Abstract
The question referred to in the title of this article is a relatively common situation when performing prostate MRI in some healthcare settings. Moreover, the answer is not always straightforward. The decisions on type of receiver coil for prostate MRI and whether or not an endorectal coil (ERC) should be used is based on several factors. These relate to the patient (e.g., body habitus, presence of metallic devices in the pelvis), the focus of the exam (diagnosis, staging, recurrence), and characteristics of the MRI system (e.g., magnetic field strength and hardware components including coil design and number of elements/channels available in the surface coil). Historically, the combined use of an ERC and a surface coil was the optimal combination for maximizing the signal-to-noise ratio (SNR), particularly for low-strength magnetic fields (1.5T). However, there are several disadvantages associated with the use of an ERC, and several studies have advocated equivalent clinical performance of modern MRI systems for diagnosis and staging of prostate cancer (PCa), either with ERC or surface alone. Accordingly, there is a wide variation in the precise imaging technique across institutions. This article focuses on the most relevant aspects of the decision of whether to use an ERC for PCa MR imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance imaging (MRI) of the prostate has become an indispensable tool for diagnosing, staging, or assessing the aggressiveness of a histologically confirmed prostate cancer (PCa) [1]. For best performance, in this approach, the MRI should be done under high quality standards [2].
The use of an endorectal coil for prostate imaging is primarily historical. The basic assumed rationale for its use is the theoretical improvement of image quality particularly for older and low magnetic field systems. Moreover, the literature has strong opinions both ways, as ERC use has well-known advantages and limitations [3,4,5,6,7]. The major factor put forward to support its use is the improved quality of images, by enhancing image resolution and SNR [8]. However, the disadvantages are also relevant and include patient discomfort, reduced workflow efficiency, longer acquisition time due to coil positioning, and coil-related artifacts (including some prostate gland deformation, distortion, or both) [9, 10].
The decision to use an ERC is multifactorial and closely related to another capital issue in prostate imaging: whether to use 1.5 or 3.0 T systems [11]. Regarding this question, two statements are commonly emphasized: (1) If a 3T system were available, then it should be favored for prostate imaging (although it is recognized that newer and more advanced 1.5T scanners may sometimes produce better quality images than some older 3T systems); and (2) Prostate MRI should not be performed on MRI systems under 1.5T [12].
Although minimal standards for prostate MR imaging have been published by multiple entities, there is no explicit recommendation for receiver coil, whether endorectal or surface (pelvic phased array) [13]. The PI-RADS Committee is cautious when assessing this issue, and on version 2.1, in order to perform state-of-art prostate MRI, both receiver coils options are admitted, but members of Committee favor the surface only approach. Regarding the scanners’ magnet strength, 3T is preferable with the surface coil and the use of an endorectal coil for low-field magnets is not formally indicated, assuming good images can be achieved with the modern 1.5T systems, along with a multichannel receiver surface coil. However, most authors actually favor the use of 3T equipment, if available, or the use of an endorectal coil when using older generation 3T or 1.5T systems [13, 14]. In fact, the PI-RADS committee reaffirms that, for some 1.5T systems, the use of an ERC is preferable [13].
Once the coil approach has been defined, it is important to define the exam protocol, as adjustments in sequence parameters might be required, especially in FOV and Matrix size.
Image quality—ERC vs non-ERC approach
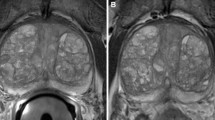
The first report of the use of an endorectal coil for prostate imaging dated from 1989, by Martin et al. [15]. First, ERC was used alone, yielding images with limited FOV and that were difficult to adjust as the signal was not uniform (being very high close to the coil and low far from the rectal wall). The combination of an ERC with a surface coil was a major advance in prostate MR image quality [16] and, currently, there is consensus that this approach does enhance image resolution (Fig. 1), regardless of the MRI system used, either 1.5 or 3.0T, by improving the SNR [17]. This improved SNR yields greater spatial resolution, which can in turn aid the assessment of crucial structures, such as the prostatic capsule and the neurovascular bundles [18]. However, the use of ERC is also associated with technical issues, such as near-field artifact and more frequent generation of susceptibility artifacts, especially for diffusion-weighted (DW) images [9], and even distortion of the gland (Fig. 2).
