Abstract
Purpose
To evaluate the feasibility of CT perfusion performed during routine multiphase contrast-enhanced CT on a 160 mm wide-coverage 256-slice scanner in patients with pancreatic ductal adenocarcinoma (PDAC).
Methods
Fifty-seven patients had a CT perfusion acquisition during their routine multiphase CT. Perfusion was performed 5 to 42.5 s (15 passes at 2.5 s intervals) after intravenous contrast administration (4.2–5 ml/s), followed by pancreatic parenchymal and portal venous phases for clinical interpretation. Perfusion maps were generated and blood flow (BF), blood volume (BV), and permeability surface area product (PS) for tumor and uninvolved pancreas were calculated using deconvolution algorithms and compared to existing similar publications. Radiation dose information was recorded and size-specific dose estimate (SSDE) was calculated using body dimensions.
Results
Diagnostic quality of standard images was unaffected by performing the perfusion acquisition. Average tumor center BF was 20.8 ± 12.1 ml/100 g/min, BV 2.5 ± 2.1 ml/100 g and PS 15.5 ± 39.4 ml/100 g/min. Average pancreas BF was 90.8 ± 50.2 ml/100 g/min, BV 11.9 ± 4.3 ml/100 g and PS 33.6 ± 27.7 ml/100 g/min. For the perfusion acquisition, mean SSDE was 57 ± 11 mGy, CTDIvol 43 ± 6 mGy and DLP 685 ± 100 mGy-cm.
Conclusion
Adding a perfusion CT acquisition to standard pancreatic CT protocol is feasible using a wide-detector 256-slice CT scanner and adds quantitative information while maintaining diagnostic quality of the standard of care examination. This novel protocol adds no time or cost to the examination and yields perfusion parameters that are comparable to existing literature using a separate dedicated perfusion protocol.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the third leading cause of cancer death in the USA, its incidence is increasing by an estimated 0.5% per year, and prognosis is dismal with 5-year survival rate of about 10% [1,2,3]. The widespread availability and advancement of CT technology has led to earlier diagnosis and better ability to determine resectability. Although this has resulted in more patients presenting with resectable disease, it has not translated into improved 5-year survival [4]. Nonetheless, surgical resection offers the only potential cure [5, 6]. Approximately 30% of patients present with locally advanced (LA) disease, usually due to vascular involvement, and receive neoadjuvant chemotherapy and radiation therapy with the hopes of ultimately achieving an R0 surgical resection [6]. Patients with LA and borderline resectable (BR) PDAC who receive neoadjuvant therapy and achieve R0 resection have similar overall survival to those who are resectable at presentation [6]. Although CT is the standard for assessing resectability at baseline, it cannot accurately distinguish between residual tumor and treatment-related fibroinflammatory tissue, thus standard response criteria (e.g., RECIST) and CT findings underestimate response and do not reliably predict histologic R0 resectability [7]. Regression of vascular contact indicates a higher likelihood of achieving R0 resection in spite of apparent residual LA tumor, but is subjective and has low interobserver agreement [5]. Noninvasive quantitative markers are needed to more accurately assess response to neoadjuvant therapy, predict R0 resection, and better correlate with survival, ideally in a way that can be acquired routinely and consistently.
Volume perfusion CT measures dynamic changes in tissue iodine concentration over time, allowing for calculation of tissue-specific parameters, including blood flow (BF), blood volume (BV), time to peak concentration (TTP), vascular permeability surface area product (PS), and permeability (Ktrans), which can be used as surrogates for tumor vascularization, vascular immaturity, and perfusion pressure [8]. Perfusion parameters have been used in other malignancies to characterize tumors at baseline, assess response to therapy, and distinguish viable from nonviable tumor [9]. For PDAC, quantifying perfusion is of particular interest since hypoenhancement on CT has been shown to be significantly associated with fibrosclerotic stroma and an independent negative predictor of survival, perhaps as a result of intratumoral hypoxia and fibrosis [10]. Perfusion parameters have already been shown to distinguish high- and low-grade PDAC and to predict response to neoadjuvant therapy, although not yet been used to predict R0 resection or correlate with long-term survival [11, 12]. High radiation dose, time added, respiratory motion and need for 2nd intravenous contrast injection have been traditional barriers to performing CT perfusion.
