Abstract
Definitive therapy for prostate cancer includes radical prostatectomy and radiation therapy. Treatment is elected based on patient preference, biological tumor factors, and underlying health. Post prostatectomy, men are surveyed for disease recurrence with serial PSA measurements, digital rectal exam, and imaging studies depending on nomogram predicted risk of local disease recurrence and distant metastasis. In men with rising PSA levels, pathologically incomplete surgical margins or, if symptoms of metastasis develop, imaging may be obtained to localize disease. In cases of known biochemical recurrence, imaging is used to target biopsy, to contour in salvage radiation therapy and to assess disease response. For local disease recurrence, the most commonly performed exams are pelvic MRI and transrectal US. CT can evaluate for lymph node metastasis, but is suboptimal in the evaluation of the prostatectomy bed. PET/CT and PET/MRI have been used successfully to evaluate for local disease recurrence. The PI-RADSv2.1 manual provides a risk level and lexicon for use in description of prostate carcinoma prior to prostatectomy, but does not address imaging features post-surgery. A detailed description of nodal, bony, and visceral metastasis is given elsewhere. This manuscript outlines the context in which appropriate imaging exams may be obtained and focuses on imaging findings concerning for local disease recurrence after prostatectomy on various imaging modalities including CT, US, MRI, and PET.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to NCCN guidelines, at diagnosis, patients with prostate cancer are risk stratified into very low, low, favorable/unfavorable intermediate, high or very high risk based on a combination of clinical, pathologic and imaging features [1]. In combination with patient preferences and life expectancy, treatment is then elected and consists of watchful waiting, active surveillance, external beam radiation therapy (EBRT), brachytherapy or radical prostatectomy with or without lymph node dissection [1]. Definitive therapy is defined as radical prostatectomy, brachytherapy and external beam radiation [1]. Radical prostatectomy is deemed appropriate in patients who have clinically localized disease that can be completely excised surgically, who have a life expectancy of greater than 10 years and who do not have a significant comorbidity that may complicate surgery [1]. Urinary incontinence, erectile dysfunction, blood loss and disease recurrence are complications which can be minimized by high-volume experienced surgeons and centers [1].
Following definitive therapy, patients are surveyed for biochemical recurrence with prostate specific antigen (PSA) levels. The frequency of imaging surveillance is informed based on individual risk, patient age, PSA doubling time (PSADT), Gleason score and patient health [1]. In patients who have undergone radical prostatectomy, the American Urological Association defines biochemical recurrence as a serum PSA of greater than or equal to 0.2 ng/ml which is confirmed with a second determination of PSA greater than or equal to 0.2 ng/ml [2]. In patients post-radiation therapy, the Phoenix criteria defines biochemical recurrence as PSA nadir + 2 ng/ml [3]. Biochemical recurrence post radical prostatectomy fall into three groups: [1] PSA levels that fail to fall to undetectable levels after radical prostatectomy (persistent disease), [2] those who achieve undetectable PSA after radical prostatectomy with a subsequent detectable PSA level on two or more measurements and [3] persistent but low PSA levels attributed to slow PSA metabolism or residual benign tissue [1]. Patients in groups 1 and 2 must be evaluated for distant metastasis. Patients in group 3 do not require evaluation until PSA increases [1]. In general, low and intermediate risk groups with low serum PSAs in the post-operative period have a very low risk of a positive bone or CT scan. In patients with biochemical recurrence, PSADT is calculated and used to risk stratify for local regional or distant metastasis to inform nomogram use and patient counseling. In addition, skeletal scintigraphy, CT chest, CT or MRI of the abdomen/pelvis, C-11 choline or F-18 fluciclovine PET/CT or PET/MR and prostate bed biopsy may be obtained to assess for distant metastasis [1]. These imaging modalities are used to re-stage as well as to guide biopsy and therapy decisions.