Image quality with and without ERC. a Axial T2-w image showing an ill-defined, intermediate signal lesion (ISUP 3 at histopathology) at left, anterior transition zone (asterisk); b and c are DWI images without and with ERC acquired in a 1.5 T scanner at the same time; and d and e are the corresponding ADC maps without and with ERC. The SNR is clearly superior in ERC images and besides the index lesion (*), a secondary lesion (arrow) is better defined in the set of images with ERC
Therefore, a crucial point here is whether this improved SNR impacts clinical results or, in other words, if more lesions are seen with the use of an ERC. On the way to addressing this essential question, in the last decade, the gap in quality between images with and without ERC has narrowed, driven by the advent of modern pelvic phased array coils, along with improvements in gradient systems, in such a way that some recent studies suggested this difference no longer has clinical significance [19].
Here, it is important to consider technical issues, as the performance of surface coils shows wide variability between vendors, depending on their design, the number of elements and channels, coil efficiency, and other technical parameters that ultimately define the image quality of the system [20, 21].
Other factors should also be considered when the focus is to optimize image quality, including patients’ characteristics. For instance, it is clear that a patient’s size affects image quality, so for those with high body mass index (BMI) or large body habitus, the use of ERC could be considered to maintain high quality standards [22]. Another important clinical situation is the presence of metallic devices within the pelvic field of view, producing susceptibility artifacts (Fig. 3); in this case, the most common are hip implants. For those, a 1.5T could be the best option as both 3T scanners and ERC are more prone to generate susceptibility artifacts [13]. Of importance, the newer DWI techniques including reduced field of view acquisitions have the potential to reduce susceptibility artifacts [23], yielding higher quality images compared to conventional DWI sequences (Fig. 4).
a and b Images from a patient with a hip implant acquired in T2-w (a) and DWI-ADC map (b) in a 3T system. The severity of susceptibility magnetic artifacts (asterisk in both images) is greater in the high-field equipment and is worse in DW images, where the prostate can barely be seen, compared to T2-w, where the prostate is well depicted (white arrows in both images). The Echo-Planar sequences, common in DWI, are prone to these artifacts
Comparison in different clinical settings
One of the major factors in deciding whether to use (or not use) an ERC is the indication for prostate MR imaging [24]. For diagnosis, one of the first studies comparing prostate cancer detection rate with and without the use of an ERC was by Heijmink et al. in 2007 [3], indicating better results with the combined use of ERC and surface coils. However, more recent studies favored the opposite (i.e., no significant difference in detection rates between systems with and without ERC) [5,6,7, 19, 25, 26], except for the study of Costa et al. [8] which indicated a better sensitivity when an ERC was added. In the most recent study focusing on this question, Mirak et al. [19] indicated that both approaches showed similar detection of overall and index prostate cancer. The index tumor detection rates for the endorectal coil and non-endorectal coil groups were 78.5% and 76.3%, respectively. However, of interest, for posterior and peripheral prostate cancers, the endorectal coil group had a significantly higher detection rate, while for the anterior and transition zone PCa, the same group showed a lower detection rate. Also, the meta-analysis of Shaish et al. [27] reported similar findings, i.e., no significant difference for index lesions, when assessing tumor grading based on quantitative ADC: the pooled sensitivity was 83% for the ERC group and 74% for the non-ERC group (p = 0.30) (pooled specificities of 71% and 80%, respectively) (p = 0.16).
For the second major indication—prostate cancer staging—the findings are similar. Heijmink et al. [3] and Futterer et al. [4], both in 2007, also indicated better performance for staging PCa using the ERC approach compared to non-ERC option. However, with improvements to MRI systems, recent studies [19, 28,29,30,31] have consistently indicated no significant difference between the two approaches. The recent meta-analysis of Tirumani et al. [32], designed to compare the performance for staging T3 lesions (or lesions with local extraprostatic involvement) between ERC versus non-ERC approaches, indicated no significant difference, with an AUC of 0.741 for the ERC group and 0.711 for the latter approach. Similar results had been reported in a previous meta-analysis of De Rooji et al. in 2016 [33]. Also, an important common point in these two meta-analyses was the limited sensitivity, in general, of MRI for assessing PCa staging. As an attenuating factor favoring the use of MRI, both meta-analyses included studies performed with old systems, for instance, from 1994 [34], which has undoubtedly impacted these results.