We sought to determine whether it was feasible to perform perfusion CT as part of the standard diagnostic CT for patients undergoing staging and restaging on a 256-slice scanner. Since multiphase CT is already the standard of care for PDAC staging and restaging, we hoped to acquire additional quantitative data without adding cost or time to the scan. Additionally, we hypothesized that using a wide-coverage 256-slice scanner with 160 mm per gantry rotation detector coverage would offer whole organ coverage, rapid acquisition, and minimal motion artifact that would simplify acquisition compared to previous efforts. We also believed we could minimize added radiation dose using adaptive statistical iterative reconstruction techniques. In addition to the clinical challenges managing PDAC, we felt the proximity to the aorta as arterial input vessel, uniform contrast in the normal gland, and inherent high contrast between the tumor and uninvolved parenchyma would offer certain advantages for performing perfusion CT. In this way, our project sought to design a single comprehensive examination for PDAC by routinely providing essential qualitative and quantitative data at each time point. To our knowledge, this is the first study attempting to acquire perfusion parameters for PDAC without using a separate scan or IV contrast injection.
Demonstrating feasibility of CT perfusion for PDAC has significant implications for clinical care and future research, particularly if added to routine examinations. Cutoff values can be used to identify responders and non-responders, determine timing of surgery, and help distinguish between residual viable and nonviable neoplasm, thus identifying patients that ordinarily would be precluded from resection. Defining standard and meaningful perfusion parameters using consistent techniques is the first step in laying the groundwork for future research, both in PDAC and other solid tumors.
Materials and methods
Patients
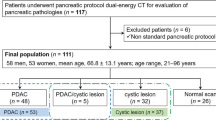
Institutional review board approval was obtained for this prospective HIPAA-compliant investigator-initiated study. Between June 2017 and February 2019, 60 patients with PDAC undergoing a pancreas staging or restaging CT were identified prospectively and written informed consent was obtained. Exclusion criteria included patients unable to provide proper informed consent, women who were pregnant or intending to become pregnant during the study, patients with body mass index greater than 40 kg/m2, and patients with a history of severe allergic-like reaction to iodinated contrast media.
Image acquisition
All CT exams were performed on a 160 mm wide-detector 256-slice scanner (Revolution CT, GE Healthcare, Waukesha, WI). A weight-based dose of intravenous contrast (Omnipaque 350; GE Healthcare, Chalfont St. Giles, UK) was administered through a dual-head power injector (Stelland D; Medrad, Warrendale, PA) at an injection rate of 5 ml/s. Patients with pre-existing implanted port catheters had contrast injected at a maximum rate of 4.2 ml/s. Beginning 5 s after intravenous contrast injection, a 37.5 s perfusion acquisition was performed from 5 to 42.5 s (15 passes at 2.5 s intervals with slow and shallow breathing) followed by fixed timing routine pancreatic parenchymal (45 s) and portal venous phase (70 s) acquisitions that were pushed to PACS and interpreted for routine clinical care (PACS; Centricity, GE Healthcare, Chicago, IL). The CT protocol and scan parameters are outlined in Table 1 and Fig. 1.