In order to describe local disease recurrence adequately, an understanding of the surgically relevant pre and post-operative anatomy of the lower male genitourinary tract and prostatectomy bed is important. The surgeon performing the prostatectomy will attempt to preserve urinary continence and sexual function while maximizing oncologic outcome. To do this, various surgical techniques have been described, including bladder neck sparing, neurovascular bundle sparing, preservation of urethral length and seminal vesicle sparing surgery [4,5,6,7,8]. To inform pre and post-operative risk assessment, radiologists should understand the location of neurovascular bundle between the prostate and the levator ani muscle laterally and posterior periprostatic/seminal vesicle fascia (Denonvilliers’ fascia; anterior to the rectum at the “retro prostatic angle”) as well as the distal urethral sphincter located at the level of the membranous urethra. The internal urethral sphincter, located at the base of the urinary bladder at the level of the urethral crest in the prostatic urethra is sacrificed during surgery. During prostatectomy, the vas deferens are transected and the bladder is unroofed from the prostate gland. Periprostatic fat, the prostate gland, prostatic urethra and seminal vesicles are most often excised en bloc. The bladder neck is anastomosed to the membranous urethra. Surgical techniques that spare the neurovascular bundle and membranous urethra (including the external sphincter) significantly improve patient quality of life by preserving urinary continence and sexual function. At MRI, normal post-surgical anatomy includes the “prostatectomy bed”—a general term that encompasses the periurethral anastomotic region at the base of the bladder and retro vesicular space that includes the prior region of the prostate and excised seminal vesicles. When disease recurs, it is most often at the margin of the surgically excised tissue in the periurethrovesicular anastomotic region, retrovesicular spaces, periurethral region, seminal vesicles, penile bulb [9] and vas deferens [10]. A depiction of the relevant surgical anatomy of the prostate and periprostatic tissues is shown in Fig. 1.
Diagram showing the key anatomic structures in the coronal and axial planes before and after prostatectomy. a Coronal pre-operative anatomy. b Axial pre-operative anatomy. c Coronal pre-operative anatomy showing resection margins. d Axial pre-operative anatomy showing resection margins. e Coronal post-operative anatomy. f Axial post-operative anatomy
The substance of this review is based on the National Comprehensive Cancer Network (NCCN) prostate cancer guidelines and specifically addresses pertinent information for radiologists regarding imaging modalities available to assess for local disease recurrence and its imaging findings in patients who have undergone radical prostatectomy [1]. A similar comprehensive document published by the European Association of Urology is available which outlines appropriate diagnostic criteria, treatment options and imaging follow-up [11]. Radiologists should be aware that practice may differ slightly based on the set of guidelines that local referring urologists, oncologists and radiation oncologists adhere.
Techniques
MRI
ACR appropriateness criteria list pelvic MR as “usually appropriate” for patients in whom there is concern for residual or recurrent disease post prostatectomy. There is expert disagreement in the appropriate use of MRI of the abdomen and pelvis in patients post prostatectomy. The ACR appropriateness criteria list these studies as “may be appropriate.” In patients with metastatic prostate cancer treated by systemic therapy, dedicated pelvic MRI and MRI of the abdomen and pelvis “may be appropriate” [12]. According to NCCN guidelines, MRI can be performed with and without administration of intravenous contrast. MRI can be used in initial evaluation of prostate carcinoma and as part of workup for disease recurrence. The NCCN guidelines state that MRI can be considered following radical prostatectomy when PSA fails to fall to undetectable levels or when a previously undetectable PSA becomes detectable and increases on two or more subsequent determinations, or after radiation therapy for rising PSA or positive digital rectal exam if the patient is a candidate for additional local therapy. According to PI-RADS v2.1, multiparametric (mpMRI) of the prostate is defined as a combination of anatomic T2W images with functional and physiological assessment with diffusion weighted images (DWI) and deritative apparent diffusion coefficient (ADC) maps and dynamic contrast-enhanced (DCE) T1W images. Sometimes, other techniques such as in vivo MR proton spectroscopy images are obtained [13]. mpMRI is the only imaging study recommended by the European Society of Urogenital Radiology (ESUR) to evaluate for local recurrence when PSA is low in the early stages of suspected local disease recurrence. Low PSA is defined as between 0.2 and 2.0 ng/ml [14].
mpMRI imaging features suspicious for local disease recurrence include T2 iso to mildly hypointense lesions with associated restricted diffusion and/or abnormal (plateau/type II or washout/type III) on DCE images [9, 10] (Figs. 2, 3, 4). These lesions typically arise at the margin of the resection cavity with up to 27% occurring at the bladder-urethral perianstomotic region [9]. Less frequent sites of recurrence in the resection cavity include the retrovesicular spaces, periurethral region, seminal vesicles, penile bulb [9] and at the vas deferens [15]. While most local disease recurrence is currently treated with blind salvage radiation therapy, localization of tumor is important because clinical target volumes can be missed or marginally treated with blind external beam radiation therapy [16, 17]. This includes tumors that recur in locations outside of the prostatectomy bed (Figs. 5, 6).
