Abstract
To examine the basis of the immune modulation induced by the anticancer agent doxorubicin (DOX), the immunophenotype, tumoricidal activity, cytokine protein and mRNA expression were determined using peritoneal exudate cells (PEC) from saline-treated (untreated) and DOX-treated mice. A greater percentage of PEC from DOX-treated mice than from untreated mice were adherent to plastic, had characteristics of granulocytes, and were positive for the NK1.1, CD11b/Mac-1, and CD3 markers. DOX decreased the percentage of CD45R/B220+ cells. PEC from DOX-treated mice had greater tumoricidal potential than those from untreated mice since IL2, LPS, or IFNγ alone increased the cytolytic activity of PEC from DOX-treated mice, whereas PEC from untreated mice required both LPS and IFNγ to become cytolytic. DOX treatment modulated the expression of specific cytokines. Following stimulation in culture, PEC from DOX-treated mice produced more TNF, IL1, and IFNγ than PEC from untreated mice. DOX treatment increased the levels of TNF, but not IL1, mRNA and decreased the levels of IL6 mRNA and protein. These data demonstrate that a single DOX injection induces specific effects in PEC and, as a consequence, increases the tumoricidal potential of cells of the macrophage and natural killer types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The chemotherapeutic drug doxorubicin (DOX) can be immunomodulatory and stimulate several types of cells that have tumoricidal activity [3, 17, 34, 37, 38]. Combination therapies of moderate doses of DOX plus cytokines such as IL2 or tumor necrosis factor α (TNF) can cure mice with established tumors [10, 12, 18, 22, 25, 40] and the therapeutic effect is dependent upon treatment-induced immunomodulation [26]. The cured mice have life-long immunity to rechallenge with that tumor [13, 18, 22, 26]. Experiments examining the efficacy of these combination therapies in mice that had been sub-lethally irradiated led to the hypothesis that DOX in the combination therapy acts both on the tumor to induce cell lysis which provides a burst of tumor associated antigens and on the immune system to increase antitumor effector activity and allow the host to overcome tumor-related anergy [14].
The mechanisms by which DOX directly induces tumor cell lysis have been studied extensively [16]. However, even though there is convincing evidence that the DOX-induced modulation of host antitumor defenses is necessary for the efficacy of the combination therapies described above, little is known about the mechanisms involved. Based on the known essential regulatory roles of cytokines in all steps of the immune response [29], it was hypothesized that DOX might be acting by inducing changes in cytokine expression.
The studies described here were designed to determine whether the mechanisms of DOX-induced immune modulations involved changes in cytokines by comparing the cytokine mRNA and protein levels with immunophenotypes and effector functions of cells from untreated mice to those from DOX-treated mice. The model chosen was to examine PEC following a single i.p. DOX (10 mg/kg) injection since we had previously shown [36, 37] using this system that increased biologically active TNF and IL1 could be detected in the conditioned media from PEC of DOX-treated mice.
Materials and methods
Animals and cell lines
Female C57BL/6Cr mice were obtained through the National Cancer Institute Animal Program (Frederick, Md.). Medium for all single-cell isolates and cell lines was supplemented RPMI-1640 complete culture medium [33]. All cell incubations were performed in a humidified atmosphere at 37°C, 5% CO2. The cultured cell lines used were Mycoplasma-negative (based on testing with products of Gen-Probe Inc, San Diego, Calif.) and included: WEHI-164 fibrosarcoma clone 13 variant, which is sensitive to lysis by TNF, YAC-1 used to assess NK activity, and P815 mastocytoma used to examine both macrophage tumoricidal activity and the specificity of NK activity
DOX treatment and isolation of PEC
Fifty mice were injected i.p. on day 0 of each experiment with 10 mg/kg DOX (a generous gift from Adria Labs, Columbus, Ohio). One hundred and twenty-five control animals (untreated) were injected i.p. with an equivalent volume of saline. Treated and control mice were matched by age. On day 7, mice were sacrificed by cervical dislocation and PEC were harvested in aseptic conditions by peritoneal lavage with 7 ml cold Hanks buffer [35]. Cells were washed once with Hanks buffer, counted, diluted, and used for further experimentation.
Generation of PEC populations by adherence culture
Following plating (six-well plates, 3.125×106 cells/well, 2 ml/well) and a 2-h incubation, some of the PEC were separated into cell populations adherent (Ad) and nonadherent (NAd) to a plastic dish [4]. Some of the PEC were left unseparated after the 2-h incubation and were considered the whole cell population (W); this was to insure that the W population was treated similarly to the Ad and NAd populations.
Immunofluorescence
Aliquots of 106 cells/sample were incubated on ice for 20 min with anti-CD3-FITC, anti-NK1.1-PE (PharMingen, San Diego, Calif.), anti-Mac-1(CD11b)-PE, and anti-Ly5(CD45R/B220)-TC (Caltag, South San Francisco, Calif.). Anti-CD32/CD16 (Fc Block, PharMingen) was used to block non-specific Fc receptor binding at 15 μl/sample and respective isotype control antibodies were used to establish background fluorescence. After labeling, samples were washed twice in PAB [cold PBS supplemented with 0.1% sodium azide (Sigma, St. Louis, Mo.) and 0.5% bovine serum albumin (Boehringer Mannheim, Indianapolis, Ind.)], and resuspended in 2% ultrapure formaldehyde (Polysciences, Inc, Warrington, Pa.) in PAB. Samples were examined on a FACScan (Becton/Dickinson, San Jose, Calif.) flow cytometer and analyzed with LYSYS and WinList software [46, 47, 48].
Morphology
Cytospin preparations of PEC were stained with Wright stain.The number of granulocytes was determined based on characteristic morphology and staining when viewed using light microscopy. Four hundred cells were examined for each sample.
Cytotoxicity tests
The 51Cr release assay was used to assess the cytolytic activity of PEC. Several phenotypes of cells with cytolytic activity were examined based on differential responses to stimulation and/or target cell sensitivity.