Diagram depicting pancreas CT perfusion protocol. A weight-based contrast dose of 100–180 ml Omnipaque 350 was used with a preferred injection rate of 5 cc/s. Patients with preexisting port catheters received a maximum injection rate of 4.2 cc/s due to rate limitations. Fifteen perfusion acquisitions were obtained every 2.5 s beginning at 5 s (grey). Pancreatic parenchymal and portal venous acquisitions occurred at 45 and 70 s
Image analysis
Perfusion data were analyzed using dedicated software (CT perfusion 4D; AW Server; GE Medical Systems) after applying a motion correction algorithm. Regions of interest (ROIs) were drawn freehand by a single radiologist (XXX. fellowship trained in abdominal imaging with 11 years of experience in radiology) and placed in the suprarenal aorta, tumor and pancreas. ROIs for the tumor were drawn on the axial image(s) where the mass was largest and/or had maximal contrast compared to adjacent pancreatic parenchyma using the native CT images. Three separate tumoral ROIs were drawn: (1) center of the tumor, (2) outer rim of the tumor, and (3) as large as possible to cover the entire tumor, all of which were drawn while avoiding encased blood vessels, dilated pancreatic duct or sidebranches, and cystic components ROIs were also placed in tumor-free uninvolved pancreas remote from the tumor, also avoiding blood vessels. Identical ROIs were propagated to all perfusion phases to generate perfusion maps from which tumor blood flow (BF), blood volume (BV), and permeability surface area product (PS) were calculated using deconvolution algorithms. These variables were selected as they are the three parameters most frequently reported and considered to be most significant for PDAC [13]. ROIs were numbered sequentially and images with the ROI sizes, shapes, and locations were saved in the perfusion software and exported as a comma separated values (CSV) file. Standard pancreatic arterial and venous phase images were reviewed in PACS as per routine clinical protocol and to record bidimensional tumor size to calculate RECIST 1.1 response. A separate radiologist (XX; fellowship-trained in abdominal imaging with 18 years of experience in radiology) independently reviewed the images specifically to assess the image quality using a four-point ordinal scale, as follows: 1, Nondiagnostic due to very high image noise, marked artifact, marked distortion of spatial or contrast resolution, minimal pancreatic parenchymal enhancement or very poor lesion conspicuity; 2, Poor but evaluable due to high image noise, moderate artifact, moderate distortion of spatial or contrast resolution, or poor edge definition. Suboptimal pancreatic parenchymal enhancement. Lesion visible, but suboptimal conspicuity. Considered diagnostic. 3, Good/sufficient image similar to routine clinical scans at institution. Minor compromise due to increased image noise, some image artifact, some distortion of spatial or contrast resolution, or fair edge definition. Good pancreatic parenchymal enhancement and lesion conspicuity. Considered diagnostic. 4, Excellent quality with very minor image noise, minimal artifact, very little distortion of spatial or contrast resolution, and good edge definition. Excellent pancreatic parenchymal enhancement and lesion conspicuity. Considered diagnostic.
Carbohydrate antigen (CA) 19-9 levels were extracted from the electronic medical record at each time point. The displayed volume CT dose index was recorded for all phases.
Since this was a feasibility study, we compared our perfusion data to existing literature acquired using a separate dedicated pancreatic perfusion CT for PDAC or perfusion CT of normal pancreas. An electronic search of PubMed was conducted for existing publications that performed perfusion CT for pancreas and PDAC, from which we extracted the technique details (scanner model and manufacturer, IV contrast type and injection rate, perfusion software package, ROI method, kinetic model and calculation method), perfusion parameters (BF, BV, and PS for the tumor or normal pancreas), and estimated radiation doses (DLP, CTDIvol, SSDE, or effective dose). Radiation doses were compared among all studies performing perfusion CT of the pancreas, while perfusion parameters were only specifically compared among those using the deconvolution model (including our data).
Statistical analysis
Continuous measures were summarized using means and standard deviations. Categorical data were summarized using counts and percentages. Size-specific dose estimate (SSDE) was calculated using the sum of the anterior–posterior and lateral dimensions at the level of the mid liver and effective dose was calculated using the conversion coefficient for abdomen [14, 15]. Student’s t-tests were used to compare the mean BF, BV, and PS between tumor and normal pancreas. A p value of 0.05 was considered statistically significant.