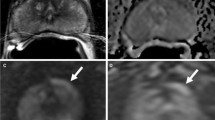
76-year-old male with a history of Gleason Grade 8 prostate adenocarcinoma post definitive therapy with radical prostatectomy and pelvic node dissection. mpMRI was performed for suspected recurrence due to increasing PSA levels. a A lesion is not able to be identified on a T2-weighted fast spin echo image or b ADC map. c Contrast-enhanced spoiled 3D gradient echo subtraction T1 image shows enhancement of a focal lesion (arrow) in the left retrovesicular space. d Color map image shows the enhancing lesion (arrow). Targeted biopsy revealed recurrent prostate adenocarcinoma
69-year-old male with a history of Gleason Grade 9 prostate adenocarcinoma post definitive therapy with radical prostatectomy and pelvic node dissection. mpMRI with use of an endocoil was performed for suspected recurrence due to elevated PSA of 2.4 ng/mL. a Contrast-enhanced spoiled 3D gradient echo subtraction T1 image showing invasive recurrent enhancing prostate adenocarcinoma (arrow) in the right lateral prostatic groove. Tumor invades the adjacent right pubococcygeus and obturator muscles as well as the pubic tubercle. b Axial T2-weighted fast spin echo image shows homogenous intermediate T2 signal corresponding to the enhancing tumor. c ADC map shows diffusion restriction within the tumor (arrow). d Coronal T2-weighted fast spin echo image reveals bladder (short arrows) and pelvic side wall invasion (long arrow). Targeted biopsy revealed recurrent prostate adenocarcinoma
82-year-old male with a history of Gleason Grade 8 prostate adenocarcinoma post definitive therapy with radical prostatectomy and pelvic node dissection. mpMRI was performed for suspected recurrence due to increasing PSA levels. a Axial T2-weighted fast spin echo image showing homogenous intermediate T2 signal intensity (arrow) corresponding to a soft tissue nodule along the left margin of the proximal aspect of the urethra. b ADC map demonstrates diffusion restriction (arrow) within the nodule. c Contrast-enhanced spoiled 3D gradient echo subtraction T1 image shows enhancement within the nodule (arrow). Color map shows the enhancing nodule. Targeted biopsy revealed recurrent prostate adenocarcinoma
62-year-old male with a history of Gleason Grade 9 prostate adenocarcinoma post definitive therapy with radical prostatectomy and node dissection. mpMRI was performed for surveillance. a Contrast-enhanced spoiled 3D gradient echo T1 subtraction image shows enhancement within a soft tissue lesion (arrow) in the right pelvic sidewall, potentially outside of the normal contour volume. b Axial T2-weighted fast spin echo image shows homogenous intermediate T2 signal intensity (arrow) corresponding to the enhancing nodule. Targeted biopsy revealed recurrent prostate adenocarcinoma
74-year-old male with a history of Gleason Grade 9 prostate adenocarcinoma post radical prostatectomy and node dissection. mpMRI was performed for dysuria and pelvic pain. a Coronal T2-weighted fast spin echo image shows numerous homogenous hypointense T2 metastases in the penile corpora. b Contrast-enhanced spoiled 3D gradient echo T1 image shows enhancing recurrence corresponding to prior findings. Targeted biopsy revealed metastatic prostate adenocarcinoma
While not incorporated into the PI-RADS v2.1 lexicon, Liauw et al. described a scoring system to describe likelihood of post-prostatectomy disease recurrence with risk assessed on a numeric scale from 0 to 4. In this system, a score of 0 corresponded with no abnormality detected on any sequence and was assessed as definitely normal; a score of 1 corresponded with small, asymmetric T2 hypointensities seen on only 1 imaging plane without corresponding abnormality on DWI or DCE sequences and was assessed as probably normal; a score of 2 was defined as small asymmetric T2 hypointensity seen on more than 1 imaging plane without any corresponding abnormality on DWI or DCE sequence and assessed as indeterminate; a score of 3 was defined as abnormality detected on either DWI or DCE and assessed as probably abnormal; a score of 4 was defined as abnormality detected on T2, DWI and DCE sequences and was assessed as definitely abnormal. A score of 3 or 4 is considered recurrence and was associated with positive operative margins [10]. Others have used PI-RADS radiologic criteria and applied the findings in the post-operative state. In this scheme, findings concerning for recurrence include soft tissue nodules in or around the prostatectomy bed on T2 weighted images, PI-RADS risk assessment score on DWI images between 3 and 5 and associated DCE showing a type II-III curve [9]. Figure 4 shows typical MRI features of prostate carcinoma recurrence with DWI restriction, contrast enhancement and a T2 intermediate nodule in the perianastomotic region.
