Abstract
There have been many publications detailing imaging features of malignant transformation of intraductal papillary mucinous neoplasms (IPMN), management and recommendations for imaging follow-up of diagnosed or presumed IPMN. However, there is no consensus on several practical aspects of imaging IPMN that could serve as a clinical guide for radiologists and enable future data mining for research. These aspects include how to measure IPMN, define reporting terminology, standardize reporting and unify guidelines for surveillance. The Society of Abdominal Radiology (SAR) created multiple Disease-Focused Panels (DFP) comprised multidisciplinary panel members who focus on a particular disease, with the goal to develop ways for radiologists to improve patient care, education, and research. DFP members met to identify the current controversies and limitations of imaging pancreatic IPMN. This paper aims to provide a practical review of the key imaging characteristics of IPMN for trainees and practicing radiologists, to guide uniformity of performance and interpretation of surveillance imaging studies, and to improve communication with clinicians by providing a lexicon and reporting template based on the experience of the SAR-DFP panel members.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The estimated prevalence of pancreatic cysts in the US population is 2.5% [1]. However, up to 44.7% of magnetic resonance cholangiopancreatography (MRCP) examinations may detect pancreatic cystic lesions with the frequency of cysts increasing with patient age [2, 3]. Incidental detection of pancreatic cystic lesions often leads to further workup with computed tomography (CT), MRI/MRCP and/or endoscopic ultrasound (EUS) with or without cyst aspiration. Incidental pancreatic cystic lesions may be characterized as benign, with no malignant potential (e.g., pseudocysts or serous cystic tumors), or as potentially malignant (e.g., mucinous neoplasms (MCN) and intraductal papillary mucinous neoplasms (IPMN)) [4]. Even after additional diagnostic assessment, some pancreatic cystic lesions cannot be definitively characterized and are presumed to represent branch duct IPMN.
IPMNs are exocrine neoplasms that arise in the pancreatic ductal system, either the main or branch ducts. They arise from mucin producing epithelial cells. Distinct from other mucinous cystic neoplasms of the pancreas, IPMNs lack ovarian-type stroma and unlike pancreatic intraepithelial neoplasm (PanIN; < 0.5 cm), they are grossly visible rather than microscopic (typically ≥ 1.0 cm but at least 0.5 cm) [5, 6]. IPMNs may be low grade (low to intermediate dysplasia) or high grade (carcinoma in situ). Low grade lesions are considered less clinically significant compared to high grade lesions, which have more frequent associations with invasive cancer [7]. IPMNs may be further classified into four subtypes based on cytoarchitecture and immunophenotype (gastric, intestinal, pancreatobiliary and oncocytic), which have variable malignant potential. Gastric subtypes are more often associated with branch duct lesions and are least likely associated with malignancy, intestinal-type may be associated with low or high grade dysplasia, whereas pancreaticobiliary and oncocytic subtypes are more often associated with high grade dysplasia and malignant transformation [6, 8]. Concomitant invasive mucinous adenocarcinomas can arise independently at a separate location in the pancreas that contains an IPMN (estimated incidence, 4.1%) [5, 9, 10].
There have been many publications detailing imaging features of malignant transformation of intraductal papillary mucinous neoplasms (IPMN), management and recommendations for imaging follow-up of diagnosed or presumed IPMN [11,12,13,14,15]. However, there is no consensus on several practical aspects of imaging IPMN that could serve as a clinical guide for radiologists and enable future data mining for research. These aspects include: how to measure IPMN, definition of reporting terminology, standardization of reporting and unification of guidelines for surveillance. The Society of Abdominal Radiology (SAR) created multiple Disease-Focused Panels (DFP) comprised multidisciplinary panel members who focus on a particular disease, with the goal to develop ways for radiologists to improve patient care, education, and research [16]. Pancreatic IPMN DFP members met to identify the current controversies and limitations of imaging pancreatic IPMN.
This paper aims to provide a practical review of the key imaging characteristics of IPMN for trainees and practicing radiologists, to guide uniformity of performance and interpretation of surveillance imaging studies, and to improve communication with clinicians by providing a lexicon and reporting template based on the experience of the SAR-DFP panel members.
Imaging protocols
MRI
MRI should be performed on at least a 1.5 T magnet equipped with phased-array coils to maximize signal-to-noise, and when available parallel imaging can be employed to increase speed of acquisition and/or improve spatial resolution. MRI protocol should include a breath-hold two-dimensional (2D or 3D) axial in- and out-of-phase T1-weighted gradient- echo (GRE) sequence, axial and coronal half-Fourier single-shot fast spin echo (FSE) breath-hold T2-weighted sequences, heavily T2- weighted 2D and/or three-dimensional (3D) T2-weighted MRCP, and breath-hold or respiratory navigated, dynamic 3D fat-suppressed T1-weighted spoiled GRE axial MR images before and after administration of intravenous gadolinium chelate contrast [17]. An axial FSE or single-shot FSE T2-weighted sequence with fat saturation may be added to increase conspicuity of pancreatic cystic lesions relative to the background (Fig. 1). Diffusion-weighted imaging (SS-EPI) using a low b value b 0, 50 s/mm2 and higher b values ≥ 500 s/mm2 has been shown useful for detecting pancreatic cancer and differentiating between malignant and benign IPMN and is routinely included in most practices [18,19,20]. The incremental value of adding intravenous secretin stimulation for detection of duct communication, intravenous glucagon to reduce bowel motion or oral contrast to suppress background signal from bowel is variable; thus, these techniques are not currently recommended on a routine basis but may be preferred by some centers [21].
Retrospective studies have suggested that an abbreviated MRI protocol without IV contrast (including at least T1W gradient echo and T2W single-shot FSE with or without 2D/3D MRCP) may be used for surveillance of previously characterized cystic lesions with no significant impact in decision-making [22,23,24,25]. There are obvious advantages to abbreviated protocols including shortening examination time and decreasing both the risk of contrast-related complications and financial cost [22, 25]. In 2017, the Korean Society of Abdominal Radiology consensus (n = 82, 90.9% agreement) stated that non-contrast MRIs could be used for serial follow-up of incidental pancreatic cystic lesions [26].
We believe that contrast-enhanced sequences using breath-hold 3D T1-weighted GRE sequences are beneficial for initial characterization and risk stratification of a pancreatic cystic lesion but follow up imaging could be performed without IV contrast in low risk patient populations (no main pancreatic duct dilation, mural nodularity or other worrisome features/high risk-stigmata; no symptoms, family history, or genetic predisposition). Contrast-enhanced imaging however, allows for a more thorough assessment of the remainder of the pancreas as the incidence of synchronous and metachronous pancreatic cancer has been shown to be higher in patients with pancreatic cystic lesions than in patients with no cystic lesions [10, 27]. In a larger cohort of retrospective studies from Japan and the US, risk of patients with BD-IPMN developing invasive pancreatic cancer (including concomitant pancreatic cancer) after 5 years of follow up ranged from 10-15.6 fold higher than the age-match general population (and higher than patients with familial risk) with baseline smaller sized cysts ≤ 1.5 cm conferring less risk in the US study (Fig. 2) [28,29,30]. Furthermore, findings of early pancreatic cancer elsewhere in the gland may be subtle especially on non-contrast imaging. IV contrast for MRI is advocated for surveillance of patients at high risk for pancreatic cancer (e.g., known genetic mutation or at least one first degree relative with pancreatic cancer) [31].
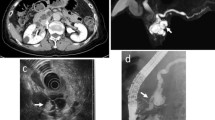
70-year-old male with known BD-PMN on surveillance. Axial T2W SSFSE image a shows a 2 cm T2 hyperintense IPMN in body of pancreas and mild parenchymal atrophy and ductal dilation upstream. b T2W FSE with fat suppression demonstrates similar findings but there is subtle T2 hypointense lesion in the body of the pancreas adjacent to the BD-IPMN (arrow) that is better appreciated on the fat suppressed image. Unenhanced 3D T1W GRE c shows the T1 hypointense cystic mass and a loss of normal T1 hyperintensity of the pancreas adjacent to the cyst. On the subtracted post contrast arterial phase image d a solid slightly hypointense mass (relative to surrounding pancreatic parenchyma) with rim enhancement concomitant pancreatic adenocarcinoma causing MPD obstruction
MDCT
A dedicated pancreatic protocol multidetector CT (MDCT) can be used as an alternative to MRI, especially in patients with contraindications to MRI as it offers easier availability, high spatial resolution and the ability to generate multiplanar reformations. A dedicated pancreatic CT protocol should include dual-phase contrast-enhanced acquisitions in the pancreatic and portal venous phases using a narrow detector configuration. At some institutions, a split-bolus technique on both MDCT and dual energy CT systems has been implemented demonstrating similar conspicuity of pancreatic parenchyma and tumors with significant reduction in radiation [32, 33]. Thin-section images should be available on a workstation that can perform 3D post processing as needed. Coronal and oblique multiplanar reconstructions along the body and tail of pancreas, 1–3 mm maximum intensity projections, and curved multiplanar reformations along the course of the pancreatic duct are helpful in detection of duct communication and characterization of pancreatic cystic lesions (Fig. 3) [4, 11]. Non-contrast CT is inadequate for characterization of pancreatic cysts. Dual Energy CT (DECT)/Spectral CT is used in some centers and has potential advantages. In a small retrospective study, DECT was found to add diagnostic value because it increased lesion conspicuity, improved visualization of the relationship of lesions to the pancreatic duct and improved differentiation of cystic from solid lesions while decreasing radiation dose [34]. In sum, it is essential to perform a dual-phase CT for initial assessment and desirable to performed dual phase imaging for follow-up but a good quality single acquisition during the early portal venous phase or DECT in the portal venous phase is also acceptable.