Results
Sixty patients were enrolled in this study and scanned with the CT perfusion protocol. Three exams were excluded due to technical errors/corrupt images on the perfusion acquisition. The final study population consisted of 57 patients. Since the primary aim was to test the feasibility of this method, our cohort included patients at varying time points during their treatment cycle. Thirty-eight patients had at least one cycle of treatment prior to perfusion. Other patient characteristics are outlined in Fig. 2. All exams were considered clinically diagnostic as part of standard workflow, as well as in the secondary image quality assessment, including the three exams where the perfusion acquisitions were unable to be post-processed. In the independent image quality assessment, the standard of care images (pancreatic parenchymal and venous phases) were rated Good (3) or Excellent (4) in 56/57 patients (mean = 3.7). One examination was rated as Poor (2) but still considered diagnostic. The mean contrast dose was 142 ml (range 115–180 ml) and the mean injection rate was 4.6 ml/s. Mean CA 19-9 value was 3644 U/ml (range < 1 to 64637) and there was no significant correlation between any perfusion parameters and CA 19-9 values. Perfusion parameters for the tumor and uninvolved pancreas are reported in Table 2 and Figs. 3 and 4. Mean BF, BV and PS was significantly lower in tumor compared to uninvolved pancreas (p < 0.001). Estimated radiation doses for the perfusion, arterial, and venous phases are shown in Table 3.
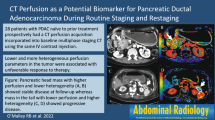
73-Year-old female with stage IV pancreatic head adenocarcinoma with metastases to the liver. a Freehand ROI encompassing the entire primary tumor on contrast-enhanced CT (mean attenuation = 75 HU). Color maps of functional parameters showing: b Average blood volume of tumor = 2.9 ml/100 g. c Average blood flow of tumor = 16.58 ml/100 g/min. d Average permeability surface area product of tumor = 13.6 ml/100 g/min
67-Year-old female with resectable pancreatic head adenocarcinoma who underwent pancreaticoduodenectomy with R0 resection. No evidence of recurrent or metastatic disease 11 months postoperatively. a Freehand ROI encompassing the entire primary tumor on contrast-enhanced CT (mean attenuation = 47 HU). Color maps of functional parameters showing: b Average blood volume of tumor = 3.4 ml/100 g. c Average blood flow of tumor = 21.53 ml/100 g/min. d Average permeability surface area product of tumor = 3.8 ml/100 g/min
Comparisons of our perfusion data (for both the tumor and uninvolved pancreas) to existing literature are shown in Table 4 and Fig. 5. Most of the technical parameters varied with all the comparisons performing a separate scan and separate injection, using different scanner models and manufacturers, timing and number of perfusion acquisitions. The patient populations also differ among all the publications, including the initial staging, types of treatment regimens, and whether patients ultimately underwent surgical resection.
Discussion
Our approach demonstrates that performing a perfusion acquisition is feasible during standard multiphase pancreatic CT using a single contrast injection. By combining perfusion CT with standard of care CT, our approach adds no time or cost to the patient, while simultaneously acquiring quantitative data and standard staging and restaging information. Moreover, using a wide-coverage 256-slice scanner mitigates many historical limitations, allowing for rapid whole organ coverage with no significant motion artifact.
Existing literature for PDAC perfusion CT includes a wide variety of technology, including scanners manufactured by Siemens, GE, Toshiba, and Philips and models ranging from 64-to 640-slice. Most importantly, all were performed separately from the standard of care CT, requiring a separate scan and IV contrast injection from the standard of care examination. Despite the technical differences and heterogeneous patient population, our perfusion parameters were comparable to what has been reported elsewhere. Notably, IV contrast injection rate for perfusion CT has historically been much higher (> 5 ml/s) than what is used for routine examinations, but our results suggest that standard weight-based injection doses and rates can be used without affecting the perfusion parameters. In phantom studies at our institution, we found no significant differences between the perfusion parameters at various injection rates. These are preliminary observations in a small number of patients, but routinely acquiring perfusion parameters in patients with PDAC offers added quantitative information with little downside. The added radiation from the perfusion sequence is the only notable consideration, but of minimal significance in this patient population with an aggressive malignancy and limited life expectancy, many of whom will receive treatment radiation doses. Of the existing literature using pancreas perfusion CT, only a few specifically report radiation dose and ours is the only study reporting SSDE. Effective dose is most often reported and our mean of 10.2 mSv is within the range of 3.60–23.37 mSv that has been reported elsewhere. Most importantly, our radiation doses were within the range predicted by our medical physicists when designing the protocol and we had no outlier scans with unexpectedly higher doses. Although radiation exposure is not a major concern for PDAC patients, this consistency and predictability is important for implementing similar perfusion protocols in other oncology patients.
