracts from autologous HER-2/neu + tumor cells we could develop five CTL lines, all of which recognized HER-2(9369) and HER-2(9435), four of five recognised HER-2(9689), whereas HER-2(9665) was recognized by one of them. In all those studies, the CTL induction protocols included repeated (4–5) stimulations over an extended period of time (4–5 weeks), which, however, does not allow us to draw conclusions regarding the immunodominance of these epitopes in the tumor-specific CTL repertoire of patients. Thus, prolonged in vitro restimulations may favor the preferential outgrowth of T-cell clones specific for a given peptide, whose precursors should not necessarily exist at high frequencies within freshly isolated PBMCs or tumor material. For instance, analysis of the TCR usage in tumor-infiltrating lymphocytes (TILs) from six patients with ovarian carcinoma using repeated autologous tumor stimulation showed strong accumulation of TCRBV-2+ and -6+ T-cell clones existing at very low levels in the freshly isolated TILs [34]. Also in TILs from melanoma patient, analyses of the TCRVB-gene usage showed critical differences before and after prolonged in vitro expansion [17]. Therefore it is reasonable to propose that comparison of epitope-specific CTLp frequencies between cancer patients and healthy donors would seem necessary to establish whether peripheral blood or other sources of antitumor CTLs, do indeed contain enhanced levels of tumor antigen–specific CTLp.
MHC class I–binding synthetic peptides derived from the HER-2/neu sequence have been also demonstrated to generate CTLs capable of recognizing autologous tumor cells in breast, ovarian, and gastric cancer patients [5, 16, 23, 37]. In addition, HER-2/neu–specific CTLs have been demonstrated to specifically lyse human colorectal and lung adenocarcinomas [6, 35]. However, in these latter studies there was no information available regarding lysis of the autologous tumors by HER-2/neu–specific CTLs. In the present study, we provide novel information for increased CTLp within the PBMCs specific to HER-2(9435), HER-2(9665), HER-2(9689), and HER-2(9952) in four patients with HER-2/neu + colorectal and four patients with HER-2/neu + lung cancer. PBMCs from these patients containing CTLp at increased frequencies could lyse autologous DCs, pulsed with the relevant HER-2/neu peptide as well as autologous tumor targets, suggesting that these peptides are naturally processed and expressed on the surface of such carcinomas.
In prostate cancer several markers such as prostate-specific antigen, prostatic acid phosphatase, prostate stem cell antigen, and prostate-specific membrane antigen, which are all preferentially expressed by prostatic epithelial cells, have been demonstrated to serve as substrate sources of immunogenic peptide epitopes recognized by CTLs [7, 10, 25, 33]. HER-2/neu has been also identified to be expressed in prostate cancer cells [38], but so far to our knowledge there is no report that supports its recognition by prostate cancer CTLs. Herein, we show that patients with prostate cancer developed increased CTLp for HER-2(9435), HER-2(9665), and also HER-2(9952) and that such peptide-specific CTLs could recognize and lyse the autologous HER-2/neu + tumor cells, suggesting that these epitopes are also naturally processed and expressed on prostate tumor cells. In general, the cytotoxic response against autologous tumor targets, although significantly higher compared with control groups, did not reach high levels even at an E/T ratio of 100. Since such cytotoxic responses were observed within only a short period of stimulation (i.e., 8-day cultures) we believe that prolonged incubation with repeated restimulations will considerably increase the percentage cytotoxicity [4, 5, 6, 37].
By using a computer program that takes into account the presence of main HLA-A2.1–specific anchor residues and specific secondary anchor residues (SYFPEITHI) we could identify sequence p777–785 (i.e., HER-2(9777)) that binds with intermediate affinity to HLA-A2.1. Two patients with breast cancer were responders to this peptide, exhibiting increased CTLp namely 40 and 84/106 PBMC. Most importantly, such PBMCs could efficiently lyse autologous DCs pulsed with HER-2(9777) and also autologous tumor cells, supporting the conclusion that this peptide is also naturally processed and presented in the context of HLA-A2.1. In this way, we have been able to detect an additional MHC class I–restricted HER-2 epitope that may be utilized in peptide-based vaccination protocols. The fact that we could not detect significant differences in the mean CTLp for this particular peptide between HER-2/neu + patients and HER-2/neu - patients or healthy donors, does not exclude the possibility that upon immunization with the same peptide or peptide mixtures also including HER-2(9777), such CTLp will not be increased (as this has already been shown to the case of both HER-2(9369) and HER-2(9689) [15, 20]).
In summary, we have evaluated specific CTLp frequencies to HER-2(9435), HER-2(9665), HER-2(9689), HER-2(9952), and HER-2(9777) in patients with HER-2/neu + tumors. Patients with preexisting immunity to these peptides have been scored in colorectal, lung, and prostate cancer, in addition to breast and ovarian cancer. The fact that increased CTLp to HER-2(9952) could be detected in almost 30% of our patient population points to the potential use of this particular peptide alone or in combination with any of the others in peptide-based vaccinations.