Ultrasound
Transabdominal ultrasound (US) offers the benefit of easy access, lower cost and potential to scan patients with contraindications to MRI and CT. It may have a role to play in follow up of cystic lesions in appropriately select patients. Sun et al. have shown that US can have a sensitivity of 78% for visualization of cystic lesions greater than 2 cm and a sensitivity of 100% for lesions greater than 3 cm [35]. Small body habitus, weight, body mass index and gender positively correlated with cyst visualization. Cysts were more frequently seen in females which may relate to the aforementioned size metrics but could also be related to the subcutaneous fat or visceral fat measurements which were not specifically calculated in this study. In a study by Jeon et al. of 938 patients with 1064 cysts, detection rate was 88.3% with median size of 13 mm in detected cysts versus 10 mm in undetected cysts. The detection rate was significantly higher when transabdominal ultrasound was performed after other modalities particularly for smaller cysts, ≤ 25 mm [36]. This study suggests that a pancreatic cystic lesion which is visible on transabdominal ultrasound can be followed with US; perhaps alternating with MRI or CT. If any change is observed, then imaging with EUS could be performed. While this approach would potentially be less expensive, reduce radiation exposure and helpful in institutions where CT/MRI and EUS are not readily available, prospective studies are warranted before its universal adoption.
Contrast enhanced ultrasound may also be helpful for characterization of pancreatic cystic lesions, potentially being able to distinguish soft tissue mural nodules from mucin and guide targeted biopsy [37]. This could be especially beneficial for patients who cannot receive intravenous iodinated or gadolinium based contrast agents. However, currently there is limited data to support this approach and the access to ultrasound contrast agents is limited, as is expertise in performing these procedures in the USA.
EUS
Endoscopic ultrasound (EUS) with fine needle aspiration (FNA) is useful for (a) differentiation between the mucinous and non-mucinous cysts, (b) assessment for duct communication, thereby aiding cystic lesion characterization and (c) performance of cytology, molecular, and biomarker analysis for characterization of mucinous versus non-mucinous cysts [38]. EUS guided FNA should be considered if there are worrisome imaging features on noninvasive imaging modalities (MRI or CT), or if the patient is symptomatic, to help differentiate neoplastic from non-neoplastic cysts [11, 12, 15]. Analysis of cyst fluid is fairly routine and can help characterize lesions by testing for tumor markers such as CEA. In some centers analysis of cyst fluid for genetic mutations such as KRAS, GNAS, RNF43, allelic imbalance (loss of heterozygosity) and aneuploidy is employed. While mutations are more numerous in malignant tumors, the sensitivity for detecting malignancy is low such that negative findings are less informative but over time, repeat sampling may detect molecular changes [39]. Studies have demonstrated greater accuracy of EUS over CT and MRI in detecting certain worrisome features such as solid components/mural nodules with one study demonstrating the incremental yield of EUS with or without FNA for diagnosis of neoplasia in cystic pancreatic masses after CT and MRI was 36% and 54%, respectively, but in this study only half of the subjects underwent MRCP and the MRCP and CT protocols were not described [38, 40]. While performance of EUS is likely to be somewhat operator dependent, the broader availability of contrast enhanced EUS will likely increase its accuracy in the future. For similar reasons as discussed above, contrast-enhanced EUS may be useful to characterize pancreatic cystic lesions and guide targeted biopsies [41].
Multiple studies have demonstrated the safety of pancreatic cyst puncture and none have demonstrated sufficient concern for needle-path seeding or intraperitoneal seeding. Current technology also allows direct cyst wall biopsy by 19 gauge needles during EUS FNA. Early results suggest that microforceps biopsy yields more accurate characterization of cyst type compared to cytology but is comparable in terms of detection of mucinous and high risk cysts [42, 43]. The promise of this modality is that it can acquire histologic material to classify cyst type and potentially use immunohistochemical sub-classification of IPMN types.
Comparison of imaging modalities
Visser et al. evaluated the relative accuracy of CT and MRI for cystic pancreatic lesion characterization using a dedicated pancreatic imaging protocol and found similar accuracies for CT and MRI, although the majority of cystic lesions in the study were symptomatic [44]. Sainani et al. showed a higher sensitivity and reader confidence of MRI for the detection of individual morphologic features like duct communication when compared to CT. However, the overall accuracy for classifying lesions based on histologic aggressiveness was equivalent with the two imaging modalities [45]. Waters et al. found MRI to be superior as compared to MDCT in demonstrating ductal connection, estimating main duct involvement, and identification of small branch duct cysts in patients with IPMN [46]. Finally, Kim et al. evaluated MRI and EUS and found the two modalities to be comparable in the characterization of cystic pancreatic lesions and prediction of malignancy [47].
Based on these studies, we suggest that MRI with MRCP is the preferred imaging modality to detect, characterize and follow pancreatic cystic lesions. The American Gastroenterology Association (AGA) guidelines encourage the use of MRI for surveillance of patients with presumed IPMN [12]. A dedicated pancreatic protocol CT with multiplanar reformations can be used as an alternative for surveillance of pancreatic cystic lesions as it has similar accuracy in detecting worrisome features in pancreatic IPMN [45, 48], though ionizing radiation is a theoretical concern with long-term surveillance. The American College of Radiology (ACR) recommendations and International Association of Pancreatology (IAP) guidelines suggest use of either MRI or CT in surveillance of IPMN is appropriate [11, 14].
Explanation of terminology used in reporting
Once a lesion is characterized as a probable IPMN, certain imaging features can help guide management based on the International Association of Pancreatology (IAP) consensus guidelines [14], and have become part of a common lexicon for healthcare teams treating patients with cystic lesions of pancreas (Table 1). In this section, we aim to define imaging features and thresholds for descriptors so they can be incorporated into a reporting template or macro (Appendices 1, 2, 3).
Size
The current surveillance guidelines for pancreatic cystic lesions are based on size and morphological features. The location and size of IPMN should be stated and measurements performed in a reproducible and consistent manner. However, there is no consensus on the methodology by which pancreatic cystic lesions are measured on imaging. Traditionally, the longest diameter of these lesions is measured in axial, coronal or sagittal plane. The choice of plane chosen for measurement is subjective and depends on the radiologist’s perception of the longest measurable diameter. This is often a challenge in daily practice given the highly variable shapes of pancreatic cystic lesions, which may appear more elongated in certain planes than others, and the ideal plane may in fact be oblique to traditional axial or coronal planes. Therefore, although the longest diameter is measured by an electronic caliper, the selection of an optimal plane of measurement is subjective. A recent study has shown excellent reproducibility, despite the inherent subjectivity in this method [49]. Dunn et al. reported improvement in reproducibility of pancreatic cyst measurement on MRI when standard methodology is followed but measurements can still vary up to 3.3 mm even after standardization [50]. The authors demonstrated improvement in reproducibility of measurement when the cyst is measured on a single coronal T2W image using outer to outer wall measurement. If the cyst is not well seen on coronal T2W image, an axial T2W image or thick slab/maximum intensity projection (MIP) MRCP image is suggested as the next alternative [50].
The single longest diameter of an IPMN is an indirect reflection of its true size as many IPMNs are not spherical. Therefore, single longest diameter may in fact over-estimate the true size of these lesions [51]. Volumetry would eliminate the subjective assessment of the longest diameter of these lesions and would take into account the non-spherical morphology of pancreatic IMPN. However, volumetry is not widely available and is not yet seamless in the clinical environment. Bi-dimensional measurement of IPMN will provide additional information regarding the shape and true size of these lesions. However, the current management guidelines for IPMN are based on the longest diameter, posing a challenge for translating recommendations for follow-up of these lesions when using 2- or 3-dimensional measurements.
Based on the pattern of growth of IPMNs, it is not uncommon to see these lesions as a cluster of multiple small cystic lesions and it can be difficult to differentiate a single multi-septated lesion from few adjacently clustered lesions (Fig. 4). In such cases, it is suggested that the entire cluster of lesions is to be reported as a single lesion, to be in agreement with lesion size reported on pathology, as most of these are reported as a single lesion on histopathology [9].
Pleomorphic branch duct IPMN with a ‘cluster of grapes” appearance on (a) coronal MIP of a 3D MRCP and a (b) 2D thick-slab MRCP. Axial (c) and coronal (d) T2W SSFE images demonstrate a cluster of tiny cysts but the extent of the lesion is better visualized on the MRCP imaging. The borders of the lesion are ill-defined but the size and extent of the lesion is best demonstrated and measured in the coronal plane (b). A different patient underwent MDCT CT (e, f) for surveillance on a BD-IPMN with lobulated, multi-septated appearance. Note the coronal images are best for measuring the maximal dimension and measurements were saved on the PACS system
In summary, cystic lesions should be given a single measurement based on the longest outer-wall to outer-wall dimension in which ever plane demonstrates the largest measurement. This can be measured on the 2D or 3D MRCP and should not include the neck or narrow connection to the main pancreatic duct. The image and series number should be documented in the report and saved on the PACS system so it can be measured similarly on follow-up scans.