Our results show that BF and BV in the tumor are very low compared to uninvolved pancreas and even lower in the center compared to the rim, which is thought to reflect the fibrosis and extracellular matrix deposition seen with PDAC [12]. Specifically, the perfusion parameters are lowest in the center of the tumor with progressively increasing perfusion when moving radially to the tumor, rim, immediately adjacent pancreas, and distant pancreas, which is similar to what has been reported elsewhere [16]. In fact, the altered perfusion in immediately adjacent “normal” pancreas is often attributed to microscopic tumor involvement that is occult by conventional CT images [17]. Hamdy et al. recently showed patients with higher baseline BF were more likely to respond to neoadjuvant CRT, suggesting worse tumor perfusion reflects a rigid extracellular matrix and impaired delivery of systemic anticancer therapy. We focused on BF, BV, and PS as they have shown the most promise in prior studies, of which BF has been reported to be the single most important parameter of tissue perfusion [18]. There is no consensus regarding PS in PDAC and the variability in our results and in literature comparisons often occur due to differences regarding delayed phase acquisition [18]. Reliable PS values typically require delayed imaging (> 40 s) allowing for contrast extravasation in the intravascular compartment. Since our protocol was designed as an add-on to the standard of care CT, we chose not to acquire additional delayed phases that would have added to the scan time. Our perfusion parameters for uninvolved pancreas were also comparable to what has been reported elsewhere, but we acknowledge that pancreatic parenchyma without any visible tumor may not be ‘normal’ and it is difficult to know what the expected perfusion parameters should be.
One of the barriers limiting widespread adoption has been lack of reference values and technical standards. It is difficult, if not impossible, to directly compare perfusion values across different scanners, mathematical models, and software used. By demonstrating the feasibility of a perfusion protocol combined with routine imaging, more standardized protocols can be utilized wherein reference standards can at least be validated among similar technology and calculation methods. Compartmental and deconvolution analysis are the most widely used kinetic models and there is still no consensus regarding which is most applicable for abdominal oncology applications, nor whether the parameters are directly interchangeable [19]. It has been suggested the deconvolution method can tolerate greater image noise and is therefore particularly well suited to measuring lower levels of perfusion, such as is seen with PDAC at baseline and after therapy [20]. Kaufmann et al. retrospectively reviewed patients undergoing perfusion CT for liver response and compared perfusion parameters in the normal pancreas for both calculation methods using three different perfusion CT (scans up to 3 months apart). They found 30% variability in the range of BF measurements irrespective of the calculation method but suggested that the deconvolution method was more robust with acceptable deviation at follow-up [21]. Accordingly, any attempts to perform perfusion CT must clearly and consistently report their technical specifications and calculation methods. Establishing standards for quantification methods is essential for ensuring reliability and comparability of the perfusion data.
Notably, our parameters were below cutoff values established using perfusion CT and deconvolution analysis to differentiate PDAC from mass-forming chronic pancreatitis (MFCP) [13]. Aslan et al. found cutoff values of BV 7.60 ml/100 g, BF 64.43 ml/100 ml/min, and PS 28.08 ml/100 ml/min, below which provided 100% sensitivity and specificity for distinguishing PDAC from MFCP [13]. Yadav et al. similarly used perfusion CT to distinguish PDAC from MFCP, but used Patlak analysis, and reported cutoff values of 5 ml/100 ml for BV (92.3% sensitivity and 67.9% specificity), 19.1 ml/100 ml/min for BF (100% sensitivity and 73.8% specificity), and 12.7 ml/100 ml/min for PS (84.6% sensitivity and 69.1% specificity), which highlights the importance of only comparing perfusion parameters derived from similar calculation methods.