NK activity was assessed [37] by contrasting the ability of cells within the NAd PEC populations to lyse the NK target YAC-1 with their ability to lyse the NK-insensitive target P815 in a 4-h assay. Following an 18-h incubation of NAd PEC (1.5×105 cells/well) with IL2 (1,000 U/ml) or without addition of cytokine, the NAd PEC were either undisturbed (NAd-W) or were separated into nonadherent cells (NAd-NAd, transferred to new plates) and adherent cells (NAd-Ad, remained on the plate). 51Cr-radiolabeled target cells (YAC-1 or P815, 5×103 cells/well) were added to these effector populations for 4 h and cytotoxicity was evaluated by the amount of radioactivity released into the supernatant.
Tumoricidal macrophage activity was assessed [27, 37] by the cytolytic activity of PEC subpopulations against P815 target cells that are sensitive to lysis by tumoricidal macrophages. Where noted, stimulants [lipopolysaccharide (LPS)derived from Escherichia coli strain 0111:B4 (Difco Laboratories, Detroit, Mich.), IL2, interferon γ (IFNγ), or combinations thereof] were added for 18 h at the following concentrations: IL2 at 1,000 U/ml, LPS at 0.01 μg/ml and IFNγ at 4 U/ml. In the 18-h assays, effector cells were incubated with targets and stimulants simultaneously for 18 h. In the 36-h assay, PEC were incubated with stimulants for 18 h, and the target cells were added for an additional 18 h. The 51Cr released into the supernatant by cell lysis was determined by γ counting.
Measurement of cytokine mRNA and cytokine protein
Reverse transcription/polymerase chain reaction (RT/PCR) assays were chosen for quantification of selected cytokine mRNA levels because these assays require less RNA than other methods (e.g., northern blot, ribonuclease protection). It was known both that (a) PEC contain little RNA compared with that present in cultured cell lines [5] and (b) cytokine mRNAs are relatively nonabundant [2, 9]. Cytokine protein levels were determined using both bioactivity assays, which measure only active (not inactive) cytokine protein, and ELISAs, which measure total antibody-reactive protein. This distinction is especially important for proteins such as IL1β, which require post-translational processing for activity; antibody-reactive IL1β, such as would be detected by ELISA, is not necessarily active protein. For each bioactivity assay, control assays demonstrated that addition of an antiserum to the cytokine of interest ablated the activity.
Quantification of cytokine mRNA by RT/PCR
RNA was extracted from W, Ad, and NAd PEC following the 2-h initial incubation and after the further 24-h incubation in the absence or presence of 0.01, 1.0, or 100 μg/ml LPS; the level of each specific cytokine mRNA was determined by quantitative RT/PCR developed specifically for this purpose [4]. Briefly, a known amount of a standard RNA was combined with cellular (e.g., PEC) RNA and subjected to RT/PCR. The standard RNA for each cytokine had been engineered to contain a single point mutation that produces a unique SmaI restriction enzyme site utilized to identify the product produced from the standard RNA. Since the standard RNA competes with the cellular mRNA of interest for the reagents of the RT/PCR reaction, the relative proportion of the products arising from the cellular RNA to those arising from the known amount of standard mRNA is used to quantify the amount of cellular cytokine mRNA present [4]. Any influence of differences in RNA recovery between samples was eliminated by normalizing to the yield of a parallel sample "spiked" with 3H-RNA [5].
Cytokine protein assays
Conditioned media (CM) samples were collected from the incubations of W, Ad, and NAd PEC after the further 24-h incubation in the absence or presence of 0.01, 1.0, or 100 μg/ml LPS. Cytokine protein levels were determined using assays based on activity and/or on antibody reactivity (i.e. ELISA).
TNF bioactivity
The amount of bioactive TNF in CM was quantitated based on TNF-induced lysis of WEHI 164 (clone 13) cells as determined by staining the viable (i.e., those with active mitochondria) cells with MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] [43]. WEHI 164 cells (5×103) were aliquoted into each well of a 96-well plate, and several dilutions of either known amounts of recombinant TNF, to be used as a standard, or of the sample to be tested for TNF activity were added and incubated (for a total of 72 h, 200 μl final volume). Cell lysis was assessed by staining with MTT for the last 5 h and the percentage of specific lysis was calculated. The amount of TNF in the unknown sample was determined by comparison to the standard TNF curve. Parallel experiments in which cell number was assessed via uptake of 3H-thymidine by viable cells [15, 44], instead of by mitochondrial dehydrogenase conversion of MTT to the colored formazan product, gave similar results. Murine recombinant TNF, used as a standard, and neutralizing monoclonal anti-murine TNF antibody, used to verify that the activity resulted from TNF, were generously provided by Asahi Chemical Industry Co. (Tokyo, Japan).
IL1
Bioactive IL1 in CM samples was measured based on the stimulation of proliferation of murine thymocytes in a standard co-mitogenic assay [15, 24, 37]. C57BL/6 mouse thymocytes (7.5×103/well, 96 well plate) were cultured for 72 h with phytohaemagglutinin (0.8 μg/ml, Wellcome Diagnostics, Dartford, UK) and either known amounts of recombinant IL1β to be used as a standard or multiple dilutions of the sample to be tested for IL1 activity (the final volume was kept constant at 200 μl). The cultured thymocytes were pulsed with 3H-thymidine (1 μCi/well, 6.7 Ci/mmol, New England Nuclear) for the last 6 h of the 72-h incubation period and harvested on to glass fiber filters (Otto Hiller cell harvester). The cell-associated radioactivity was measured by scintillation counting (Beckman Model LS 1801). The determination of the amount of active IL1 in the CM samples was by comparison of their stimulation of thymocyte proliferation with that induced by known amounts of IL1. Recombinant murine IL1β, used as a standard, and neutralizing monoclonal anti-murine IL1β antibody, used to verify that the activity resulted from IL1β, were gifts from Dr. J.J. Huang [28].
Cytokine ELISA assays
Sandwich ELISA assays were used to detect IL1, TNF, IL6, and IFNγ. Capture and detection monoclonal antibodies were purchased from PharMingen (San Diego, Calif.) and assays were according to the protocol provided by the manufacturer. Briefly, the plates were incubated overnight with the rat anti-mouse capture (primary) antibody, washed, blocked and CM samples were added and incubated overnight prior to washing. A biotinylated rat anti-mouse detection (secondary) antibody was then added, washed, avidin-peroxidase was added, washed and substrate (ABTS/H2O2) was added. The intensity of the color was indicative of the amount of cytokine present in the sample and was quantified by comparison with a standard curve containing known amounts of the cytokine examined by ELISA in parallel.