Interval growth
Cyst size of 3 cm or above is considered a “worrisome” feature and impacts management. Currently, reporting the single longest dimensional “outer wall to outer wall” measurement in any plane is accepted as the standard despite the limitations as discussed above. A threshold for reporting “interval growth” is not defined by the AGA. Revised international consensus Fukuoka guidelines consider rate of cyst growth of more than 5 mm in 2 years as a worrisome feature [15] whereas European Guidelines use a threshold of ≥ 5 mm/1 year [52] as indicating significant growth (Fig. 5). The ACR incidental pancreatic cyst management recommendations recommend the following stratified criteria to define growth: lesions < 0.5 cm require 100% increase in long-axis diameter; lesions ≥ 0.5 cm and < 1.5 cm require 50% increase in long-axis diameter; lesions ≥ 1.5 cm require a 20% increase in long axis diameter [11]. In retrospective studies, cysts > 2 cm in size at baseline were more likely to develop features that would lead to consideration of resection including growth up to greater than 3 cm, development of main duct involvement and mural nodularity [53]. Cysts with growth rate of greater than 2 mm/year have significantly higher risk of malignancy with a 5-year risk of 45.5% versus 1.5% when that rate of growth was not observed [54] and these data are supported by other retrospective studies [55]. Kwong et al. found a growth rate of 2 mm/year of BD-IPMN had a sensitivity and specificity of 78% and 90% and an accuracy of 88% for identifying malignancy [56] and a hazard ratio of 19.5 (95% CI 2.4-157.8) for developing malignancy. In this same study, total BD-IPMN growth was also associated with increased risk of malignancy with growth of at least 10 mm prior to cancer diagnosis [56]. ACR recommends that size should be reported so growth rate can be documented [11].
Cyst size is an important feature to measure over time. As in this case axial T2W SSFSE images at (c) baseline (b) 4 years and (c) 6 years later demonstrating a growth rate exceeding ≥ 5 mm/2 year (worrisome) and has grown to > 3 cm in maximum dimension (high risk stigmata) based on several guidelines (see Table 1)
It should be noted that cyst growth is not uncommon. In a study of 131 patients with low to intermediate risk BD-IPMN < 3.5 cm followed for ≥ 4 years, 56% of cysts increased in size based on a interval growth threshold of ≥ 20% or ≥ 2 mm after a minimum of 12 months [57]. Despite the majority of lesions increasing in size in that study, growth rate did not exceed 1.7 mm/year, less than 10% of cysts developed worrisome features, only one lesion (3%) had high-grade dysplasia, and none developed adenocarcinoma [57]. In similar studies with long-term follow up (77–96 months), observation of an increase in cyst size has been reported in the range of 38–41% [58, 59]. While the majority of cysts increase in size within the first year, 11-30% may exhibit delayed growth after 1 or even after 5 years [57, 60]. Other studies have shown delayed growth and development of worrisome features, high-risk stigmata or invasive carcinoma even after 5 years, thus supporting long-term follow-up with imaging [61, 62]. ACR recommends following lesions for 9 to 10 years in most patients up to the age of 80 [11].
Multiplicity
Multifocal (> 1 distinct lesion) cystic pancreatic lesions are present in 14-41% of patients (Fig. 6) [63,64,65]. In a study of 150 patients who underwent 156 operations for IPMN, among the BD-IPMNs, 59% were unifocal, 41% multifocal, 83% were localized to one segment of the pancreas and 17% were “diffuse” (involved more than one segment). The authors observed that the mean number of BD-IPMNs found in patients without an invasive component was 2.7 versus 1.6 (p = 0.03) among those with BD-IPMN with an invasive component suggesting the possibility that multiplicity of BD-IPMN favors benignity. In another study of 145 patients with BD-IPMN, 14% had multifocal lesions with no significant difference between benign and malignant IPMN [64]. Another study with similar findings of no significant difference in rate of malignancy between patients with multi- or uni-focal cysts also demonstrated that progression in patients with multifocal pancreatic IPMNs was more likely in the dominant cyst [66]. Thus, decisions about surgery and surveillance should be based on the characteristics of individual cysts, symptoms and risk factors [67]. Total pancreatectomy is rarely required. Partial pancreatectomy, i.e., pancreas sparing surgery is favored with resection performed on the most suspicious/highest risk lesions.
Reporting multifocality of pancreatic cystic lesions and whether they are present in one segment of the pancreas (localized) versus multiple segments (diffuse) is helpful but each cyst should be evaluated individually; the most concerning cyst(s), whether by size or presence of intermediate or high-risk features should be described in detail to determine if surveillance or intervention is warranted.
Pancreatic duct dilation
IPMNs produce mucin that may inspissate and obstruct the flow of normal pancreatic secretions. Diffuse dilation of the main pancreatic duct (MPD) may be associated with parenchymal atrophy and calcifications and mimic chronic pancreatitis. Duct size is typically greater in the presence of main duct IPMN as compared to duct dilation in chronic pancreatitis. The absence of a history of pancreatitis as well as more importantly, an underlying cause such as stone or stricture for the duct dilation, favors a main duct IPMN. Involvement of the main pancreatic duct, e.g., mixed branch duct or main duct IPMN, increases the risk of invasive carcinoma. Direct communication of the cyst with the main pancreatic duct may not always be clear, although every possible effort should be made to establish or exclude this relationship. When there is duct dilation, whether segmental or diffuse, it is used as an indirect sign of communication.
Seo et al. found the median caliber of the MPD to be significantly higher in patients with MD-IPMN (7–7.5 mm) as compared to BD-IPMN (2 mm) [68]. An isolated finding of MPD caliber of 5 mm at the level of the pancreatic head may be a normal variant in the absence of an associated cystic lesion, abrupt duct caliber change, or interval change from prior imaging; however, the main pancreatic duct upstream in the body and tail should remain ≤ 3 mm in diameter [69]. Dilation of the MPD either downstream only or both downstream and upstream to a cystic lesion suggests mucin overproduction and should raise suspicion for mixed-type IPMN. Dilation of the main pancreatic duct upstream to a lesion with abrupt cut-off of the duct at the level of the lesion in the absence of history of pancreatitis should raise concern for invasive carcinoma causing ductal obstruction.
The main pancreatic duct should be measured perpendicular to its long axis and as mentioned above the duct may be slightly larger in caliber in the head of the pancreas compared to the body and tail. Similar to measuring the cystic lesion itself, coronal and axial T2W sequences are typically used and if those are suboptimal, the thick slab/maximum intensity projection (MIP) MRCP image. The MRCP is very helpful in allowing the radiologist to view the entire length of the main pancreatic duct and appreciate more caliber changes. The pancreas is obliquely oriented so the pancreatic duct is not typically seen on a simple imaging slice. Review of multiple slices and planes is usually required to insure visualization of the entire pancreatic duct throughout the gland.
Based on the 2017 international consensus guidelines, main pancreatic duct caliber of 5–9 mm is considered a “worrisome” feature requiring further evaluation with EUS, while MPD ≥ 10 mm is considered “high-risk stigmata” of malignancy requiring referral for potential surgical resection if the patient is a surgical candidate (Fig. 7) [14, 15]. ACR recommendations suggest using a cut off of 7 mm or greater as worrisome. Various studies have shown MPD dilatation to be a predictor for malignancy in IPMNs. However, the definition of MPD dilatation ranged from 5 to 10 mm in these studies [68, 70,71,72]. Although MPD dilatation (≥ 5–7 mm) was associated with malignancy in BD-IPMN in their meta-analysis, Kim et al. noted that the overall diagnostic value of this finding was weak with a pooled specificity of 67% [70]. In contrast, Seo et al. showed a high specificity (98.1%) and accuracy (86.7%) when the MPD caliber exceeded 10 mm on CT and MRI in BD-IPMN, but with a very low sensitivity (12.5%) [68].
Although absolute measurements of the various imaging features may be useful to predict malignancy in IPMNs and hence guide management per the 2017 international consensus guidelines, increase in cyst size or MPD diameter compared with a previous examination may be the most concerning feature. For example, new ductal dilatation to 4 mm in the pancreatic tail adjacent to an IPMN may warrant EUS for sampling even though the absolute size of the MPD remains below the threshold to be categorized as a worrisome feature.
Evaluating whether the cyst is in communication with the main pancreatic duct can be challenging. Communication is defined as direct visualization of the cystic lesion in continuity with the main duct with no septum in between. This observation is important for characterization and risk stratification as this finding is characteristic of BD-IPMN and only rarely seen in the setting of MCNs [73]. MRI and EUS have shown similar accuracy in determining if a cyst communicates with the main duct with excellent interobserver agreement [47, 74]. MRI however, has shown better accuracy when compared to CT [75].
Mural nodularity
Mural nodules are defined as papillary excrescences ≥ 3 mm within dilated MD or BD-IPMNs and are associated with malignancy (in situ and invasive carcinoma) especially in combination with other worrisome features or high-risk stigmata [76]. If solid nodule enhancement can be confidently identified with a nodule larger than 5 mm, it would be considered high-risk stigmata, and the patient should be referred for surgical resection if clinically appropriate (Fig. 8) [14]. Like the cystic lesion itself, the largest dimension of the nodule or the largest nodule should be measured on the sequence and plane where it is seen best and documented in the report. For enhancing nodules, this would be the post contrast T1W GRE sequence in the portal venous of delayed phase whereas non-enhancing nodules may be best measured on the T2W images. A note of caution, flow effects on T2W images can mimic nodules on the T2W sequences so it is best to confirm the nodule is present in two different planes.
68-year-old male with known main duct IPMN. Axial heavily T2W MRCP image shows a a 1 cm non-enhancing mural nodule within the dilated main pancreatic duct in head of pancreas (arrow). Axial T1W fat suppressed post contrast image b show no enhancement (arrow) within the lesion representing inspissated mucin
“Non-enhancing mural nodules” within a cystic pancreatic lesion or enhancing nodules < 5 mm on CT or MRI are considered worrisome features that require further evaluation (Fig. 9). Comparing pre and post contrast MRI images can identify an enhancing (worrisome nodule) from a non-enhancing (not worrisome) nodule, the result of a “mucin ball”. Mucin balls are more common than enhancing nodules and they are almost always on the dependent surface of the cyst. Endoscopic contrast-enhanced ultrasound shows promise for differentiation but more studies are warranted [77].