Prior studies have shown that RECIST and other metrics using serial tumor size change are unreliable for PDAC, especially after neoadjuvant therapy. Hence, it is often very challenging to determine if PDAC patients are responding favorably and if/when borderline locally advanced tumors should undergo resection. Routinely acquiring perfusion parameters at baseline and during therapy could provide more specific tumor characteristics and response assessment, including predictive features at baseline, assess ongoing response to therapy and distinguish viable from nonviable tumor after neoadjuvant therapy. Our ongoing work seeks to derive meaningful parameters before and during therapy that can be used to guide management and correlate with survival.
Other potential uses for combining perfusion CT with a routine CT protocol could include troubleshooting when the diagnosis of PDAC is uncertain, such as cases of MFCP vs. PDAC, unexplained pancreatitis, or in cases of unexplained pancreatic duct obstruction [13, 18]. Color perfusion maps have also been shown to be useful at detecting PDACs that are isoenhancing compared to pancreatic parenchyma and not visible on the native CT images [18].
This study has several important limitations. First, our sample size was limited to 57 patients with PDAC at various stages of disease and management. As such, we focused entirely on protocol feasibility and did not attempt to correlate with outcomes. A larger series with a more homogenous population would be required to assess treatment response and correlate with outcomes. Second, this protocol was performed on a wide-detector scanner from only one vendor, which represents our clinical workflow, but the results may not translate to other scanner types or scan parameters. Moreover, the perfusion protocol on this scanner could not accommodate patients with body mass index greater than 40 kg/m2, which limits the relevance for such patients. Third, the data processing used specific software utilizing the deconvolution algorithm and the results are likely not interchangeable with other kinetic models. Fourth, a single reader performed all of the perfusion ROIs, which limits the reproducibility of the findings beyond our practice. Future studies with multiple readers are necessary to ensure this process can be repeated and has broader relevance. Lastly, generating and analyzing perfusion parameters requires software separate from the clinical PACS, which adds time for the radiologist and is a consideration if perfusion acquisitions are performed more routinely.
Conclusions
In conclusion, it is feasible to perform a perfusion CT acquisition with standard of care CT scans using a single contrast injection and without detrimentally affecting the standard pancreatic and venous phases. Using the latest CT technology mitigates historical concerns regarding respiratory motion, image noise, and added radiation dose, such that perfusion CT can be considered as an add-on for patients with PDAC with very little downside. The tumoral perfusion parameters in our cohort are similar to what has been reported previously using the deconvolution method, in spite of other technical differences. However, reliable comparison can only be made among perfusion parameters calculated using the same kinetic model (Patlak/maximum slope or deconvolution). By acquiring this information as part of a single routine scan, we hope to be able to provide more specific details regarding individual tumors and response assessment.
References
Nelson DW, Chang S-C, Grunkemeier G, et al (2018) Resectable Distal Pancreas Cancer: Time to Reconsider the Role of Upfront Surgery. Ann Surg Oncol 25:4012–4019.
Zhong J, Switchenko J, Behera M, et al (2018) Chemotherapy with or Without Definitive Radiation Therapy in Inoperable Pancreatic Cancer. Ann Surg Oncol 25:1026–1033.
Cancer Stat Facts: Pancreatic Cancer [SEER Cancer Stat Facts Web site]. Available at: http://seer.cancer.gov/statfacts/html/pancreas.html. Accessed June 10, 2020.
Luberice K, Downs D, Sadowitz B, et al (2017) Has survival improved following resection for pancreatic adenocarcinoma? Am J Surg 214:341–346.
Cassinotto C, Mouries A, Lafourcade J-P, et al (2014) Locally advanced pancreatic adenocarcinoma: reassessment of response with CT after neoadjuvant chemotherapy and radiation therapy. Radiology 273:108–116.
Ferrone CR, Marchegiani G, Hong TS, et al (2015) Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 261:12–17.
Katz MHG, Fleming JB, Bhosale P, et al (2012) Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 118:5749–5756.
Klotz E, Haberland U, Glatting G, et al (2015) Technical prerequisites and imaging protocols for CT perfusion imaging in oncology. Eur J Radiol 84:2359-2367.