Results
Multiple characteristics of PEC were examined, including: number, morphology, phenotype, cytokine mRNA levels, cytokine protein levels, and tumoricidal activity. In each instance, aliquots of a pool of PEC harvested from mice 7 days following 10 mg/kg i.p. DOX treatment were compared with aliquots of a pool of PEC from saline-treated (untreated) mice to examine changes induced by DOX. Three independent experiments (i.e. harvests of PEC from untreated and DOX-treated mice) were carried out.
Cell number, morphology, and immunophenotype
When considered on a per mouse basis, twice as many PEC were recovered following DOX treatment than were recovered from untreated mice (7.8±1.2×106 compared to 3.8±0.9×106, p=0.003 by Student's t test). Aliquots of PEC were separated into Ad and NAd cells following a 2-h incubation on tissue culture plates. A greater percentage of PEC from DOX-treated mice than from untreated mice were Ad after 2 h (66.6%±5.3% and 53.7%±7.9%, respectively, p=0.002 by Student's t test). The Ad PEC from DOX-treated mice appeared to be larger and more "spread out" than those from untreated mice (Fig. 1).
Ad PEC from DOX-treated mice have different morphology than those from untreated mice. PEC were harvested from untreated mice and from mice 7 days after injection with 10 mg/kg DOX i.p. PEC were plated and incubated. Some of the PEC were separated into Ad and NAd subpopulations: vigorous pipetting was used to remove the NAd cells and cells which remained after washing in warm media were considered Ad. This Ad subpopulation was photographed. For this figure only, incubation prior to separation was 24 h. For all other experiments, incubation was for 2 h. a Ad PEC from untreated mice. b Ad PEC from DOX-treated mice
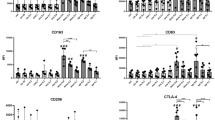
Further evaluation of the size and granularity of the PEC subpopulations was performed by flow cytometric analysis of forward scatter (FSC) and side scatter (SSC) parameters. Incubation on plastic for 2 h did not change the PEC appreciably when the FSC/SSC pattern of the W PEC, from either untreated or DOX treated mice, was compared with the respective PEC population analyzed directly following harvest from the mice (data not shown). DOX treatment resulted in a whole cell population that was enriched in cells with a high degree of SSC compared with the population from untreated mice (Fig. 2a). The FSC/SSC pattern data were analyzed by gating on four subpopulations (Fig. 2a): gate 1 contained predominantly small lymphocytes, gate 2 contained predominantly large lymphocytes and some macrophages, gate 3 contained predominantly macrophages and some large lymphocytes and gate 4 contained predominantly granulocytes. Based on these assignments, DOX-treatment increased the percent of the cells in gate 4 sevenfold compared with W PEC from untreated mice (Fig. 2b). There was a concomitant decrease in the percent of the cells in gates 1 and 2 after DOX treatment; the percent of the cells in gate 3 was unchanged. Similar DOX-induced changes were apparent when the Ad and the NAd subpopulations were examined (data not shown). Granulocytes were assessed in Wright's stained cytospin preparations. PEC from untreated mice contained 1% granulocytes whereas PEC from DOX-treated mice contained 4% granulocytes. When these percentages are multiplied by the respective PEC yields per mouse, DOX treatment increased the average number of granulocytes in PEC over eightfold, from 38,000/mouse to 312,000/mouse. Increased numbers of granulocytes were also detected by this method in both the Ad and NAd subpopulations following DOX treatment (data not shown). The immunophenotype of the PEC subpopulations was determined by flow cytometric analysis following incubation with fluorescently labelled antibodies specific for CD3, NK1.1, CD11b/Mac-1, and CD45R/B220 (Table 1). DOX treatment increased the percentage of CD3+ cells, NK1.1+ cells, and CD11b+ cells in the W, Ad, and NAd populations. DOX treatment decreased the percentage of CD45R+ cells in the W, Ad and NAd populations.
PEC from DOX-treated mice have a different distribution of cells than do PEC from untreated mice when evaluated flow cytometrically by forward (FSC) and side scatter (SSC) parameters. a Following 2 h on plastic, W PEC from both untreated and DOX-treated mice were recovered and examined for FSC/SSC properties on a flow cytometer. Ten thousand events were analyzed. Similar data were obtained in two additional independent experiments. The numbers indicate the established gates: gate 1 contained predominantly small lymphocytes, gate 2 contained predominantly large lymphocytes and some macrophages, gate 3 contained predominantly macrophages and some large lymphocytes and gate 4 contained predominantly granulocytes. b Quantification of the percentage of the 10,000 events collected that fall into each of the four gates. The averages of the results for each of three independent PEC harvests ± the standard deviations are shown. Differences that are statistically significant as judged by a two-tailed Student's t test are marked with an asterisk. *P<0.05, **P<0.01.
Tumoricidal activity
The tumoricidal (activated macrophage) potential of the PEC populations was determined by 51Cr release assays with P815 cells as targets and IL2, IFNγ, and/or LPS as stimulants (Fig. 3). When stimulated with a single agent, PEC from DOX-treated mice had higher levels of cytotoxic activity against P815 cells in an 18-h assay than did PEC from untreated mice (Fig. 3a). In both the 18-h and 36-h assays, the maximal cytolytic activity (attained following stimulation with both IFNγ and LPS) of PEC from DOX-treated mice was similar to that obtained with PEC from untreated mice (Fig. 3). However, PEC from DOX-treated mice displayed maximal cytolytic activity against P815 when stimulated with IL2 or LPS; incubation of PEC from untreated mice with these stimulants did not result in maximal cytolytic activity (Fig. 3b). Similar differences between the tumoricidal activity of cells from DOX-treated mice and cells from untreated mice were evident when the Ad populations were compared with each other as well as when the NAd populations were compared (data not shown).