In addition to evaluation of recurrence in the prostatectomy bed, mpMRI is used to evaluate for local regional nodal metastasis. According to PI-RADS v2.1, assessment of lymph nodes is limited to size, morphology, shape and enhancement pattern. Positive nodes are greater than 0.8 cm in short axis [13]. A meta-analysis of 24 studies revealed a pooled sensitivity of MR in detection of nodal metastasis at 39% (22–56%, 95% CI) with specificity of 82% (79–83%, 95% CI). The difference between CT and MR was not statistically significant [18]. Unfortunately, and despite use of novel contrast agents and DWI, mpMRI does not perform well for evaluation of nodal disease with standard of care remaining surgical lymphadenectomy [19]. An example case of nodal recurrence based on size, morphology, and heterogenous enhancement is shown in Fig. 7. An example cases of tumor recurrence post external beam radiation is shown in Fig. 8.
65-year-old male with a history of Gleason Grade 7 prostate adenocarcinoma post definitive therapy with radical prostatectomy and pelvic node dissection. mpMRI was performed for increasing PSA and suspected recurrence of prostate cancer. a Wide field-of-view contrast-enhanced spoiled 3D gradient echo T1 image shows a rounded, enlarged enhancing right external iliac lymph node (arrows). b ADC map shows diffusion restriction (arrow) corresponding to prior findings. A catheter is in the urethra (small arrow). Targeted biopsy revealed recurrent prostate adenocarcinoma
69-year-old male with a history of Gleason Grade 3 + 4 prostate adenocarcinoma post definitive therapy with external beam radiation. mpMRI was performed for rising PSA (5.02 ng/ml). Targeted biopsy revealed Gleason Grade 4 + 3 adenocarcinoma. a Contrast-enhanced spoiled 3D gradient echo T1 image shows enhancement (arrow) in the left mid-gland anterior transition zone, b T2-weighted echo planar fast spin echo image showing a non-circumscribed, homogenous, moderately hypointense lesion (arrow) corresponding to the findings in (a). c ADC map showing marked diffusion restriction (arrow). d Color map image shows the enhancing lesion (arrow)
PET
Three PET radiotracers are approved by the FDA for men with prostate cancer including C-11 choline, F-18 sodium fluoride and F-18 fluciclovine. While investigational in the USA and not currently FDA approved, Ga-68 PMSA is widely available in Europe. F-18 has a half-life of 110 min and thus can be produced with regional cyclotrons. C-11 has a half-life of 20 min and may be produced on site. Ga-68 has a half-life of 68 min and is produced in a generator [1]. ACR appropriateness criteria list C-11 choline and F-18 fluciclovine as “usually appropriate” for patients in whom there is concern for residual or recurrent disease post prostatectomy [12]. The NCCN panel suggests use of F-18 fluciclovine and C-11 choline in PET/CT or PET/MRI in men with biochemical recurrence as a second modality after bone scan, CT or MRI. C-11 choline or F-18 fluciclovine are used to detect disease in soft tissue and bone [20, 21]. When disease is suggested, biopsy is usually recommended to confirm a true positive result. While the PET tracers may show earlier metastasis than conventional imaging techniques, the NCCN panel cautions against assuming that this will result in improved overall survival or oncologic outcome and may indeed result in use of newer and more expensive therapies without proven benefit. Use of PET/CT or PET/MRI for staging of small volume recurrent or metastatic prostate cancer with tracers such as C-11 choline or F-18 fluciclovine remains an area of active research. The panel states that due to FDA clearance and approved reimbursement mechanisms, gold-standard multicenter clinical trials are unlikely to be performed to document improved patient outcomes.