Prezzi D, Khan A, Goh V (2015) Perfusion CT imaging of treatment response in oncology. Eur J Radiol 84:2380–2385.
Fukukura Y, Takumi K, Higashi M, et al (2014) Contrast-enhanced CT and diffusion-weighted MR imaging: Performance as a prognostic factor in patients with pancreatic ductal adenocarcinoma. Eur J Radiol 83:612–619.
D’Onofrio M, Gallotti A, Mantovani W, et al (2013) Perfusion CT can predict tumoral grading of pancreatic adenocarcinoma. Eur J Radiol 82:227–233.
Hamdy A, Ichikawa Y, Toyomasu Y, et al (2019) Perfusion CT to Assess Response to Neoadjuvant Chemotherapy and Radiation Therapy in Pancreatic Ductal Adenocarcinoma: Initial Experience. Radiology 292:628-635.
Aslan S, Nural MS, Camlidag I, et al (2019) Efficacy of perfusion CT in differentiating of pancreatic ductal adenocarcinoma from mass-forming chronic pancreatitis and characterization of isoattenuating pancreatic lesions. Abdom Radiol 44:593–603.
AAPM Task Group 204. AAPM Report No. 204 Size-Specific Dose Estimates (SSDE) in Pediatric and Adult Body CT Examinations. 2011. Available at: https://www.aapm.org/pubs/reports/rpt_204.pdf. Accessed June 10, 2020.
AAPM Task Group 23. AAPM Report No. 96 The Measurement, Reporting, and Management of Radiation Dose in CT. 2008. Available at: https://www.aapm.org/pubs/reports/RPT_96.pdf. Accessed June 10, 2020.
Delrue L, Blanckaert P, Mertens D, et al (2011) Assessment of Tumor Vascularization in Pancreatic Adenocarcinoma Using 128-Slice Perfusion Computed Tomography Imaging. J Comput Assist Tomogr 35:434–438.
Xu J, Liang Z, Hao S, et al (2009) Pancreatic adenocarcinoma: dynamic 64-slice helical CT with perfusion imaging. Abdom Imaging 34:759–766.
Yadav AK, Sharma R, Kandasamy D, et al (2016) Perfusion CT – Can it resolve the pancreatic carcinoma versus mass forming chronic pancreatitis conundrum? Pancreatology 16:979–987.
Schneeweiß S, Horger M, Grözinger A, et al (2016) CT-perfusion measurements in pancreatic carcinoma with different kinetic models: Is there a chance for tumour grading based on functional parameters? Cancer Imaging 16:43.
Miles KA (2003) Perfusion CT for the assessment of tumour vascularity: which protocol? Br J Radiol 76:S36–S42.
Kaufmann S, Schulze M, Horger T, et al (2015) Reproducibility of VPCT Parameters in the Normal Pancreas. Acad Radiol 22:1099–1105.
Bao J, Liu A, Zhao C, et al (2019) Correlation between dual-energy computed tomography single scan and computed tomography perfusion for pancreatic cancer patients: initial experience. J Comput Assist Tomogr 43:599–604. https://doi.org/10.1097/RCT.0000000000000878.
Xie Q, Wu J, Tang Y, et al (2013) Whole-organ CT perfusion of the pancreas: impact of iterative reconstruction on image quality, perfusion parameters and radiation dose in 256-slice CT-preliminary findings. PloS One 8:e80468. https://doi.org/10.1371/journal.pone.0080468.
Funding
This research was supported by GE Healthcare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All author declare that they have no conflict of interest.
Consent to participate
All subjects signed written informed consent before participating.
Ethical approval
IRB approval was obtained for this prospective research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
O’Malley, R.B., Soloff, E.V., Coveler, A.L. et al. Feasibility of wide detector CT perfusion imaging performed during routine staging and restaging of pancreatic ductal adenocarcinoma. Abdom Radiol 46, 1992–2002 (2021). https://doi.org/10.1007/s00261-020-02786-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02786-y