PEC from DOX-treated mice have greater cytolytic activity against 51Cr-labeled P815 mastocytoma than do PEC from untreated mice. a 18-h cytolytic assay. W PEC (1.5x105/well) from untreated and DOX-treated mice were incubated with 51Cr-labeled P815 cells (5x103/well) for 18 h in the absence or presence of the stimulants noted. Stimulants were used at the following concentrations: IL2 at 1,000 U/ml, LPS at 0.01 μg/ml, and IFNγ at 4 U/ml. The 51Cr released into the supernatant upon cell lysis was determined by γ counting. b 36-h cytolytic assay. Conditions were as described in a except that the W PEC were incubated without (control) or with the listed stimulants for 18 h prior to the addition of the 51Cr-labeled P815 cells for an additional 18 h. For both a and b, the average ± the standard deviation (n=4) is shown. Similar data were obtained in two additional independent experiments
The cytolytic (NK) potential of the NAd PEC was also determined after an 18-h in vitro incubation with IL2 followed by 4-h 51Cr release assays using YAC-1 and P815 cells as targets (Fig. 4). For this experiment, NAd PEC were incubated with IL2 and a portion of those cells was separated further into NAd and Ad subpopulations at the end of the 18-h incubation with IL2 (denoted as NAd-NAd and NAd-Ad, respectively). Both the total population (NAd-W) and the NAd-NAd subpopulation of PEC from DOX-treated mice generated higher levels of YAC-1 lysis than did the corresponding cells from untreated mice (Fig. 4). Aliquots of these IL2-treated cells had no appreciable level of cytolytic activity against P815 (Fig. 4) and, without IL2 treatment, the cells had no cytolytic activity against either YAC-1 or P815 (data not shown).
Subpopulations of NAd PEC from DOX-treated mice have greater cytolytic activity against 51Cr-labeled YAC-1 than do PEC from untreated mice. NAd PEC (1.5x105 cells/well) were incubated with 1,000 U/ml IL2 for 18 h. Cells were undisturbed (NAd-W) or separated into Ad and NAd subpopulations (NAd-Ad and NAd-NAd, respectively). YAC-1 (left panel) or P815 cells (right panel) that had been prelabeled with 51Cr were added (5x103 cells/well) for 4 h and cytotoxicity was measured by the release of 51Cr into the supernatant determined by γ counting. The average ± the standard deviation (n=4) is shown
Cytokine mRNA and protein levels
To examine the levels of selected cytokine mRNAs, total RNA was purified from the W PEC and the separated NAd and Ad populations following a 2-h adherence to plastic incubation and from each of these populations following a further 24-h incubation. To examine levels of cytokine produced by the respective cell populations, the conditioned medium (CM) was removed from the cells following the 24-h incubation. Total RNA was then prepared from the cells. During the 24-h incubation, cells were either untreated or treated with 0.01, 1.0, or 100 μg/ml LPS. The levels of mRNA for selected cytokines were determined by quantitative RT/PCR and the levels of protein for selected cytokines were determined by bioassays and ELISAs.
When RNAs obtained from W PEC immediately after the 2-h adherence step were analyzed, TNF mRNA levels were ~7.5-fold higher in PEC from DOX-treated mice than they were in the corresponding populations from untreated mice (Fig. 5, two separate experiments are shown). DOX also increased TNF mRNA in the Ad PEC subpopulation (Fig. 5). After the 24-h culture, the TNF mRNA level in the PEC from DOX-treated mice was similar to the level at 2 h and was still greater than that in PEC from untreated mice (compare W PEC of Fig. 5 with −LPS of Fig. 6). LPS, at the concentrations tested, induced even higher levels of TNF mRNA in PEC from DOX-treated mice but had no effect on the levels of TNF mRNA in PEC from untreated mice (Fig. 6).
PEC from DOX-treated mice have higher TNF mRNA than do PEC from untreated mice. An RT/PCR assay was used to quantify the TNF mRNA present in total RNA of W and Ad PEC. The results of two independent experiments are shown; similar data were obtained in a third independent experiment. The differences between the respective −DOX/+DOX pairs are statistically significant based on the Student's t test (P<0.05)
LPS treatment increases the TNF mRNA levels of PEC from DOX-treated mice but not PEC from untreated mice. An RT/PCR assay was used to quantify the TNF mRNA present in total RNA of W PEC from untreated and DOX-treated mice following a 2-h adherence to plastic and a subsequent 24-h incubation in the absence or presence of increasing concentrations of LPS as indicated. The total RNA assayed was a pool from three independent experiments. The data shown are the results of a series of RT/PCR assays. Similar results were obtained upon repetition of the quantitative RT/PCRs. As previously published [4], the typical range of the standard deviation of this TNF RT/PCR assay for W PEC RNA is ~0.5-7.2 molecules/cell
The levels of bioactive TNF in CM from PEC are shown in Fig. 7. Specifically, as shown by the open bars (inset), (1) the levels of TNF in CM from cells which had not been incubated with LPS were low, (2) CM from PEC of DOX-treated animals had even lower levels of TNF than were found in the CM from PEC of untreated mice, and (3) incubation with LPS, as shown by the filled bars, dramatically increased the TNF level in CM from PEC of DOX-treated mice, but incubation with LPS did not increase the TNF level in CM from PEC of untreated mice. Similar results were obtained when the level of TNF protein in the CM was examined by ELISA (Table 2).
PEC from DOX-treated mice produce more TNF than do PEC from untreated mice. W, Ad, and NAd PEC from untreated and DOX-treated (10 mg/kg, i.p., 7 days prior) mice were incubated for 24 h in the absence or presence of increasing concentrations of LPS as indicated. The conditioned medium (CM) was removed from the cells and the amount of bioactive TNF in the CM was determined based on cytotoxicity towards WEHI-164, clone 13 cells. The inset is a magnified view of the data obtained without LPS treatment (note that the scale on the ordinate is pg/ml for the inset). Similar data were obtained in two additional independent experiments. The standard deviation of the assay is ~ 10–15%
The levels of IL1α and IL1β mRNAs were determined in RNA purified from W, Ad, and NAd PEC after the 2-h adherence culture. In three separate experiments, there was little or no difference between the IL1β mRNA levels determined for W PEC from DOX-treated mice and untreated mice; the level ranged from 7,500 to 12,000 molecules of IL1β mRNA/cell. The levels also did not differ significantly between the PEC of DOX-treated and untreated animals for the Ad and NAd subpopulations (data not shown). For both PEC from untreated and PEC from DOX-treated mice, a 24-h stimulation with LPS resulted in an increased level of IL1β mRNA (Fig. 8). The level of IL1β mRNA in PEC from DOX-treated animals was lower in all cases than that in the corresponding sample from untreated animals. IL1α mRNA levels, which were ~50-fold less than IL1β mRNA levels, displayed changes similar to those of IL1β mRNA (data not shown).