A third specific situation relevant to the use of ERC is for performing MR spectroscopic imaging (MRSI). Although, not currently included in the majority of clinical protocols, MRSI [mainly proton based (H+)] is used mostly for research purposes [35, 36]. In this particular situation, the SNR is even more fundamental than in conventional imaging and, although some clinical studies have shown that this difference is non-significant for 3T systems [37], clinical and experimental studies have demonstrated that improved SNR with ERC is essential for optimizing spectral data in MRSI, particularly when using a 1.5 system [38,39,40]. Table 1 summarizes the main studies in this topic.
Patient preparation
The decision to use ERC or surface coils affects the patient preparation for undergoing a prostate MRI, regardless of the indication. If a surface coil is used alone, some studies have advocated bowel preparation prior to prostate MRI [41, 42], although there is no clear consensus on this [43]. The distended rectum is associated with increased DWI distortion and consequently impaired DWI image quality due to susceptibility artifacts [42]. Generally, DWI involves echo-planar imaging (EPI), a sequence historically known for its susceptibility artifacts that can be greater at 3T magnets and around air-soft tissue interfaces, as the rectum/posterior aspect of prostate (Fig. 5), which is troublesome as the majority of prostate cancers arise around this location [44]. In addition to degrading DWI image quality, rectum distention also affects T2 images. The overdistended rectum might increase motion artifacts on T2-weighted images, as demonstrated by Padhani et al. [45].
a This T2-w, sagittal image, acquired in a 3T system with a surface receiver coil only, demonstrates an overdistended rectal ampullae (R). b Axial DWI (b = 1400 s/mm2) image is heterogenous and the visualization of left lobe (*) is impaired due to susceptibility artifacts; c In the corresponding ADC map, from the same patient, a curved white stripe is seen, at left (arrows), also derived from susceptibility artifacts
Some approaches have been described for bowel preparation prior to prostate MRI performed only with surface coil. Some of these are less invasive, including switching patients to a prone position to induce air displacement for a non-dependent position, away from the prostate-rectum interface, or even asking patients to defecate prior to MRI examination [46]. Another option is to use a rectal enema. Griethuysen et al. [42] described an important reduction of both incidence and severity of the susceptibility artifacts in patients who self-administered a bowel enema immediately before an MRI exam in a 1.5 T scanner. In their study, clinically relevant gas artifacts were over six times more common in patients without bowel cleansing compared to those who have used it. However, although some pieces of evidence favor the use of bowel preparation when ERC is not used, there is no specific recommendation for that in PI-RADS v2.1 [14].
On the other hand, if the choice is the ERC approach, an important issue is how to fill and correctly position the coil. Filling with air was the first option described in the literature. However, several studies have shown that perfluorocarbon or barium sulfate might significantly reduce susceptibility artifacts, which can be very helpful for DWI images and MRSI [47, 48].
When used, correct positioning of ERC is essential for optimizing image quality (Fig. 6). The coil should be inflated with 60–80 ml of air, perfluorocarbon, or barium sulfate and the receiver’s face of the coil placed towards the anterior rectal wall/posterior prostate, in such a way to ensure coverage of the whole prostate. The correct placement can be assessed on a sagittal scout image. Scout images are also useful for verifying that there is no excessive tilt of the coil (more than 20o) relative to the prostate in the axial plane [39, 49].
Endorectal coil positioning. a T2-w sagittal view showing the distended endorectal coil adequately positioned. The whole prostate (red lines) lies within the limits of the coil; b Axial T2-w image. The receiver face of the coil where detectors can be inferred by the more intense signal in the anterior face (arrows) towards to anterior rectal wall and peripheral zone of the prostate
Conclusion
The decision of whether to use an ERC is multifactorial. Users should make this decision not only based on a complete knowledge of the MRI system and type of surface coil available, but also on essential patient information, such as the indication for the exam, body habitus, and presence of metallic artifacts in the pelvis. Although, some degree of improvement in image quality is achieved with the use of an ERC, modern MRI systems provide high-quality images using only a surface, phased array coil. Also, important, this choice should be followed by specific details on patient preparation, either for using surface coil solely or for the combined use, with an ERC, as well as a dedicated protocol for optimizing imaging in both circumstances.
References
Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: a review. JAMA 2017;317:2532e42. https://doi.org/10.1001/jama.2017.7248.