Several studies have shown that the presence of a mural nodule or solid component is a significant predictor of malignancy whether seen on EUS, MRI, or CT [68, 70,71,72]. In a study of 180 IPMN specimens, an enhancing mural nodule was detected in 44% of all IPMN, 93% in IPMNs with high grade dysplasia, 59% with an invasive component, 36% with intermediate grade and 19% with low grade dysplasia [78]. Based on endoscopic ultrasound, larger mural nodules (16 mm versus 4 mm) correlated with high-risk IPMNs [79]. Other studies have also shown mural nodule size to be predictive of malignancy with cutoff values ranging between 6 and 9 mm, whether in BD-IPMN or MD-IPMN [68, 80].
When cystic lesions are small (< 2 cm) and/or spatial resolution is limited, it can be difficult to distinguish between mural nodularity and confluent septa but nonetheless, it is best to describe the finding if suspected, because either feature warrants short-term follow up or endoscopic ultrasound evaluation and fluid sampling.
Thickened enhancing wall and septa
There is no well-defined threshold for wall thickening; some authors have defined it as > 2 mm while others define it as > 3 mm (Fig. 10) [81, 82]. Wall thickening may manifest as focal or diffuse, smooth or irregular. Choi et al. demonstrated that there is a statistically significant association of cyst wall thickening and malignancy in IPMNs [72]. The diagnostic value of this “worrisome” feature for diagnosing malignant BD-IPMN is limited, with a pooled specificity of 60% and a diagnostic odds ratio of 2.3 (95% CI 0.9–5.5) based on a meta-analysis of 23 articles in 1373 patients [70]. Not surprisingly, given lack of a concrete objective definition for wall thickening, this finding is associated with a high degree of interobserver variability among radiologists [83, 84]. While there is no set definition, we can extrapolate from renal cyst imaging to define wall thickening as anything more than a “thin and imperceptible” wall or a “pencil-thin” wall, ≤ 2 mm [85]. Of note, perceived wall thickness can change based on modality used for interpretation. Similar to renal cysts, MRI may show apparent wall thickening particularly on T2-weighted images due to lower spatial resolution and other subtle artifacts [86]. Of note, in certain cases, a thick enhancing wall may be the only suggestion that the lesion is in fact a different type of pancreatic lesion such as a ductal adenocarcinoma with central necrosis, pancreatic neuroendocrine tumor with cystic degeneration, MCN, or metastasis mimicking an IPMN due to central cystic change or necrosis.
Although some IPMNs may be unilocular, many are multiloculated containing multiple septa coursing through the cystic lesion which may or may not enhance [87]. It is important to differentiate between thin septae and thick enhancing septae. Thickened septae, defined as measuring > 2 mm, have been shown to predict malignancy [70, 71]. While thick enhancing septa are not specifically mentioned as high risk stigmata or a “worrisome” feature, their presence, especially if new, should prompt evaluation with EUS.
Lymph node enlargement
Lymph node enlargement is considered a worrisome feature based on international guidelines [15]. The definition is short axis > 1 cm with round morphology, heterogeneity, or central necrosis, and is extrapolated from the pancreas adenocarcinoma literature [88]. In one retrospective study including 140 patients with IPMN involving the main duct treated with resection, of 137 who had follow up, 41% (24/58) of those diagnosed with IPMN containing invasive carcinoma had positive lymph nodes. Their survival was worse than that of patients with invasive cancer and negative lymph nodes but the comparison did not reach statistical significance [89]. Other studies are in agreement that positive lymph nodes and a > 2 cm invasive component impart poorer prognosis in patients with invasive IPMN [90]. However, there is no prospective literature with regards to lymphadenopathy and BD-IPMN.
Carbohydrate antigen 19-9
Carbohydrate antigen 19-9 (CA 19-9) is an independent predictor of stage and survival in patients with resectable pancreatic ductal adenocarcinoma. A recent meta-analysis of 15 studies in 1629 patients found a pooled sensitivity and specificity of 52% and 88% for detecting invasive carcinoma in IPMN and 40% and 89%, respectively, for malignant IPMN [91]. CA 19-9 may not be routinely obtained or available to the radiologist upon interpretation of imaging studies. International guidelines suggest a serum CA 19-9 level > 37 μ/ml is a worrisome feature, but other authors suggest that a higher threshold of 100 μ/ml may be more useful [92] to improve accuracy.
Obstructive jaundice
Most branch duct IPMNs are asymptomatic at presentation. There is a weak correlation between symptoms and malignancy with an odds ratio of 1.6 (CI 1.0–2.6). The most common symptoms in order of incidence include: abdominal pain, weight loss, pancreatitis, jaundice, back pain, palpable mass and post prandial fullness [93, 94]. Obstructive jaundice, although not commonly seen at presentation, has a high association with high grade dysplasia (HGD) and invasive cancer (IC), with a hazard ratio of 9.3 (CI of 2.4–35.4) [95]. Other symptoms such as recent onset diabetes is associated with both an increased risk of high grade dysplasia/invasive cancer and higher likelihood of more aggressive histologic subtypes [28, 96] and considered a relative indication for surgical resection in the European guidelines [52].
Reporting template for known or suspected IPMN
Management of pancreatic IPMNs is best determined using a multidisciplinary approach including radiologists, gastroenterologists, oncologists, pathologists, and pancreaticobiliary surgeons. To facilitate management decisions, diagnostic imaging reporting of IPMNs should be tailored to the practice patterns of the multidisciplinary group with our proposed structure reports in the form of a macro(s) presented in Appendices 1 and 2. A macro is also provided with management recommendations based on the ACR 2017 white paper (Appendix 3) but other management recommendations could be listed if preferred by local referrers. The size threshold for including cystic lesions within imaging reports and the maximum number of lesions reported should be determined in this context.
Location of the lesion should be reported as this allows for localization during EUS and surgical planning and follow-up. In addition to reporting specific features of the cystic lesion as discussed above, it is important to closely examine the rest of the gland as patients with IPMN may present with synchronous or metachronous ductal adenocarcinoma elsewhere in the pancreas [10, 97]. Pancreatic parenchymal and extra-pancreatic changes should be observed and reported as these findings potentially represent more ominous signs of infiltrative neoplasm or acute/chronic pancreatitis as alternate diagnoses. Lastly, calcifications may be seen in up to 20% of IPMNs and have been described as punctate (87%), coarse (33%) or eggshell (rarely seen in IPMNs, more often seen in mucinous cystic neoplasm, MCNs) based on CT data [98]. Calcifications in IPMN are more often seen peripherally in the wall of the cyst. In larger retrospective studies of subjects with IPMN, calcification was more likely seen in larger cysts but was not associated with histologic type or aggressiveness [98]. Coarse calcification were associated with malignancy but not in isolation. Additional worrisome and/or high risk features were observed in addition to the calcification. Calcification is included in the provided standardized reports as it is relevant to the differential diagnosis but is not considered a worrisome or high risk feature.
Guidelines/recommendations
Guidelines and recommendations have been published addressing management of pancreatic IPMNs between 2006 and 2018 and these are summarized and compared in Table 2 [11,12,13,14,15, 26, 52, 94, 99, 100]. The first international consensus guidelines for the management of pancreatic IPMNs were established in 2006, also referred to as the Sendai guidelines. These were subsequently revised in 2012 and in 2017 and are commonly referred to as the Fukuoka guidelines [13, 14]. Based on the presumed risk of malignant change, the Fukuoka Guidelines classified IPMNs into low, intermediate and high-risk groups. This categorization was determined by the absence of concerning imaging features (low risk); the presence of worrisome features (intermediate risk) and high risk stigmata (high risk) (Table 1). Low risk lesions could be followed with serial imaging, intermediate risk lesions should undergo EUS and FNA for further characterization and high risk lesions should be considered for surgical resection. The interval of follow up imaging is stratified based on size of lesions and is summarized in Table 2. Patients are to be followed until they are unlikely to benefit from surgical intervention, whether due to overall health status or limited life expectancy.
There are several early studies with small patient cohorts validating the superiority of the Fukuoka guidelines over the Sendai guidelines. These reported high positive predictive value (PPV) (74–57%) and high negative predictive value (NPV) of 100% and 96% when using imaging features for determination of malignant change [101, 102]. Goh et al. performed a systematic review of the literature between 2012 and 2014 and reported that the PPV of the tumors that met resection criteria using the Fukuoka guidelines was low: ranging between 27 and 62% with an overall PPV of only 36% whereas the NPV was high, ranging from 83 to 100% [103].
The European Study Group on Cystic Tumors of the Pancreas published their consensus statement on management of pancreatic cystic lesions in 2013 [99] and updated in 2018 [52] with the focus on surgical indications. The consensus was achieved using an evidence-based approach including a Grading of Recommendations Assessment, Development and Evaluation (GRADE) rating for the quality of the evidence and the strength of the recommendation. They divide lesions into those with no indication, relative, and absolute indications for surgery. The absolute indications for resection were positive cytology for malignant/high grade dysplasia, solid mass, tumor related jaundice, enhancing mural nodules ≥ 5 mm and main pancreatic duct (MPD) dilatation of ≥ 10 mm. The relative indications for resection were rapid growth rate (≥ 5 mm/year), and elevated serum CA 19-9 levels (≥ 37 U/ml, MPD dilation 5–9.9 mm, cyst diameter ≥ 4 cm, enhancing mural nodules < 5 mm, new onset diabetes or acute pancreatitis due to the cystic lesion. The follow up recommendations for non-resected lesions favored long-term surveillance with the surveillance intervals of 6 months in the first year and annual surveillance subsequently including monitoring clinical symptoms, serum CA 19-9 levels and re-examining the lesions with MRI and/or EUS. Patients are to be followed until they are no longer appropriate surgical candidates.