LPS treatment increases the IL1β mRNA levels in PEC from both DOX-treated and untreated mice. An RT/PCR assay was used to quantify the IL1β mRNA present in total RNA of W PEC from untreated and DOX-treated mice following a 2-h adherence to plastic and a subsequent 24-h incubation in the absence or presence of increasing concentrations of LPS as indicated. The total RNA assayed was a pool from three independent experiments. The data shown are the results of a series of RT/PCR assays. Similar results were obtained upon repetition of the quantitative RT/PCRs
The levels of bioactive IL1 in CM from cells that were not treated with LPS were low in all PEC populations (Fig. 9, inset); the levels of IL1 detected in CM of PEC populations from DOX-treated mice were consistently found to be somewhat lower than those in CM of PEC from untreated mice. However, with LPS stimulation, considerable amounts of IL1 were present in the CM of W, Ad, and NAd PEC from DOX-treated mice (levels from 200 to 900 pg/ml, Fig. 9). CM of PEC from untreated mice, even when the PEC were incubated with LPS, did not contain appreciable IL1 (maximal levels were ≤22 pg/ml). Addition of a neutralizing anti-IL1β antibody to the bioassay of the CM ablated the IL1 activity measured (data not shown). Results estimating the levels of IL1 protein in the CM by ELISA (Table 2) indicated changes that were consistent with those observed using the bioactivity assay.
PEC from DOX-treated mice produce more IL1 than do PEC from untreated mice. W, Ad, and NAd PEC from untreated and DOX-treated mice were incubated for 24 h in the absence or presence of increasing concentrations of LPS as indicated. The conditioned media (CM) was removed from the cells and the amount of bioactive IL1 in the CM was determined based on stimulation of thymocyte proliferation. The inset is a magnified view of the data obtained without LPS treatment. Similar data were obtained in two additional independent experiments. The standard deviation of the assay is ~ 5–10%
Quantitative RT/PCR assays demonstrated that the level of IL6 mRNA was lower in W PEC of DOX-treated mice than in PEC of untreated mice: an average of four separate RT/PCR titrations ± the standard deviation of 558±269 IL6 mRNA molecules/cell for PEC from DOX-treated mice and 5,338±536 IL6 mRNA molecules/cell for PEC from untreated mice. Measurements of IL6 protein levels by ELISA also indicated that the level was lower in CM from PEC of DOX-treated mice than in CM from PEC of untreated mice (Table 2). A higher level of IFNγ protein was found in CM from PEC of DOX-treated mice than in CM from PEC of untreated mice (Table 2).
Discussion
The data reported herein provide a greater understanding of the DOX-induced effects on host defenses by quantitating specific changes in PEC population characteristics resulting from a single injection of DOX. These changes concern: (1) cell number, morphology, and immunophenotype, (2) tumoricidal activity, and (3) cytokine mRNA and protein expression.
Following a single i.p. DOX injection, twice as many PECs were present. This is consistent with the demonstration that i.p. DOX increases the number of peritoneal mononuclear cells [20, 37]. Whether DOX induces proliferation in situ or recruitment of cells from other sites has not been determined. In contrast, it has also been reported that PEC yields in rats are lower after i.p. DOX treatment [42], but this was observed at 24 h, not at 7 days post injection.
Several pieces of evidence indicate that the PEC population from DOX-treated animals is enriched in cells whose characteristics are consistent with their having arisen from the myelomonocytic stem cell lineage and that these cells are primed. First, a greater percentage of PEC from mice treated with DOX are adherent after a 2-h incubation on plastic. Second, flow cytometry indicates that these PEC contain more cells with a high degree of side scatter; this characteristic is indicative of cells with a high degree of granularity (Fig. 2). Third, Ad cells from DOX-treated mice are spread out with the morphological appearance of primed macrophages (Fig. 1). Fourth, flow cytometric analysis demonstrates that a greater percentage of the PEC from DOX-treated mice bind the CD11b antibody; CD11b is a marker of cells of the myelomonocytic lineage (Table 1). Fifth, PEC from DOX-treated mice have greater numbers of granulocytes as judged by Wright's staining.
In contrast to the increase in CD11b+ PEC, the single i.p. DOX injection decreased the percentage of the total cell population that bound the antibody to the B-cell marker CD45R/B220 (Table 1). However, it should be noted that, since DOX treatment increased the number of PEC/mouse by ~twofold, there was no decrease in the total number of CD45R+ cells following DOX treatment. In fact, the actual number of CD45R+ cells per mouse increased slightly. PEC from DOX-treated mice contained more cells that were CD3+ (Table 1). This indicates that T cells were also increased by DOX.
DOX treatment increased the percentage of PEC that were positive for the expression of NK1.1, a marker of NK cells. There were NK1.1+ PEC whose FSC and SSC characteristics were not those of classical small lymphocytes. These data, together with the large increases in the percentage of granulocytic cells, support the hypothesis that the large granulocytic lymphocyte (LGL) type of NK cell [21, 39] was increased in PEC from DOX-treated mice. These data are consistent with the published data indicating that NK activity in PEC is augmented following DOX administration [37].
DOX treatment stimulated tumoricidal macrophage activity in the W PEC (Fig. 3). Following stimulation in culture with IL2, LPS, or IFNγ alone, PEC from DOX-treated C57BL/6 mice generally have increased cytolytic activity against P815 cells compared with PEC from untreated mice. P815 mastocytoma cells are targets for tumoricidal macrophages but not NK cells. Following stimulation with LPS plus IFNγ, identical numbers of PEC from untreated and DOX-treated mice attain a similar level of maximal tumoricidal macrophage activity (although the increased numbers of PEC in the DOX-treated mice suggest that the total activity in the treated animals is greater than that in untreated animals). The ability of the pretumoricidal cells in the PEC from DOX-treated animals to become fully tumoricidal in response to a single stimulus suggests that they are "primed." Based on the known ability of IFNγ to activate ("prime") macrophages, this may be the consequence of response to IFNγ since, without ex vivo stimulation, its levels were increased in CM from PEC of DOX-treated mice (Table 2). Activated cells within either the CD3+ or the NK1.1+ subpopulation may have contributed to the increased IFNγ production.