Panebianco V, Valerio MC, Giuliani A et al. Clinical Utility of Multiparametric Magnetic Resonance Imaging as the First-line Tool for Men with High Clinical Suspicion of Prostate Cancer. Eur Urol Oncol. 2018;1(3):208-214. doi: 10.1016/j.euo.2018.03.008
Heijmink SW, Futterer JJ, Hambrock T, et al. Prostate cancer: body- array versus endorectal coil MR imaging at 3T comparison of image quality, localization, and staging performance. Radiology 2007;244:184e95.
Futterer JJ, Engelbrecht MR, Jager GJ, et al. Prostate cancer: comparison of local staging accuracy of pelvic phased-array coil alone versus integrated endorectal-pelvic phased-array coils. Local staging accuracy of prostate cancer using endorectal coil MR imaging. Eur Radiol 2007;17:1055e65.
Turkbey B, Merino MJ, Gallardo EC, et al. Comparison of endorectal coil and non-endorectal coil T2W and DW MRI at 3T for localizing prostate cancer: correlation with whole-mount histopathology. J Magn Reson Imaging 2014;39:1443e8. https://doi.org/10.1002/jmri.24317.
Shah ZK, Elias SN, Abaza R, et al. Performance comparison of 1.5-T endorectal coil MRI with 3.0-T non endorectal coil MRI in patients with prostate cancer. Acad Radiol 2015;22:467e74.
Barth BK, Rupp NJ, Cornelius et al. A Diagnostic Accuracy of a MR Protocol Acquired with and without Endorectal Coil for Detection of Prostate Cancer: A Multicenter Study. Curr Urol. 2019;12(2):88-96. https://doi.org/10.1159/000489425.
Costa DN, Yuan Q, Xi Y et al. Comparison of prostate cancer detection at 3-T MRI with and without an endorectal coil: A prospective, paired-patient study. Urol Oncol. 2016;34(6):255.e7-255.e13. doi: 10.1016/j.urolonc.2016.02.009.
Barth BK, Cornelius A, Nanz D, et al. Comparison of image quality and patient discomfort in prostate MRI: pelvic phased array coil vs. an endorectal coil. Abdom Radiol 2016;41(11):2218e26.
Stocker D, Manoliu A, Becker AS, et al. Image quality and geometric distortion of modern diffusion-weighted imaging sequences in magnetic resonance imaging of the prostate. Invest Radiol. 2018;53:200–206. doi: 10.1097/RLI.0000000000000429
Stabile A, Giganti F, Emberton M, Moore CM. MRI in prostate cancer diagnosis: do we need to add standard sampling? a review of the last 5 years. Prostate Cancer Prostatic Dis 2018;21(4):473–487
Padhani AR, Barentsz J, Villeirs G et al. PI-RADS Steering Committee: The PI-RADS Multiparametric MRI and MRI-directed Biopsy Pathway. Radiology. 2019 Aug;292(2):464-474. doi: 10.1148/radiol.2019182946.
Weinreb JC, Barentsz JO, Choyke PL et al. PI-RADS Prostate Imaging Reporting and Data System: 2015, version 2. Eur Urol 2016;69(1):16–40
Turkbey B, Rosenkrantz AB, Haider MA et al. Prostate Imaging Reporting and Data System version 2.1: 2019 update of Prostate Imaging Reporting and Data System version 2. Eur Urol 2019, 76:340-351. doi:10.1016/j.eururo.2019.02.033.
Martin JF, Hajek P. Baker L, Gylys-Morin V. Fitzmonnis-Glass R, Mattrey RR. Inflatable surface coil for MR imaging of the prostate. Radiology 1988; 167:268-270.
Bloch BN, Rofsky NM, Baroni RH, Marquis RP, Pedrosa I, Lenkinski RE. 3 Tesla magnetic resonance imaging of the prostate with combined pelvic phased-array and endorectal coils; Initial experience. Acad. Radiol. 2004; 11:863–867. DOI: 10.1016/j.acra.2004.04.017.
Ullrich T, Quentin M, Oelers C et al. Magnetic resonance imaging of the prostate at 1.5 versus 3.0T: A prospective comparison study of image quality. Eur J Radiol. 2017 May;90:192-197. https://doi.org/10.1016/j.ejrad.2017.02.044
Husband JE, Padhani AR, MacVicar AD, Revell P. Magnetic resonance imaging of prostate cancer: comparison of image quality using endorectal and pelvic phased array coils. Clin Radiol, 1998; 53: 673-681.