The AGA published their guidelines in 2015, which focus only on incidental, asymptomatic pancreatic neoplastic cysts [12, 100]. They too used an evidence-based GRADE framework [104]. The guidelines do not evaluate the impact of symptoms on the management of cysts nor do they consider other neoplastic cysts such as solid papillary neoplasms, cystic adenocarcinomas, neuroendocrine tumors, or main duct IPMN without side branch involvement. The three features used in AGA guidelines for risk stratification of incidental cysts are presence of main pancreatic ductal dilation, solid nodule and cyst size ≥ 3 cm. If cysts do not have more than 1 of these features, they can be followed up with MRI for surveillance. For cysts with at least 2 high risk features, AGA guidelines recommend EUS and FNA. If cytology from FNA is concerning for malignancy, surgical resection is to be considered and if FNA is not concerning for malignancy, the cysts can be followed with imaging. The AGA recommendations do not support continued surveillance beyond 5 years if there is no change in cyst size or appearance or if the subject is no longer a surgical candidate.
The American College of Gastroenterology (ACG) published their guidelines on surveillance of IPMN and MCN in 2018 also using an evidence-based GRADE rating approach [94]. The guidelines rely on the radiologist’s report, but history, symptoms and laboratory data play a central role in decision-making such as jaundice secondary to the cyst, new onset or worsening diabetes mellitus and pancreatitis secondary to the cyst or elevated CA 19-9. EUS and FNA play a major role in stratifying risk especially if there are intermediate type risk factors such as MPD > 5 mm or more concerning features. Multidisciplinary discussion is recommended if there are more concerning features such as MPD involvement or patulous ampulla, high grade dysplasia at cytology, or mural nodule. Threshold size of nodules or the main pancreatic duct is not specified in the ACG guidelines. If the cystic lesion is not definitively characterized as serous cystadenoma after EUS and is suspected to be a mucinous tumor (IPMN or MCN), follow up with MRI is recommended for small < 1 cm cysts every 2 years, annually for cysts 1–2 cm and MRI or EUS for 2–3 cm cysts at 6 months to 1 year. During follow up, increase in size ≥ 3 mm/year or new suspicious feature prompts further evaluation with shorter interval MRI follow up, EUS or multidisciplinary discussion. Surveillance may be discontinued if patient is no longer a surgical candidate based on informed discussion when patient is > 75 years.
The ACR’s incidental findings committee on pancreatic lesions published a revised white paper on the management of incidental (asymptomatic) pancreatic cysts in 2017 [11]. ACR recommendations incorporate the Fukuoka guidelines with respect to cysts with high risk stigmata prompting surgical resection and cysts with worrisome features prompting EUS and FNA. As compared to Fukuoka guidelines where low risk IPMN follow up is stratified based only on lesion size, ACR guidelines for follow up of lesions are stratified based on lesion size as well as patient age and are summarized in Table 2. In general, for most patients under 80 years of age, ACR guidelines recommend a 9 to 10-year follow up, terminating at the age of 80 years or if the patient is no longer a candidate for surgery. One exception is the so-called “white dot” (< 5 mm) lesions seen on T2-weighted MRI. Based on limited clinical and published experience, ACR recommendations suggest that one follow-up CT or MRI at 2 years demonstrating stability is sufficient to stop surveillance or some may choose not to report these observations in patients > 75 years.
Given the lack of clinical trials, these various guidelines are based largely on retrospective data and expert opinion, resulting in low grade recommendations. The management of pancreatic IPMN is therefore strongly influenced by local expertise and experience, as well as variable practice settings, with patient preference, demographics, comorbidities, and imaging features informing the decision-making process. There is clearly a need for prospective studies and cost effectiveness analyses. ACR 2017 recommendations were developed to assist radiologists in clinical practice who may have limited time to access the multiple guidelines and recommendations and/or local expertise for management of these patients. While the guidelines do vary, there are many common elements and further effort towards a single universal guideline is warranted. In general practice, the DFP encourages the use of ACR recommendations by default unless a practice has consulted with their referrers and the institutions have chosen to follow alternate guidelines.
Conclusion
In summary, in an effort to standardize the reporting of pancreatic cystic lesions that require intervention and/or imaging surveillance, we have reviewed definitions of key features that should be reported, demonstrated how measurements should be performed through illustrative examples and offer a downloadable reporting template that can be implemented and tailored to local clinical practice. We also review the latest management guidelines from the IAP, European Study Group, AGA, ACG and ACR that can be incorporated into template reports and used in daily practice to help guide radiologists, referring clinicians and inform multidisciplinary discussions for patients with pancreatic cystic lesions.
References
Gardner TB, Glass LM, Smith KD, Ripple GH, Barth RJ, Klibansky DA, Colacchio TA, Tsapakos MJ, Suriawinata AA, Tsongalis GJ, Pipas JM, Gordon SR (2013) Pancreatic cyst prevalence and the risk of mucin-producing adenocarcinoma in US adults. The American journal of gastroenterology 108 (10):1546-1550. https://doi.org/10.1038/ajg.2013.103
Girometti R, Intini S, Brondani G, Como G, Londero F, Bresadola F, Zuiani C, Bazzocchi M (2011) Incidental pancreatic cysts on 3D turbo spin echo magnetic resonance cholangiopancreatography: prevalence and relation with clinical and imaging features. Abdom Imaging 36 (2):196-205. https://doi.org/10.1007/s00261-010-9618-4
Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y (1995) Analysis of small cystic lesions of the pancreas. Int J Pancreatol 18 (3):197-206. https://doi.org/10.1007/BF02784942
Sahani DV, Kambadakone A, Macari M, Takahashi N, Chari S, Fernandez-del Castillo C (2013) Diagnosis and management of cystic pancreatic lesions. AJR American journal of roentgenology 200 (2):343-354. https://doi.org/10.2214/ajr.12.8862
Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, Brosens LA, Fukushima N, Goggins M, Hruban RH, Kato Y, Klimstra DS, Kloppel G, Krasinskas A, Longnecker DS, Matthaei H, Offerhaus GJ, Shimizu M, Takaori K, Terris B, Yachida S, Esposito I, Furukawa T, Baltimore Consensus M (2015) A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol 39 (12):1730-1741. https://doi.org/10.1097/PAS.0000000000000533
Castellano-Megias VM, Andres CI, Lopez-Alonso G, Colina-Ruizdelgado F (2014) Pathological features and diagnosis of intraductal papillary mucinous neoplasm of the pancreas. World J Gastrointest Oncol 6 (9):311-324. https://doi.org/10.4251/wjgo.v6.i9.311
Reid MD, Lewis MM, Willingham FF, Adsay NV (2017) The Evolving Role of Pathology in New Developments, Classification, Terminology, and Diagnosis of Pancreatobiliary Neoplasms. Arch Pathol Lab Med 141 (3):366-380. https://doi.org/10.5858/arpa.2016-0262-SA
Pittman ME, Rao R, Hruban RH (2017) Classification, Morphology, Molecular Pathogenesis, and Outcome of Premalignant Lesions of the Pancreas. Arch Pathol Lab Med 141 (12):1606-1614. https://doi.org/10.5858/arpa.2016-0426-RA
Adsay V, Mino-Kenudson M, Furukawa T, Basturk O, Zamboni G, Marchegiani G, Bassi C, Salvia R, Malleo G, Paiella S, Wolfgang CL, Matthaei H, Offerhaus GJ, Adham M, Bruno MJ, Reid MD, Krasinskas A, Kloppel G, Ohike N, Tajiri T, Jang KT, Roa JC, Allen P, Fernandez-del Castillo C, Jang JY, Klimstra DS, Hruban RH (2016) Pathologic Evaluation and Reporting of Intraductal Papillary Mucinous Neoplasms of the Pancreas and Other Tumoral Intraepithelial Neoplasms of Pancreatobiliary Tract: Recommendations of Verona Consensus Meeting. Annals of surgery 263 (1):162-177. https://doi.org/10.1097/sla.0000000000001173
Yamaguchi K, Kanemitsu S, Hatori T, Maguchi H, Shimizu Y, Tada M, Nakagohri T, Hanada K, Osanai M, Noda Y, Nakaizumi A, Furukawa T, Ban S, Nobukawa B, Kato Y, Tanaka M (2011) Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas 40 (4):571-580. https://doi.org/10.1097/MPA.0b013e318215010c
Megibow AJ, Baker ME, Morgan DE, Kamel IR, Sahani DV, Newman E, Brugge WR, Berland LL, Pandharipande PV (2017) Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. Journal of the American College of Radiology : JACR 14 (7):911-923. https://doi.org/10.1016/j.jacr.2017.03.010
Scheiman JM, Hwang JH, Moayyedi P (2015) American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 148 (4):824-848.e822. https://doi.org/10.1053/j.gastro.2015.01.014
Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S (2006) International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al] 6 (1-2):17-32. https://doi.org/10.1159/000090023
Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K (2012) International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al] 12 (3):183-197. https://doi.org/10.1016/j.pan.2012.04.004