References
Bargmann CI, Hung MC, Weinberg RA (1986) The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature 319:226
Baxevanis CN, Reclos GJ, Papamichail M (1990) Decreased HLA-DR antigen expression on monocytes causes impaired suppressor cell activity in multiple sclerosis. J Immunol 144:4166
Baxevanis CN, Dedoussis GV, Papadopoulos NG, Missitzis I, Stathopoulos GP, Papamichail M (1994) Tumor specific cytolysis by tumor infiltrating lymphocytes in breast cancer. Cancer 74:1275
Baxevanis CN, Voutsas IF, Tsitsilonis OE, Gritzapis AD, Sotiriadou R, Papamichail M (2000) Tumor-specific CD4+ T lymphocytes from cancer patients are required for optimal induction of cytotoxic T cells against the autologous tumor. J Immunol 164:3902
Baxevanis CN, Gritzapis AD, Tsitsilonis OE, Katsoulas HL, Papamichail M (2002) HER-2/neu-derived peptide epitopes are also recognized by cytotoxic CD3(+)CD56(+) (natural killer T) lymphocytes. Int J Cancer 98:864
Brossart P, Stuhler G, Flad T, Stevanovic S, Rammensee HG, Kanz L, Brugger W (1998) Her-2/neu-derived peptides are tumor-associated antigens expressed by human renal cell and colon carcinoma lines and are recognized by in vitro induced specific cytotoxic T lymphocytes. Cancer Res 58:732
Correale P, Walmsley K, Zaremba S, Zhu M, Schlom J, Tsang KY (1998) Generation of human cytolytic T lymphocyte lines against prostate-specific antigen (PSA) employing a PSA oligoepitope peptide. J Immunol 161:3186
Costello R, Imbert J, Olive D (1993) Interleukin-7, a major T-lymphocyte cytokine. Eur Cytokine Netw 4:253
Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, Seeburg PH, Libermann TA, Schlessinger J, Francke U, Levinson A, Ullrich A (1985) Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science 230:1132
Dannull J, Diener PA, Prikler L, Furstenberger G, Cerny T, Schmid U, Ackermann DK, Groettrup M (2000) Prostate stem cell antigen is a promising candidate for immunotherapy of advanced prostate cancer. Cancer Res 60:5522
Disis ML, Calenoff E, McLaughlin G, Murphy AE, Chen W, Groner B, Jeschke M, Lydon N, McGlynn E, Livingston RB, Moe R, Cheever MA (1994) Existent T cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res 54:16
Disis ML, Cheever MA (1997) HER-2/neu protein: a target for antigen-specific immunotherapy of human cancer. Adv Cancer Res 71:343
Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA (1997) High titer HER-2/neu protein specific antibody immunity can be detected in patients with early stage breast cancer. J Clin Oncol 15:3363
Disis ML, Knutson KL, Schiffman K, Rinn K, McNeel DG (2000) Pre-existent immunity to the HER-2/neu oncogenic protein in patients with HER-2/neu overexpressing breast and ovarian cancer. Breast Cancer Res Treat 62:245
Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K (2002) Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol 20:2624
Fisk B, Blevins TL, Wharton JT, Ioannides CG (1995) Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med 181:2109
Halapi E, Jeddi-Tehrani M, Osterborg A, Mellstedt H (1999) T cell receptor usage in malignant diseases. Springer Semin Immunopathol 21:19
Jager E, Jager D, Knuth A (2002) Clinical cancer vaccine trials. Curr Opin Immunol 14:178
Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, Appella E, Rosenberg SA. (1994) Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med 180:347
Knutson KL, Schiffman K, Disis ML (2001) Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest 107:477
Knutson KL, Schiffman K, Cheever MA, Disis ML (2002) Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide, p369–377, results in short-lived peptide-specific immunity. Clin Cancer Res 8:1014
Kono K, Halapi E, Hising C, Petersson M, Gerdin E, Vanky F, Kiessling R (1997) Mechanisms of escape from CD8+ T-cell clones specific for the HER-2/neu proto-oncogene expressed in ovarian carcinomas: related and unrelated to decreased MHC class 1 expression. Int J Cancer 70:112
Kono K, Rongcun Y, Charo J, Ichihara F, Celis E, Sette A, Appellia E, Sekikawa T, Matsumoto Y, Kiessling R (1998) Identification of HER2/neu-derived peptide epitopes recognized by gastric cancer-specific cytotoxic T lymphocytes. Int J Cancer 78:202
Linehan DC, Goedegebuure PS, Peoples GE, Rogers SO, Eberlein TJ (1995) Tumor-specific and HLA-A2-restricted cytolysis by tumor-associated lymphocytes in human metastatic breast cancer. J Immunol 155:4486
Lu J, Celis E (2002) Recognition of prostate tumor cells by cytotoxic T lymphocytes specific for prostate-specific membrane antigen. Cancer Res 62: 5807