DOX treatment also stimulated NK tumoricidal activity in the NAd PEC (Fig. 4). When the NAd PEC population was incubated in vitro with IL2, the NAd-PEC from DOX-treated mice possessed greater lytic activity against YAC-1, the NK1.1 target, than PEC from untreated mice. This lytic activity has the characteristics of activated NK cells, rather than of LAK cells, since there was no lysis of P815 cells. P815 are insensitive to lysis by NK cells but sensitive to lysis by LAK cells. Furthermore, in vivo DOX treatment (8 mg/kg i.p.) of C57BL/6 mice has been reported to decrease in vitro LAK activity for 6–9 days post injection [23].
Specific regulation of cytokine mRNA and protein expression was measured following a single i.p. DOX injection. Because of the low level of RNA in PEC compared with the level in cultured cells [5], and the relative nonabundance of cytokine transcripts, cytokine mRNA levels were quantified by the sensitive RT/PCR method developed for that purpose [4]. The effects of DOX treatment documented in this study were not due to nonspecific stimulation of expression of all cytokines since DOX treatment decreased the levels of IL6 mRNA and protein (data in text and Table 2).
Published studies suggest that administration of DOX can lead to increased TNF protein [37, 41], but they do not address whether this occurs via an increase in TNF mRNA. Data contained herein demonstrate that, after a single i.p. DOX injection, PEC contained ~7.5-fold more TNF mRNA than was present in PEC from untreated mice (Fig. 5). However, there was a slight decrease in the TNF protein produced by PEC from DOX-treated mice compared with that produced by PEC from untreated mice (Fig. 7). This suggests that PEC from DOX-treated mice, although "primed" to make TNF, were not constitutively making detectable levels of TNF. Following in vitro LPS stimulation, the elevated levels of TNF mRNA present in the PEC from DOX-treated mice were increased even further (Fig. 6), and this increase was mirrored by a very large increase in the levels of TNF protein produced by these cells (Table 2, Fig. 7). In contrast, the PEC from untreated mice were not stimulated by the concentrations of LPS used and the levels of both TNF mRNA and protein remained unchanged. This suggests that the "primed" PEC from DOX-treated mice, upon receiving an appropriate second signal, become fully activated and make TNF. Furthermore, these data are consistent with DOX-induced changes in TNF production occurring at the level of TNF mRNA.
The following data suggest that a DOX-induced TNF increase may have therapeutic relevance: (1) when TNF plus DOX were used in limb perfusion of sarcoma-bearing rats, the inclusion of TNF led to increased accumulation of DOX in tumor tissue [45], (2) treatment of human bone marrow cells in vitro with TNF (or IL1β) protects them from DOX toxicity [11] and (3) two patients who experienced prolonged objective response after treatment with DOX plus IL2 had unusually elevated TNF levels [31].
There was essentially no difference between PEC from mice with and mice without DOX treatment in terms of the IL1α and IL1β PEC mRNA levels or the IL1 protein production (Table 2, Fig. 9). Stimulation in vitro with LPS, however, dramatically increased the level of IL1 protein produced by PEC from DOX-treated mice but not from untreated mice (Table 2, Fig. 9). This is consistent with previously published data indicating that IL1 protein increases following both in vitro and in vivo DOX treatment ([1, 6, 7, 8, 30, 37) although the in vivo effects of DOX are not always evident when studying the effect of DOX applied to a cell population in culture [41]). Inclusion of a neutralizing antibody against IL1β ablated the IL1 bioactivity, indicating that the IL1 protein produced was predominantly IL1β and not IL1α (data not shown). Interestingly, the administration of IL1 protects mice from DOX-induced (1) depletion of myeloid and erythroid cells in the bone marrow [19], (2) atrophy of the thymus and secondary lymphoid organs [19] and (3) lethality [32].
Although changes in both IL1 mRNA and protein levels were documented (Figs. 8, 9), the changes in mRNA levels did not parallel the changes in protein levels. Several hypotheses are consistent with these data. The production of high levels of IL1 protein by PEC from DOX-treated mice without obvious increases in the levels of IL1 mRNA may indicate regulation of protein production at the translational/post-translational level. Alternatively, the IL1 mRNA could have been increased initially and then decreased by the 24-h time point examined. Thus, PEC "priming" as a result of exposure to DOX can produce elevated amounts of both IL1 and TNF, albeit by different mechanisms.
The results reported herein, together with information available in the literature, support the model of DOX-induced immunomodulation outlined in Fig. 10. In this model, DOX administration initiates and sustains a cytokine-regulated cascade of events. DOX treatment in vivo changes the level of certain cytokines expressed by resident PEC. These changes lead both to recruitment and to priming of subsets of PEC, including macrophages and LGL. In addition, they provide second signals towards the activation of tumoricidal macrophages and NK cells. This cascade of events was shown to be initiated by a single injection of DOX; it is likely that the cascade is sustained by DOX that is found associated with PEC for up to 2 weeks after the initial injection [47]. Overall, these data indicate that DOX treatment in vivo stimulates specific components of the host defenses and results in the generation of "primed" macrophages and lymphoid (T and natural killer) cells as assessed by increases in the number of cells that express surface markers indicative of these phenotypes and/or increases in their cytotoxic activity. This activation of effector functions following in vivo DOX treatment likely results in a self-sustaining cascade initiated by the documented changes in both the level of certain cytokines and the cohort of cells present in the PEC population. Given the fact that treatments with DOX plus certain cytokines exert immunomodulation-dependent therapeutic effects in mouse tumor models [13, 18, 22, 26], it is likely that the functional and cellular changes in PEC populations demonstrated in this report have therapeutic relevance.