Tanaka M, Fernandez-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL (2017) Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al] 17 (5):738-753. https://doi.org/10.1016/j.pan.2017.07.007
Silverman SG, Megibow AJ, Fletcher JG (2017) Society of Abdominal Radiology Disease-Focused Panel Program: rationale for its genesis and status report. Abdominal radiology (New York) 42 (8):2033-2036. https://doi.org/10.1007/s00261-017-1115-6
Tirkes T, Menias CO, Sandrasegaran K (2012) MR imaging techniques for pancreas. Radiologic clinics of North America 50 (3):379-393. https://doi.org/10.1016/j.rcl.2012.03.003
Jang KM, Kim SH, Min JH, Lee SJ, Kang TW, Lim S, Choi D (2014) Value of diffusion-weighted MRI for differentiating malignant from benign intraductal papillary mucinous neoplasms of the pancreas. AJR American journal of roentgenology 203 (5):992-1000. https://doi.org/10.2214/AJR.13.11980
Kang KM, Lee JM, Shin CI, Baek JH, Kim SH, Yoon JH, Han JK, Choi BI (2013) Added value of diffusion-weighted imaging to MR cholangiopancreatography with unenhanced mr imaging for predicting malignancy or invasiveness of intraductal papillary mucinous neoplasm of the pancreas. Journal of magnetic resonance imaging : JMRI 38 (3):555-563. https://doi.org/10.1002/jmri.24022
Kim M, Mi Jang K, Kim SH, Doo Song K, Jeong WK, Kang TW, Kim YK, Cha DI, Kim K, Yoo H (2017) Diagnostic accuracy of diffusion restriction in intraductal papillary mucinous neoplasm of the pancreas in comparison with “high-risk stigmata” of the 2012 international consensus guidelines for prediction of the malignancy and invasiveness. Acta Radiol 58 (10):1157-1166. doi:10.1177/0284185116685921 Kim M, Mi Jang K, Kim SH, Doo Song K, Jeong WK, Kang TW, Kim YK, Cha DI, Kim K, Yoo H (2017) Diagnostic accuracy of diffusion restriction in intraductal papillary mucinous neoplasm of the pancreas in comparison with “high-risk stigmata” of the 2012 international consensus guidelines for prediction of the malignancy and invasiveness. Acta Radiol 58 (10):1157-1166. https://doi.org/10.1177/0284185116685921
Purysko AS, Gandhi NS, Walsh RM, Obuchowski NA, Veniero JC (2014) Does secretin stimulation add to magnetic resonance cholangiopancreatography in characterising pancreatic cystic lesions as side-branch intraductal papillary mucinous neoplasm? European radiology 24 (12):3134-3141. doi:10.1007/s00330-014-3355-yPurysko AS, Gandhi NS, Walsh RM, Obuchowski NA, Veniero JC (2014) Does secretin stimulation add to magnetic resonance cholangiopancreatography in characterising pancreatic cystic lesions as side-branch intraductal papillary mucinous neoplasm? European radiology 24 (12):3134-3141. https://doi.org/10.1007/s00330-014-3355-y
Pozzi-Mucelli RM, Rinta-Kiikka I, Wunsche K, Laukkarinen J, Labori KJ, Anonsen K, Verbeke C, Del Chiaro M, Kartalis N (2017) Pancreatic MRI for the surveillance of cystic neoplasms: comparison of a short with a comprehensive imaging protocol. European radiology 27 (1):41-50. https://doi.org/10.1007/s00330-016-4377-4
Nougaret S, Reinhold C, Chong J, Escal L, Mercier G, Fabre JM, Guiu B, Molinari N (2014) Incidental pancreatic cysts: natural history and diagnostic accuracy of a limited serial pancreatic cyst MRI protocol. European radiology 24 (5):1020-1029. https://doi.org/10.1007/s00330-014-3112-2
Macari M, Lee T, Kim S, Jacobs S, Megibow AJ, Hajdu C, Babb J (2009) Is gadolinium necessary for MRI follow-up evaluation of cystic lesions in the pancreas? Preliminary results. AJR American journal of roentgenology 192 (1):159-164. https://doi.org/10.2214/AJR.08.1068
Kang HJ, Lee DH, Lee JM, Yoo J, Weiland E, Kim E, Son Y (2020) Clinical Feasibility of Abbreviated Magnetic Resonance With Breath-Hold 3-Dimensional Magnetic Resonance Cholangiopancreatography for Surveillance of Pancreatic Intraductal Papillary Mucinous Neoplasm. Invest Radiol 55 (5):262-269. https://doi.org/10.1097/RLI.0000000000000636
Lee ES, Kim JH, Yu MH, Choi SY, Kang HJ, Park HJ, Park YS, Byun JH, Shin SS, Lee CH, Korean Society of Abdominal R (2019) Diagnosis and Surveillance of Incidental Pancreatic Cystic Lesions: 2017 Consensus Recommendations of the Korean Society of Abdominal Radiology. Korean J Radiol 20 (4):542-557. https://doi.org/10.3348/kjr.2018.0640
Tanno S, Nakano Y, Sugiyama Y, Nakamura K, Sasajima J, Koizumi K, Yamazaki M, Nishikawa T, Mizukami Y, Yanagawa N, Fujii T, Obara T, Okumura T, Kohgo Y (2010) Incidence of synchronous and metachronous pancreatic carcinoma in 168 patients with branch duct intraductal papillary mucinous neoplasm. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al] 10 (2-3):173-178. https://doi.org/10.1159/000231982
Gausman V, Kandel P, Van Riet PA, Moris M, Kayal M, Do C, Poneros JM, Sethi A, Gress FG, Schrope BA, Luk L, Hecht E, Jovani M, Bruno MJ, Cahen DL, Wallace MB, Gonda TA (2018) Predictors of Progression Among Low-Risk Intraductal Papillary Mucinous Neoplasms in a Multicenter Surveillance Cohort. Pancreas 47 (4):471-476. https://doi.org/10.1097/MPA.0000000000001027
Pergolini I, Sahora K, Ferrone CR, Morales-Oyarvide V, Wolpin BM, Mucci LA, Brugge WR, Mino-Kenudson M, Patino M, Sahani DV, Warshaw AL, Lillemoe KD, Fernandez-Del Castillo C (2017) Long-term Risk of Pancreatic Malignancy in Patients With Branch Duct Intraductal Papillary Mucinous Neoplasm in a Referral Center. Gastroenterology 153 (5):1284-1294 e1281. https://doi.org/10.1053/j.gastro.2017.07.019
Oyama H, Tada M, Takagi K, Tateishi K, Hamada T, Nakai Y, Hakuta R, Ijichi H, Ishigaki K, Kanai S, Kogure H, Mizuno S, Saito K, Saito T, Sato T, Suzuki T, Takahara N, Morishita Y, Arita J, Hasegawa K, Tanaka M, Fukayama M, Koike K (2020) Long-term Risk of Malignancy in Branch-Duct Intraductal Papillary Mucinous Neoplasms. Gastroenterology 158 (1):226-237 e225. https://doi.org/10.1053/j.gastro.2019.08.032
Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen G, Klein AP, Axilbund J, Griffin C, Syngal S, Saltzman JR, Mortele KJ, Lee J, Tamm E, Vikram R, Bhosale P, Margolis D, Farrell J, Goggins M, American Cancer of the Pancreas Screening C (2012) Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 142 (4):796-804; quiz e714-795. https://doi.org/10.1053/j.gastro.2012.01.005
Brook OR, Gourtsoyianni S, Brook A, Siewert B, Kent T, Raptopoulos V (2013) Split-bolus spectral multidetector CT of the pancreas: assessment of radiation dose and tumor conspicuity. Radiology 269 (1):139-148. https://doi.org/10.1148/radiol.13121409
Muenzfeld H, Mahjoub S, Roehle R, Pelzer U, Bahra M, Boening G, Hamm B, Geisel D, Auer TA (2019) Split-bolus vs. multiphasic contrast bolus protocol in patients with pancreatic cancer or cholangiocarcinoma. Eur J Radiol 119:108626. https://doi.org/10.1016/j.ejrad.2019.07.027
Chu AJ, Lee JM, Lee YJ, Moon SK, Han JK, Choi BI (2012) Dual-source, dual-energy multidetector CT for the evaluation of pancreatic tumours. Br J Radiol 85 (1018):e891-898. https://doi.org/10.1259/bjr/26129418
Sun MRM, Strickland CD, Tamjeedi B, Brook A, Mortele KJ, Brook OR, Kane RA, Siewert B (2017) Utility of transabdominal ultrasound for surveillance of known pancreatic cystic lesions: prospective evaluation with MRI as reference standard. Abdominal radiology (New York). https://doi.org/10.1007/s00261-017-1269-2
Jeon JH, Kim JH, Joo I, Lee S, Choi SY, Han JK (2018) Transabdominal Ultrasound Detection of Pancreatic Cysts Incidentally Detected at CT, MRI, or Endoscopic Ultrasound. AJR American journal of roentgenology 210 (3):518-525. https://doi.org/10.2214/AJR.17.18449
Fan Z, Yan K, Wang Y, Qiu J, Wu W, Yang L, Chen M (2015) Application of Contrast-Enhanced Ultrasound in Cystic Pancreatic Lesions Using a Simplified Classification Diagnostic Criterion. BioMed research international 2015:974621. https://doi.org/10.1155/2015/974621
Khashab MA, Kim K, Lennon AM, Shin EJ, Tignor AS, Amateau SK, Singh VK, Wolfgang CL, Hruban RH, Canto MI (2013) Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas 42 (4):717-721. https://doi.org/10.1097/MPA.0b013e3182883a91
Winner M, Sethi A, Poneros JM, Stavropoulos SN, Francisco P, Lightdale CJ, Allendorf JD, Stevens PD, Gonda TA (2015) The role of molecular analysis in the diagnosis and surveillance of pancreatic cystic neoplasms. JOP 16 (2):143-149. https://doi.org/10.6092/1590-8577/2941
Lu X, Zhang S, Ma C, Peng C, Lv Y, Zou X (2015) The diagnostic value of EUS in pancreatic cystic neoplasms compared with CT and MRI. Endosc Ultrasound 4 (4):324-329. https://doi.org/10.4103/2303-9027.170425
Yamashita Y, Ueda K, Itonaga M, Yoshida T, Maeda H, Maekita T, Iguchi M, Tamai H, Ichinose M, Kato J (2013) Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: a single-center prospective study. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 32 (1):61-68
Mittal C, Obuch JC, Hammad H, Edmundowicz SA, Wani S, Shah RJ, Brauer BC, Attwell AR, Kaplan JB, Wagh MS (2018) Technical feasibility, diagnostic yield, and safety of microforceps biopsies during EUS evaluation of pancreatic cystic lesions (with video). Gastrointest Endosc 87 (5):1263-1269
Basar O, Yuksel O, Yang DJ, Samarasena J, Forcione D, DiMaio CJ, Wagh MS, Chang K, Casey B, Fernandez-Del Castillo C, Pitman MB, Brugge WR (2018) Feasibility and safety of microforceps biopsy in the diagnosis of pancreatic cysts. Gastrointest Endosc 88 (1):79-86. https://doi.org/10.1016/j.gie.2018.02.039
Visser BC, Yeh BM, Qayyum A, Way LW, McCulloch CE, Coakley FV (2007) Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. AJR American journal of roentgenology 189 (3):648-656.