Lustgarten J, Theobald M, Labadie C, LaFace D, Peterson P, Disis ML, Cheever MA, Sherman LA (1997) Identification of Her-2/Neu CTL epitopes using double transgenic mice expressing HLA-A2.1 and human CD.8. Hum Immunol 52:109
Maxwell-Armstrong CA, Durrant LG, Scholefield JH (1998) Colorectal cancer vaccines. Br J Surg 85:149
McNeel DG, Nguyen LD, Storer BE, Vessela R, Lange PH, Disis ML (2000) Antibody immunity to prastate cancer-associated antigens can be detected in the serum of patients with prostate cancer. J Urol 164:1825
Nanda NK, Secarz EE (1995) Induction of anti-self-immunity to cure cancer. Cell 82:13
Pawelec G, Zeuthen J, Kiessling R (1997) Escape from host-antitumor immunity. Crit Rev Oncog 8:111
Perez SA, Sotiropoulou PA, Sotiriadou NN, Mamalaki A, Gritzapis AD, Echner H, Voelter V, Pawelec G, Papamichail M, Baxevanis CN (2002) HER-2/neu derived peptide 884–899 is expressed in human breast, colorectal and pancreatic adenocarcinomas and is recognized by in-vitro-induced specific CD4+ T cell clones. Cancer Immunol Immunother 50:615
Perez SA, Sotiropoulou PA, Gkika DG, Mahaira LG, Niarchos DK, Gritzapis AD, Kavalakis YG, Antsaklis AI, Baxevanis CN, Papamichail M (2003) A novel myeloid-like NK cell progenitor in human umbilical cord blood. Blood (in press)
Peshwa MV, Shi JD, Ruegg C, Laus R, van Schooten WC (1998) Induction of prostate tumor-specific CD8+ cytotoxic T-lymphocytes in vitro using antigen-presenting cells pulsed with prostatic acid phosphatase peptide. Prostate 36:129
Peoples GE, Davey MP, Goedegebuure PS, Schoof DD, Eberlein TJ (1993) HLA-A2 presents shared tumor-associated antigens derived from endogenous proteins in ovarian cancer. J Immunol 151:5481
Peoples GE, Smith RC, Linehan DC, Yoshino I, Goedegebuure PS, Eberlein TJ (1995) Shared T cell epitopes in epithelial tumors. Cell Immunol 164:279
Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S (1999) SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50:213. SYFPEITHI database. http://www.uni-tuebingen.de/uni/kxi/database.html. Cited 29 Aug 2003
Rongcun Y, Salazar-Onfray F, Charo J, Malmberg KJ, Evrin K, Maes H, Kono K, Hising C, Petersson M, Larsson O, Lan L, Appella E, Sette A, Celis E, Kiessling R (1999) Identification of new HER2/neu-derived peptide epitopes that can elicit specific CTL against autologous and allogeneic carcinomas and melanomas. J Immunol 163:1037
Scher HI (2000) HER2 in prostate cancer-a viable target or innocent bystander? J Natl Cancer Inst 92:1866
Seliger B, Rongcun Y, Atkins D, Hammers S, Huber C, Storkel S, Kiessling R (2000) HER-2/neu is expressed in human renal cell carcinoma at heterogeneous levels independently of tumor grading and staging and can be recognized by HLA-A2.1-restricted cytotoxic T lymphocytes. Int J Cancer 87:349
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science (Wash.DC) 235:177
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783
Sercarz EE (2000) Driver clones and determinant spreading. J Autoimmun 14:275
Sotiriadou R, Perez SA, Gritzapis AD, Sotiropoulou PA, Echner H, Heinzel S, Mamalaki A, Pawelec G, Voelter W, Baxevanis CN, Papamichail M (2001) Peptide HER2(776–788) represents a naturally processed broad MHC class II-restricted T cell epitope. Br J Cancer 85:1527
Trinchieri G (1995) Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adoptive immunity. Annu Rev Immunol 13:251
Trinchieri G (1997) Cytokines acting on or secreted by macrophages during intracellular infection. Curr Opin Immunol 9:17
Wang RF, Rosenberg SA (1999) Human tumor antigens for cancer vaccine development. Immunol Rev 170:85
Ward RL, Hawkins N, Coomber D, Disis ML (1999) Antibody immunity to the HER-2/neu oncogenic protein in patients with colorectal cancer. Human Immunology 60:510
Yamanaka Y, Friess H, Kobrin MS, Buchler M, Kunz J, Beger HG, Korc M (1993) Overexpression of HER2/neu oncogene in human pancreatic carcinoma. Hum Pathol 24:1127
Yoshino I, Goedegebuure PS, Peoples GE, Parikh AS, DiMaio JM, Lyerly HK, Gazdar AF, Eberlin TJ (1994) HER2/neu-derived peptides are shared antigens among human non-small lung cancer and ovarian cancer. Cancer Res 54:3387
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sotiropoulou, P.A., Perez, S.A., Voelter, V. et al. Natural CD8+ T-cell responses against MHC class I epitopes of the HER-2/neu oncoprotein in patients with epithelial tumors. Cancer Immunol Immunother 52, 771–779 (2003). https://doi.org/10.1007/s00262-003-0420-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-003-0420-9