Abbreviations
- Ad:
-
Adherent
- CM:
-
Conditioned media
- CTL:
-
Cytolytic T lymphocyte
- DOX:
-
Doxorubicin
- FSC:
-
Forward scatter
- IFNγ:
-
Interferon γ
- IL:
-
Interleukin
- LAK:
-
Lymphokine-activated killer
- LGL:
-
Large granulocytic lymphocyte
- LPS:
-
Lipopolysaccharide
- MTT:
-
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
- NAd:
-
Nonadherent
- NK:
-
Natural killer cell
- PBL:
-
Peripheral blood lymphocyte
- PCR:
-
Polymerase chain reaction
- PEC:
-
Peritoneal exudate cells
- RT:
-
Reverse transcription
- SSC:
-
Side scatter
- TNF:
-
Tumor necrosis factor α
- W:
-
Whole
References
Abdul Hamied TA, Parker D, Turk JL (1987) Effects of Adriamycin, 4-hydroperoxycyclophosphamide and ASTA Z 7557 (INN mafosfamide) on the release of IL-2 and IL-1 in vitro. Int J Immunopharmacol 9:355
Akashi M, Shaw G, Hachiya M, Elstner E, Suzuki G, Koeffler P (1994) Number and location of AUUUA motifs: role in regulating transiently expressed RNAs. Blood 83:3182
Arinaga S, Akiyoshi T, Tsuji H (1986) Augmentation of the generation of cell-mediated cytotoxicity after a single dose of adriamycin in cancer patients. Cancer Res 46:4213
Berleth ES, Ujhazy P, Dolnick BJ, Ehrke MJ (1999) Measurement of low-abundance cytokine mRNAs in cells of murine lymphoid organs: a new quantitative RT/PCR method. Cancer Immunol Immunother 48:471
Berleth ES, Dolnick BJ, Ehrke MJ, Mihich E (1994) RNA recovery efficiency for cultured cells and organ-derived cell suspensions. Nucleic Acids Res 22:1925
Bravo-Cuellar A, Martin-Ruiz JL, Liu XH, Orbach-Arbouys S (1990) Enhanced production of interleukin 1 by mouse peritoneal macrophages after aclacinomycin administration. Immunol Lett 23:165
Bravo L, Legendre AM, Hahn KA, Rohrbach BW, Abraha T, Lothrop CD Jr (1996) Serum granulocyte colony-stimulating factor (G-CSF) and interleukin-1 (IL-1) concentrations after chemotherapy-induced neutropenia in normal and tumor-bearing dogs. Exp Hematol 24:11
Bricio T, Molina A, Martin A, Mampaso F (1994) In vitro modulation of interleukin-1 beta secretion by cultured rat doxorubicin-stimulated whole glomeruli and dissociated mesangial glomerular cells. Immunology 81:53
Brown CY, Lagnado CA, Vadas MA, Goodall GJ (1996) Differential regulation of the stability of cytokine mRNAs in lipopolysaccharide-activated blood monocytes in response to interleukin-10. J Biol Chem 271:20108
Cabanes A, Even-Chen S, Zimberoff J, Barenholz Y, Kedar E, Gabizon A (1999) Enhancement of antitumor activity of polyethylene glycol-coated liposomal doxorubicin with soluble and liposomal interleukin 2. Clin Cancer Res 5:687
Colinas RJ, Burkart PT, Lawrence DA (1995) The effects of interleukin-1 beta and tumor necrosis factor- alpha on in vitro colony formation by human hematopoietic progenitor cells exposed to doxorubicin or hydroquinone. Exp Hematol 23:1247
Ehrke MJ, Verstovsek S, Ujhazy P, Meer JM, Eppolito C, Maccubbin DL, Mihich E (1998) Doxorubicin plus tumor necrosis factor alpha combination treatments in EL4-lymphoma-bearing C57BL/6 mice. Cancer Immunol Immunother 45:287
Ehrke MJ, Verstovsek S, Maccubbin DL, Ujhazy P, Berleth ES, Zaleskis G, Mihich E (2000) Protective specific immunity induced by doxorubicin plus TNF-α combination treatment of EL4 lymphoma-bearing C57BL/6 mice. Int J Cancer 87:101
Ehrke MJ (1991) Effect of cancer therapy on host response and immunobiology. Curr Opin Oncol 3:1070
Ehrke MJ, Ho RL, Hori K. Species-specific TNF induction of thymocyte proliferation. Cancer Immunol Immunother 27:103
Ehrke MJ, Mihich E, Berd D, Mastrangelo MJ (1989) Effects of anticancer drugs on the immune system in humans. Semin Oncol 16:230
Ehrke MJ, Ryoyama K, Cohen SA (1984) Cellular basis for adriamycin-induced augmentation of cell- mediated cytotoxicity in culture. Cancer Res 44:2497
Ehrke MJ, Verstovsek S, Zaleskis G, Ho RL, Ujhazy P, Maccubbin DL, Mihich E (1996) Specific anti-EL4-lymphoma immunity in mice cured 2 years earlier with doxorubicin and interleukin-2. Cancer Immunol Immunother 42:221
Eppstein DA, Kurahara CG, Bruno NA, Terrell TG (1989) Prevention of doxorubicin-induced hematotoxicity in mice by interleukin 1. Cancer Res 49:3955
Eppstein DA, van der Pas MA, Marsh YV, Bruno NA, Kurahara CG (1988) Enhanced antitumor efficacy of adriamycin in combination with interferon-gamma: effects on macrophages. J Interferon Res 8:263
Fukui H, Overton WR, Herberman RB, Reynolds CW (1987) Natural killer cell activity in the rat. VI. Characterization of rat large granular lymphocytes as effector cells in natural killer and antibody-dependent cellular cytotoxic activities. J Leukoc Biol 41:130
Gautam SC, Chikkala NF, Ganapathi R, Hamilton TA (1991) Combination therapy with Adriamycin and interleukin 2 augments immunity against murine renal cell carcinoma. Cancer Res 51:6133
Gazit Z, Kedar E (1994) Chemotherapy-induced modulation of natural killer and lymphokine-activated killer cell activity in euthymic and athymic mice. Cancer Immunol Immunother 38:243
Gery I, Gershon RK, Waksman BH (1972) Potentiation of theT-lymphocyte response to mitogens. I. The responding cell. J Exp Med 136:128
Ho RL, Maccubbin D, Zaleskis G, Krawczyk C, Wing K, Mihich E, Ehrke MJ (1993) Development of a safe and effective adriamycin plus interleukin 2 therapy against both adriamycin-sensitive and -resistant lymphomas. Oncol Res 5:373