Mirak SA, Shakeri S, Bajgiran AM, et al. Three Tesla Multiparametric Magnetic Resonance Imaging: Comparison of Performance with and without Endorectal Coil for Prostate Cancer Detection, PI-RADS™ version 2 Category and Staging with Whole Mount Histopathology Correlation. J Urol. 2019;201(3):496-502. doi: 10.1016/j.juro.2018.09.054.
Gelber ND, Ragland RL, Knorr JR. Surface coil MR imaging: utility of image intensity correction filter. Am J Roentgenol. 1994;162(3):695–7. doi:10.2214/ajr.162.3.8109524.
Golshan HM, Hasanzadeh RP, Yousefzadeh SC. An MRI denoising method using image data redundancy and local SNR estimation. Magn Reson Imaging. 2013;31(7):1206–1217.
Carucci LR. Imaging obese patients: problems and solutions. Abdom Imaging 2013, 38:630–646. DOI: 10.1007/s00261-012-9959-2
Warndahl BA, Borisch EA, Kawashima A, Riederer SJ, Froemming AT. Conventional vs. reduced field of view diffusion weighted imaging of the prostate: Comparison of image quality, correlation with histology, and inter-reader agreement. Magn Reson Imaging. 2018;47:67-76. https://doi.org/10.1016/j.mri.2017.10.01
Engels RM, Israel B, Padhani AR, Barentsz J. Multiparametric Magnetic Resonance Imaging for the Detection of Clinically Significant Prostate Cancer: What Urologists Need to Know. Part 1: Acquisition. European Urology ahead of print. https://doi.org/10.1016/j.eururo.2019.09.021
Baur AD, Daqqaq T, Wagner M, et al. T2- and diffusion-weighted magnetic resonance imaging at 3T for the detection of prostate cancer with and without endorectal coil: An intraindividual comparison of image quality and diagnostic performance. Eur J Radiol, 2016; 85: 1075-1084.
Gawlitza J, Reiss-Zimmermann M, Thörmer G et al. Impact of the use of an endorectal coil for 3 T prostate MRI on image quality and cancer detection rate. Sci Rep. 2017 1;7:40640. doi: 10.1038/srep40640.
Shaish H, Kang SK, Rosenkrantz AB. The utility of quantitative ADC values for differentiating high-risk from low-risk prostate cancer: a systematic review and meta-analysis. Abdom Radiol 2017, 42:260–270 DOI: 10.1007/s00261-016-0848-y
Torricelli P, Cinquantini F, Ligabue G, et al. Comparative evaluation between external phased array coil at 3 T and an endorectal coil at 1.5 T: preliminary results. J Comp Assist Tomogr 2006;30(3):355e61.
Kim BS, Kim TH, Kwon TG, et al. Comparison of pelvic phased-array versus endorectal coil magnetic resonance imaging at 3 Tesla for local staging of prostate cancer. Yonsei Med J. 2011; 53:550–556. doi: 10.3349/ymj.2012.53.3.550.
Lee SH, Park KK, Choi KH, et al. Is endorectal coil necessary for the staging of clinically localized prostate cancer? Comparison of non-endorectal versus endorectal MR imaging. World J Urol. 2010; 28(6):667-72. doi: 10.1007/s00345-010-0579-6
Pooli A, Isharwal S, Cook G, Oliveto JM, LaGrange CA. Does endorectal coil MRI increase the accuracy of preoperative prostate cancer staging? Can J Urol. 2016; 23(6):8564-8567.
Tirumani SH, Suh CH, Kim KW, Shinagare AB, Ramaiya NH, Fennessy FM.Head-to-head comparison of prostate MRI using an endorectal coil versus a non-endorectal coil: meta-analysis of diagnostic performance in staging T3 prostate cancer. Clin Radiol. 2019 9: S0009-9260(19)30590-2. https://doi.org/10.1016/j.crad.2019.09.142.
de Rooij M, Hamoen EH, Witjes JA, Barentsz JO, Rovers MM. Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur Urol. 2016;70(2):233-45. doi: 10.1016/j.eururo.2015.07.029.
Tempany CM, Zhou X, Zerhouni EA, et al. Staging of prostate cancer: results of Radiology Diagnostic Oncology Group project comparison of three MR imaging techniques. Radiology 1994;192(1):47e54.