In aggregate, the FDA approved PET tracers show similar sensitivity and specificity in detection of small volume disease. In post-prostatectomy patients with biochemical recurrence, C-11 choline sensitivity ranges from 32 to 93% with a specificity of 40–93%, while F-18 fluciclovine sensitivity ranges from 37 to 90% and specificity from 40 to 100% [22,23,24,25,26,27,28,29,30,31,32]. Of the FDA approved PET tracers, small scale studies have shown that F-18 fluciclovine is more sensitive than skeletal scintigraphy for detection of osseous metastases [33]. F-18 fluorocholine and F-18 sodium fluoride showed equal sensitivity (89%) but F-18 fluorocholine showed increased specificity compared to F-18 fluoride (96% vs 91%, p = 0.033) in detection of osseous disease [34]. In a prospective series of 89 patients, F-18 fluciclovine and C-11 choline showed 85% agreement with F-18 fluciclovine considered superior to C-11 choline in patients with biochemical relapse after prostatectomy [27]. An example case of perianastomotic recurrence with C-11 choline is shown in Fig. 9.
58-year-old male with a history of Gleason Grade 8 prostate adenocarcinoma post definitive therapy with radical prostatectomy and pelvic node dissection. mpMRI was performed for increasing PSA levels. a ADC image shows diffusion (arrow) restriction in the left periurethreal region. b Axial T2 weighted fast spin echo image reveals homogenous intermediate T2 signal intensity (arrow) corresponding to the enhancing nodule. c Axial fused CT and C11-Choline PET image shows uptake corresponding to the prior findings. Targeted biopsy revealed recurrent prostate adenocarcinoma
While the NCCN practice guidelines do not detail use of Ga-68 PSMA presumably due to its investigational status in the USA, EAU guidelines provide a weak level 2b recommendation to preform a Ga-68 PSMA PET if the patient is post prostatectomy and PSA is greater than or equal to 1 ng/ml and the patient is at risk for non-locoregional metastatic disease. If Ga-68 PSMA is not available, the EAU guidelines recommend a C-11 choline PET [11]. Several studies have demonstrated a high sensitivity of Ga-68 PSMA in detection of disease in patients with biochemical recurrence and very low PSA [35]. A metanalysis of 16 studies including 1309 patients showed disease in 42% of patients with PSA between 0 and 0.2 ng/ml, 58% of patients with PSA between 0.2 and 1.0 ng/ml, 76% of patients with PSA between 1.0 and 2.0 ng/ml and 95% of patients with PSA greater than 2.0 ng/ml. On a per patient analysis, G-68 PSMA both sensitivity and specificity were estimated at 86%. On a per lesion basis, sensitivity was estimated at 80% with specificity of 97% [36]. In a prospective trial comparing Ga-68 PSMA to F-18 fluciclovine, detection rates were significantly higher at the patient level in the Ga-68 PSMA group (OR 4.8, 95% CI 1.6–19.2, p = 0.003) [37]. These results are promising and suggest Ga-68 PSMA detects a higher proportion of disease compared to other already FDA approved agents.
Ultrasound
Compared to digital rectal exam, transrectal ultrasound (TRUS) has been shown to improve sensitivity of assessment of local disease recurrence post prostatectomy [38]. Sensitivity of anastomotic biopsy with TRUS guidance has been reported between 14 and 45% for PSA levels less than 1 ng/ml and 40–71% for PSA levels greater than 1 ng/ml [38,39,40,41,42]. Following prostatectomy, there is variable and normal fibrous scar tissue at the vesicouretheral anastomosis with more soft tissue thickening anteriorly [43]. Imaging features associated with local recurrence include asymmetric hypoechoic masses at the level of the vesicourethral anastomosis, the bladder neck and retrotrigone with isoechoic thickening at the level of the anastomosis [44]. Due to narrow field of view and limited assessment at ultrasound for local disease recurrence, 6-core systematic biopsy is often performed at the vesicouretheral anastomosis in addition to biopsy of any TRUS abnormality and visualized seminal vesicle remnants [39, 45]. In addition to diagnostic imaging features, TRUS has the added benefit of being a dynamic exam where the operator can use real-time imaging feedback to guide biopsy position.