Ho RL, Maccubbin DL, Ujhazy P, Zaleskis G, Eppolito C, Mihich E, Ehrke MJ (1993) Immunological responses critical to the therapeutic effects of adriamycin plus interleukin 2 in C57BL/6 mice bearing syngeneic EL4 lymphoma. Oncol Res 5:363
Hori K, Mihich E, Ehrke MJ (1989) Role of tumor necrosis factor and interleukin 1 in gamma- interferon-promoted activation of mouse tumoricidal macrophages. Cancer Res 49:2606
Huang JJ, Newton RC, Rutledge SJ, Horuk R, Matthew JB, Covington M, Lin Y (1988) Characterization of murine IL-1 beta. Isolation, expression, and purification. J Immunol 140:3838
Janeway CA, Travers P (1994) Immuno biology: the immune system in health and disease. Garland Publishing Inc, New York
Klaich GM, Kanter PM (1994) Induction of dog IL-1 by free and liposomal encapsulated doxorubicin. Anti-Cancer Drugs 5:355
Le Cesne A, Vassal G, Farace F, Spielmann M, Le Chevalier T, Angevin E, Valteau-Couanet D, Fizazi K, Cojean I, Llombard A, Tursz T, Escudier B (1999) Combination interleukin-2 and doxorubicin in advanced adult solid tumors: circumvention of doxorubicin resistance in soft-tissue sarcoma? J Immunother 22:268
Lynch DH, Rubin AS, Miller RE, Williams DE (1993) Protective effects of recombinant human interleukin-1 alpha in doxorubicin-treated normal and tumor-bearing mice. Cancer Res 53:1565
Maccubbin DL, Mace KF, Ehrke MJ, Mihich E (1989) Modification of host antitumor defense mechanisms in mice by progressively growing tumor. Cancer Res 49:4216
Maccubbin DL, Wing KR, Mace KF, Ho RL, Ehrke MJ, Mihich E (1992) Adriamycin-induced modulation of host defenses in tumor-bearing mice. Cancer Res 52:3572
Mace KF, Ehrke MJ, Hori K, Maccubbin DL, Mihich E (1988) Role of tumor necrosis factor in macrophage activation and tumoricidal activity. Cancer Res 48:5427
Mace K, Mayhew E, Mihich E, Ehrke MJ (1985) Production of a soluble mediator with tumor lytic activity by adherent peritoneal exudate cells from mice treated with Adriamycin or liposome-encapsulated Adriamycin. J Leukocyte Biol 38:68
Mace K, Mayhew E, Mihich E, Ehrke MJ (1988) Alterations in murine host defense functions by adriamycin or liposome-encapsulated adriamycin. Cancer Res 48:130
Orsini F, Pavelic Z, Mihich E (1977) Increased primary cell-mediated immunity in culture subsequent to adriamycin or daunorubicin treatment of spleen donor mice. Cancer Res 37:1719
Punturieri A, Velotti F, Piccoli M, Herberman RB, Frati L, Santoni A (1989) Overnight incubation of mouse spleen cells in recombinant IL-2 generates cytotoxic cells with NK characteristics from precursors enriched with or devoid of LGL. Clin Exp Immunol 75:155
Salup RR, Wiltrout RH (1986) Treatment of adenocarcinoma in the peritoneum of mice: chemoimmunotherapy with IL-2-stimulated cytotoxic lymphocytes as a model for treatment of minimal residual disease. Cancer Immunol Immunother 22:31
Shi F, MacEwen EG, Kurzman ID (1993) In vitro and in vivo effect of doxorubicin combined with liposome-encapsulated muramyl tripeptide on canine monocyte activation. Cancer Res 53:3986
Storm G, Steerenberg PA, Van Borssum WM, Crommelin D. J (1996) Effects of intraperitoneal administration of free and liposome-entrapped doxorubicin on rat peritoneal exudate cell populations. J Drug Target 4:255
Ujhazy P, Chen YF, Fredericks W, Ho RL, Mihich E, Baker RM, Ehrke MJ (1990) The relationship between multidrug resistance and tumor necrosis factor resistance in an EL4 cell line model. Cancer Commun 2:181
Ujhazy P, Klobusicka M, Babusikova O, Strausbauch P, Mihich E, Ehrke MJ (1994) Ecto-5'-nucleotidase (CD73) in multidrug-resistant cell lines generated by doxorubicin. Int J Cancer 59:83
van der Veen AH, de Wilt JH, Eggermont AM, van Tiel ST, Seynhaeve AL, ten Hagen TL (2000) TNF-alpha augments intratumoural concentrations of doxorubicin in TNF-alpha-based isolated limb perfusion in rat sarcoma models and enhances anti-tumour effects. Br J Cancer 82:973
Zaleskis G, Berleth E, Ehrke MJ, Mihich E (1994) Doxorubicin induced DNA degradation in murine thymocytes. Mol Pharmacol 46:901
Zaleskis G, Ho RL, Diegelman P, Maccubbin D, Ujhazy P, Mihich E, Ehrke MJ (1994) Intracellular doxorubicin kinetics in lymphoma cells and lymphocytes infiltrating the tumor area in vivo: a flow cytometric study. Oncol Res 6:183
Zaleskis G, Verstovsek S, Tzai TS, Mihich E, Ehrke MJ (1995) Doxorubicin and cyclosporin A affect murine lymphoid cells expressing different antigenic determinants. Oncol Res 7:307
Acknowledgements
The authors would like to acknowledge Cheryl Eppolito and Jane Meer for their expert technical assistance. These studies were supported, in part, by NIH NCI grants CA15142 (R01) and CA16056 (center grant).
Author information
Authors and Affiliations
Corresponding author
Additional information
P. Ujhazy and G. Zaleskis contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ujhazy, P., Zaleskis, G., Mihich, E. et al. Doxorubicin induces specific immune functions and cytokine expression in peritoneal cells. Cancer Immunol Immunother 52, 463–472 (2003). https://doi.org/10.1007/s00262-003-0391-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-003-0391-x