Sainani NI, Saokar A, Deshpande V, Fernandez-del Castillo C, Hahn P, Sahani DV (2009) Comparative performance of MDCT and MRI with MR cholangiopancreatography in characterizing small pancreatic cysts. AJR American journal of roentgenology 193 (3):722-731.
Waters JA, Schmidt CM, Pinchot JW, White PB, Cummings OW, Pitt HA, Sandrasegaran K, Akisik F, Howard TJ, Nakeeb A, Zyromski NJ, Lillemoe KD (2008) CT vs MRCP: optimal classification of IPMN type and extent. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 12 (1):101-109. https://doi.org/10.1007/s11605-007-0367-9
Kim YC, Choi JY, Chung YE, Bang S, Kim MJ, Park MS, Kim KW (2010) Comparison of MRI and endoscopic ultrasound in the characterization of pancreatic cystic lesions. AJR American journal of roentgenology 195 (4):947-952.
Lee HJ, Kim MJ, Choi JY, Hong HS, Kim KA (2011) Relative accuracy of CT and MRI in the differentiation of benign from malignant pancreatic cystic lesions. Clinical radiology 66 (4):315-321. https://doi.org/10.1016/j.crad.2010.06.019
Do RK, Katz SS, Gollub MJ, Li J, LaFemina J, Zabor EC, Moskowitz CS, Klimstra DS, Allen PJ (2014) Interobserver agreement for detection of malignant features of intraductal papillary mucinous neoplasms of the pancreas on MDCT. AJR American journal of roentgenology 203 (5):973-979.
Dunn DP, Brook OR, Brook A, Revah G, Jawadi S, Sun M, Lee KS, Mortele KJ (2016) Measurement of pancreatic cystic lesions on magnetic resonance imaging: efficacy of standards in reducing inter-observer variability. Abdominal radiology (New York) 41 (3):500-507.
Aghaei Lasboo A, Rezai P, Yaghmai V (2010) Morphological analysis of pancreatic cystic masses. Academic radiology 17 (3):348-351.
European Study Group on Cystic Tumours of the P (2018) European evidence-based guidelines on pancreatic cystic neoplasms. Gut 67 (5):789-804.
Han Y, Lee H, Kang JS, Kim JR, Kim HS, Lee JM, Lee KB, Kwon W, Kim SW, Jang JY (2018) Progression of Pancreatic Branch Duct Intraductal Papillary Mucinous Neoplasm Associates With Cyst Size. Gastroenterology 154 (3):576-584.
Kang MJ, Jang JY, Kim SJ, Lee KB, Ryu JK, Kim YT, Yoon YB, Kim SW (2011) Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 9 (1):87-93.
Kolb JM, Argiriadi P, Lee K, Liu X, Bagiella E, Gupta S, Lucas AL, Kim MK, Kumta NA, Nagula S, Sarpel U, DiMaio CJ (2018) Higher Growth Rate of Branch Duct Intraductal Papillary Mucinous Neoplasms Associates With Worrisome Features. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 16 (9):1481-1487.
Kwong WT, Lawson RD, Hunt G, Fehmi SM, Proudfoot JA, Xu R, Giap A, Tang RS, Gonzalez I, Krinsky ML, Savides TJ (2015) Rapid Growth Rates of Suspected Pancreatic Cyst Branch Duct Intraductal Papillary Mucinous Neoplasms Predict Malignancy. Digestive diseases and sciences 60 (9):2800-2806.
Kayal M, Luk L, Hecht EM, et al. (2017) Long-Term Surveillance and Timeline of Progression of Presumed Low-Risk Intraductal Papillary Mucinous Neoplasms. AJR American journal of roentgenology 209(2):320–326. https://doi.org/10.2214/ajr.16.17249
Handrich SJ, Hough DM, Fletcher JG, Sarr MG (2005) The natural history of the incidentally discovered small simple pancreatic cyst: long-term follow-up and clinical implications. AJR American journal of roentgenology 184(1):20–23. https://doi.org/10.2214/ajr.184.1.01840020
Arlix A, Bournet B, Otal P, et al. (2012) Long-term clinical and imaging follow-up of nonoperated branch duct form of intraductal papillary mucinous neoplasms of the pancreas. Pancreas 41(2):295–301. https://doi.org/10.1097/MPA.0b013e3182285cc8
Brook OR, Beddy P, Pahade J, et al. (2016) Delayed Growth in Incidental Pancreatic Cysts: Are the Current American College of Radiology Recommendations for Follow-up Appropriate? Radiology 278(3):752–761. https://doi.org/10.1148/radiol.2015140972
Crippa S, Pezzilli R, Bissolati M, et al. (2017) Active Surveillance Beyond 5 Years Is Required for Presumed Branch-Duct Intraductal Papillary Mucinous Neoplasms Undergoing Non-Operative Management. The American journal of gastroenterology 112(7):1153–1161. https://doi.org/10.1038/ajg.2017.43
Khannoussi W, Vullierme MP, Rebours V, Maire F, Hentic O, Aubert A, Sauvanet A, Dokmak S, Couvelard A, Hammel P, Ruszniewski P, Levy P (2012) The long term risk of malignancy in patients with branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al] 12 (3):198-202. https://doi.org/10.1016/j.pan.2012.03.056
Ohtsuka T, Kono H, Tanabe R, et al. (2012) Follow-up study after resection of intraductal papillary mucinous neoplasm of the pancreas; special references to the multifocal lesions and development of ductal carcinoma in the remnant pancreas. Am J Surg 204(1):44–48. https://doi.org/10.1016/j.amjsurg.2011.04.007
Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, Thayer SP, Lauwers GY, Capelli P, Mino-Kenudson M, Razo O, McGrath D, Pederzoli P, Fernandez-Del Castillo C (2007) Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology 133 (1):72-79; quiz 309-310. https://doi.org/10.1053/j.gastro.2007.05.010
Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, Howard TJ, Zyromski NJ, Nakeeb A, DeWitt JM, Akisik FM, Sherman S, Pitt HA, Lillemoe KD (2007) Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Annals of surgery 246 (4):644-651; discussion 651-644. https://doi.org/10.1097/sla.0b013e318155a9e5
Rosenblatt R, Dorfman V, Epelboym I, et al. (2015) Demographic features and natural history of intermediate-risk multifocal versus unifocal intraductal papillary mucinous neoplasms. Pancreas 44(3):478–483. https://doi.org/10.1097/MPA.0000000000000264
Farrell JJ, Fernandez-del Castillo C (2013) Pancreatic cystic neoplasms: management and unanswered questions. Gastroenterology 144(6):1303–1315. https://doi.org/10.1053/j.gastro.2013.01.073
Seo N, Byun JH, Kim JH, et al. (2016) Validation of the 2012 International Consensus Guidelines Using Computed Tomography and Magnetic Resonance Imaging: Branch Duct and Main Duct Intraductal Papillary Mucinous Neoplasms of the Pancreas. Annals of surgery 263(3):557–564. https://doi.org/10.1097/sla.0000000000001217
Mortele KJ, Rocha TC, Streeter JL, Taylor AJ (2006) Multimodality imaging of pancreatic and biliary congenital anomalies. Radiographics: a review publication of the Radiological Society of North America, Inc 26 (3):715-731. https://doi.org/10.1148/rg.263055164
Kim KW, Park SH, Pyo J, et al. (2014) Imaging features to distinguish malignant and benign branch-duct type intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Annals of surgery 259(1):72–81. https://doi.org/10.1097/SLA.0b013e31829385f7
Sahani DV, Kadavigere R, Blake M, et al. (2006) Intraductal papillary mucinous neoplasm of pancreas: multi-detector row CT with 2D curved reformations–correlation with MRCP. Radiology 238(2):560–569. https://doi.org/10.1148/radiol.2382041463
Choi SY, Kim JH, Yu MH, et al. (2017) Diagnostic performance and imaging features for predicting the malignant potential of intraductal papillary mucinous neoplasm of the pancreas: a comparison of EUS, contrast-enhanced CT and MRI. Abdominal radiology (New York) 42(5):1449–1458. https://doi.org/10.1007/s00261-017-1053-3
Morel A, Marteau V, Chambon E, Gayet B, Zins M (2009) Pancreatic mucinous cystadenoma communicating with the main pancreatic duct on MRI. Br J Radiol 82(984):e243–245. https://doi.org/10.1259/bjr/98185084
Kim JH, Eun HW, Park HJ, Hong SS, Kim YJ (2012) Diagnostic performance of MRI and EUS in the differentiation of benign from malignant pancreatic cyst and cyst communication with the main duct. Eur J Radiol 81(11):2927–2935. https://doi.org/10.1016/j.ejrad.2011.12.019
Song SJ, Lee JM, Kim YJ, et al. (2007) Differentiation of intraductal papillary mucinous neoplasms from other pancreatic cystic masses: comparison of multirow-detector CT and MR imaging using ROC analysis. Journal of magnetic resonance imaging: JMRI 26(1):86–93. https://doi.org/10.1002/jmri.21001