Payne GS. Clinical applications of in vivo magnetic resonance spectroscopy in oncology. Phys Med Biol. 2018 26;63(21):21TR02. https://doi.org/10.1088/1361-6560/aae61e
Mazaheri Y, Shukla-Dave A, Goldman DA. Characterization of prostate cancer with MR spectroscopic imaging and diffusion-weighted imaging at 3 Tesla. Magn Reson Imaging. 2019;55:93-102. doi: 10.1016/j.mri.2018.08.025.
Ma C, Chen L, Scheenen TW, Lu J, Wang J.Three-dimensional proton magnetic resonance spectroscopic imaging with and without an endorectal coil: a prostate phantom study. Acta Radiol. 2015;56(11):1342-9. doi: 10.1177/0284185114556704.
Hoffner MK, Huebner F, Scholtz JE et al. Impact of an endorectal coil for 1H-magnetic resonance spectroscopy of the prostate at 3.0T in comparison to 1.5T: Do we need an endorectal coil? Eur J Radiol. 2016; 85(8):1432-8. https://doi.org/10.1016/j.ejrad.2016.05.019
Yakar D, Heijmink SW, Hulsbergen-van de Kaa CA et al. Initial results of 3-dimensional 1H-magnetic resonance spectroscopic imaging in the localization of prostate cancer at 3 Tesla: should we use an endorectal coil? Invest Radiol. 2011; 46(5):301-6. https://doi.org/10.1097/rli.0b013e3182007503.
Verma S, Rajesh A, Futterer J, et al. Prostate MRI and 3D MR Spectroscopy: How We Do It. Am J Roentgen. 2010;194:1414-1426. 10.2214/AJR.10.4312
Caglic I, Hansen NL, Slough RA, Patterson AJ, Barrett T. Evaluating the effect of rectal distension on prostate multiparametric MRI image quality. Eur J Radiol, 90 (2017), 174-180
van Griethuysen JJM, Bus EM, Hauptmann M, et al. Gas-induced susceptibility artefacts on diffusion-weighted MRI of the rectum at 1.5 T - effect of applying a micro-enema to improve image quality. Eur J Radiol 2018;99:131e7. https://doi.org/10.1016/j.ejrad.2017.12.020.
Lim C, Quon J, McInnes M, Shabana WM, El-Khodary M, Schieda N. Does a cleansing enema improve image quality of 3T surface coil multiparametric prostate MRI? J Magn Reson Imaging. 2015;42(3):689-97. https://doi.org/10.1002/jmri.24833.
Mazaheri Y, Vargas HA, Nyman G, Akin O, Hricak H. Image Artifacts on Prostate Diffusion-weighted Magnetic Resonance Imaging: Trade-offs at 1.5 Tesla and 3.0 Tesla. Acad Radiol. 2013; 20(8): 1041–1047. https://doi.org/10.1016/j.acra.2013.04.005
Padhani AR, Khoo VS, Suckling J, et al. Evaluating the effect of rectal distension and rectal movement on prostate gland position using cine MRI. Int J Radiat Oncol Biol Phys 1999;44:525e33. https://doi.org/10.1016/S0360-3016(99)00040-1
Caglic I, Barret T. Optimising prostate mpMRI: prepare for success. Clinical Radiology 74 (2019) 831e840. https://doi.org/10.1016/j.crad.2018.12.003.
Rosen Y, Bloch BN, Lenkinski RE, et al. 3 T MR of the prostate: reducing susceptibility gradients by inflating the endorectal coil with a barium sulfate suspension. Magn Reson Med. 2007;57:898–904. doi: 10.1002/mrm.21166.
Haesun Choi, Jingfei Ma. Use of Perfluorocarbon Compound in the Endorectal Coil to Improve MR Spectroscopy of the Prostate. American Journal of Roentgenology. 2008;190: 1055-1059. 10.2214/AJR.07.299.
Choi YJ, Kim JK, Kim N, Kim KW, Choi EK, Cho KS. Functional MR imaging of prostate cancer. Radiographics. 2007;27:63–75
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muglia, V.F., Vargas, H.A. Doctor, a patient is on the phone asking about the endorectal coil!. Abdom Radiol 45, 4003–4011 (2020). https://doi.org/10.1007/s00261-020-02528-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02528-0