Sugiyama M, Atomi Y (1998) Intraductal papillary mucinous tumors of the pancreas: imaging studies and treatment strategies. Annals of surgery 228(5):685–691
Fujita M, Itoi T, Ikeuchi N, et al. (2016) Effectiveness of contrast-enhanced endoscopic ultrasound for detecting mural nodules in intraductal papillary mucinous neoplasm of the pancreas and for making therapeutic decisions. Endosc Ultrasound 5(6):377–383. https://doi.org/10.4103/2303-9027.190927
Oda Y, Aishima S, Shindo K, Fujino M, Mizuuchi Y, Hattori M, Miyazaki T, Tanaka M, Oda Y (2017) SLC2A1/GLUT1 expression in mural nodules of intraductal papillary mucinous neoplasm of the pancreas. Human Pathology 65 (Supplement C):71-78. doi:https://doi.org/10.1016/j.humpath.2017.03.008
Kobayashi N, Sugimori K, Shimamura T, Hosono K, Watanabe S, Kato S, Ueda M, Endo I, Inayama Y, Maeda S, Nakajima A, Kubota K (2012) Endoscopic ultrasonographic findings predict the risk of carcinoma in branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al] 12 (2):141-145. doi:https://doi.org/10.1016/j.pan.2011.12.008
Hirono S, Kawai M, Okada KI, et al. (2017) Factors Associated With Invasive Intraductal Papillary Mucinous Carcinoma of the Pancreas. JAMA surgery 152(3):e165054. https://doi.org/10.1001/jamasurg.2016.5054
Chiu SS, Lim JH, Lee WJ, et al. (2006) Intraductal papillary mucinous tumour of the pancreas: differentiation of malignancy and benignancy by CT. Clinical radiology 61(9):776–783. https://doi.org/10.1016/j.crad.2006.04.008
Hwang DW, Jang JY, Lim CS, et al. (2011) Determination of malignant and invasive predictors in branch duct type intraductal papillary mucinous neoplasms of the pancreas: a suggested scoring formula. Journal of Korean medical science 26(6):740–746. https://doi.org/10.3346/jkms.2011.26.6.740
de Jong K, Nio CY, Mearadji B, et al. (2012) Disappointing interobserver agreement among radiologists for a classifying diagnosis of pancreatic cysts using magnetic resonance imaging. Pancreas 41(2):278–282. https://doi.org/10.1097/MPA.0b013e31822899b6
Kim SH, Lee JM, Lee ES, et al. (2015) Intraductal papillary mucinous neoplasms of the pancreas: evaluation of malignant potential and surgical resectability by using MR imaging with MR cholangiography. Radiology 274(3):723–733. https://doi.org/10.1148/radiol.14132960
Silverman SG, Pedrosa I, Ellis JH, et al. (2019) Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology 292(2):475–488. https://doi.org/10.1148/radiol.2019182646
Gulani V, Adusumilli S, Hussain HK, et al. (2008) Apparent wall thickening of cystic renal lesions on MRI. Journal of magnetic resonance imaging: JMRI 28(1):103–110. https://doi.org/10.1002/jmri.21376
Gupta R, Mortele KJ, Tatli S, et al. (2008) Pancreatic intraductal papillary mucinous neoplasms: role of CT in predicting pathologic subtypes. AJR American journal of roentgenology 191(5):1458–1464. https://doi.org/10.2214/AJR.07.3302
Al-Hawary MM, Francis IR, Chari ST, et al. (2014) Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 270(1):248–260. https://doi.org/10.1148/radiol.13131184
Salvia R, Fernandez-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, Pederzoli P, Warshaw AL (2004) Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Annals of surgery 239 (5):678-685; discussion 685-677. https://doi.org/10.1097/01.sla.0000124386.54496.15
Turrini O, Waters JA, Schnelldorfer T, et al. (2010) Invasive intraductal papillary mucinous neoplasm: predictors of survival and role of adjuvant therapy. HPB: the official journal of the International Hepato Pancreato Biliary Association 12(7):447–455. https://doi.org/10.1111/j.1477-2574.2010.00196.x
Wang W, Zhang L, Chen L, et al. (2015) Serum carcinoembryonic antigen and carbohydrate antigen 19-9 for prediction of malignancy and invasiveness in intraductal papillary mucinous neoplasms of the pancreas: A meta-analysis. Biomed Rep 3(1):43–50. https://doi.org/10.3892/br.2014.376
Morales-Oyarvide V, Fong ZV, Fernandez-Del Castillo C, Warshaw AL (2017) Intraductal Papillary Mucinous Neoplasms of the Pancreas: Strategic Considerations. Visc Med 33(6):466–476. https://doi.org/10.1159/000485014
Anand N, Sampath K, Wu BU (2013) Cyst features and risk of malignancy in intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 11 (8):913-921; quiz e959-960. https://doi.org/10.1016/j.cgh.2013.02.010
Elta GH, Enestvedt BK, Sauer BG, Lennon AM (2018) ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. The American journal of gastroenterology 113(4):464–479. https://doi.org/10.1038/ajg.2018.14
Hsiao CY, Yang CY, Wu JM, Kuo TC, Tien YW (2016) Utility of the 2006 Sendai and 2012 Fukuoka guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas: A single-center experience with 138 surgically treated patients. Medicine (Baltimore) 95(38):e4922. https://doi.org/10.1097/MD.0000000000004922
Morales-Oyarvide V, Mino-Kenudson M, Ferrone CR, Sahani DV, Pergolini I, Negreros-Osuna AA, Warshaw AL, Lillemoe KD, Fernandez-Del Castillo C (2017) Diabetes mellitus in intraductal papillary mucinous neoplasm of the pancreas is associated with high-grade dysplasia and invasive carcinoma. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al] 17 (6):920-926. https://doi.org/10.1016/j.pan.2017.08.073
Chernyak V, Flusberg M, Haramati LB, Rozenblit AM, Bellin E (2015) Incidental pancreatic cystic lesions: is there a relationship with the development of pancreatic adenocarcinoma and all-cause mortality? Radiology 274(1):161–169. https://doi.org/10.1148/radiol.14140796
Perez-Johnston R, Narin O, Mino-Kenudson M, Ingkakul T, Warshaw AL, Fernandez-Del Castillo C, Sahani VD (2013) Frequency and significance of calcification in IPMN. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al] 13 (1):43-47. https://doi.org/10.1016/j.pan.2012.11.306
Del Chiaro M, Verbeke C, Salvia R, et al. (2013) European experts consensus statement on cystic tumours of the pancreas. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 45(9):703–711. https://doi.org/10.1016/j.dld.2013.01.010
Vege SS, Ziring B, Jain R, Moayyedi P, Clinical Guidelines C, American Gastroenterology A (2015) American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 148 (4):819-822; quize812-813. https://doi.org/10.1053/j.gastro.2015.01.015
Aso T, Ohtsuka T, Matsunaga T, et al. (2014) “High-risk stigmata” of the 2012 international consensus guidelines correlate with the malignant grade of branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas 43(8):1239–1243. https://doi.org/10.1097/mpa.0000000000000199
Roch AM, Ceppa EP, DeWitt JM, et al. (2014) International Consensus Guidelines parameters for the prediction of malignancy in intraductal papillary mucinous neoplasm are not properly weighted and are not cumulative. HPB: the official journal of the International Hepato Pancreato Biliary Association 16(10):929–935. https://doi.org/10.1111/hpb.12305
Goh BK, Lin Z, Tan DM, et al. (2015) Evaluation of the Fukuoka Consensus Guidelines for intraductal papillary mucinous neoplasms of the pancreas: Results from a systematic review of 1,382 surgically resected patients. Surgery 158(5):1192–1202. https://doi.org/10.1016/j.surg.2015.03.021
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Group GW (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926. https://doi.org/10.1136/bmj.39489.470347.AD
Funding
Non funded.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1

Appendix 2

Appendix 3: Simplified version of the ACR 2017 management recommendations that could be incorporated into a standardized report

Rights and permissions
About this article
Cite this article
Hecht, E.M., Khatri, G., Morgan, D. et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: recommendations for Standardized Imaging and Reporting from the Society of Abdominal Radiology IPMN disease focused panel. Abdom Radiol 46, 1586–1606 (2021). https://doi.org/10.1007/s00261-020-02853-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02853-4