CT
American College of Radiology (ACR) appropriateness criteria list CT of the abdomen and pelvis with IV contrast as “may be appropriate” for patients in whom there is concern for residual or recurrent disease post prostatectomy. CT of the chest with or without contrast and CT of the abdomen and pelvis without contrast are “usually not appropriate.” In patients with metastatic prostate cancer treated by systemic therapy, the ACR appropriateness criteria list contrast-enhanced CT of the chest and/or abdomen and pelvis as “usually appropriate.” Unenhanced CT is “usually not appropriate” in patients treated for prostate cancer metastasis [12]. In contrast, NCCN guidelines state that CT may be performed with or without oral and intravenous contrast. According to NCCN criteria, cross sectional imaging is recommended in patients with nomogram predicted probability of nodal involvement greater than 10%. CT can be used to examine the abdomen and pelvis for initial evaluation and as part of the workup for recurrence or progression. While the ACR appropriateness criteria indicate that there is no evidence to support the use of CT without IV contrast or multiphasic scanning, patients with renal dysfunction or allergies to iodinated contrast may be scanned in clinical practice at some centers.
Sensitivity and specificity of CT in evaluation for local disease recurrence following radical prostatectomy is underreported in our literature. A retrospective study of 22 patients with confirmed local recurrence following radical prostatectomy showed a true positive rate of detection for CT of 36% (8 cases) and a false negative rate of 41% (9 cases). An additional 23% (5 cases) were reportedly equivocal for scar or tumor, complicating post hoc calculation of sensitivity. In this study, positive cases showed tumors averaging nearly 2.0 cm in size. Axial CT slices ranged from 6.5 to 10 mm and under-staging was estimated in 41% of cases [46]. Another study of 86 CT scans showed a positive result in 14% of patients with biochemical recurrence within 3 years after radical prostatectomy. The slice thickness used in this study was 7–10 mm [47]. Based on our institutional experience, CT findings concerning for local disease recurrence include enhancing or non-enhancing soft tissue within the prostatectomy bed and an infiltrative appearance of soft tissue within the pelvis. A common pitfall includes post-operative scarring in the pelvis, which can lead to ambiguity and confusion in interpretation. An example case of local recurrence on CT is shown in Fig. 10. Additional findings on CT concerning for local disease recurrence include enlarged rounded regional lymph nodes. A meta-analysis of 24 studies revealed a pooled sensitivity of CT in detection of nodal metastasis at 42% (26–56%, 95% CI) with specificity of 82% (80–83%, 95% CI) [18]. Given improvements in CT technology, a new analysis of CT sensitivity for local recurrence may be necessary.
67-year-old male with a history of Gleason Grade 9 prostate adenocarcinoma post definitive therapy with radical prostatectomy and pelvic node dissection. CT was performed for suspected recurrence due to increasing PSA levels. Contrast-enhanced CT shows an enhancing nodule in the periurethral region in the prostatectomy bed. Targeted biopsy revealed recurrent prostate cancer
Conclusion
Prostate cancer is a common disease often treated with radical prostatectomy. Radiologist understanding of pre and post-operative anatomy as well as the surgical technique can avoid pitfalls in interpretation. CT has limited utility in assessment of the operative bed but is very useful in detection of visceral and nodal metastasis. mpMRI is the most useful modality to assess for local disease recurrence. PET/CT and PET/MRI can be used in cases for trouble shooting.
References
NCCN Clinical Practice Guidelines in Oncology (NCCN Gudelines) Prostate Cancer Version 3.2019 [Internet]. National Comprehensive Cancer Network; 2019 [cited 2019 Aug 14]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D’Amico AV, Dmochowski RR, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007 Feb;177(2):540–5.
Roach M, Hanks G, Thames H, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006 Jul 15;65(4):965–74.
Nyarangi-Dix JN, Radtke JP, Hadaschik B, Pahernik S, Hohenfellner M. Impact of complete bladder neck preservation on urinary continence, quality of life and surgical margins after radical prostatectomy: a randomized, controlled, single blind trial. J Urol. 2013 Mar;189(3):891–8.
Walsh PC, Lepor H, Eggleston JC. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. The Prostate. 1983;4(5):473–85.
Coakley FV, Eberhardt S, Kattan MW, Wei DC, Scardino PT, Hricak H. Urinary continence after radical retropubic prostatectomy: relationship with membranous urethral length on preoperative endorectal magnetic resonance imaging. J Urol. 2002 Sep;168(3):1032–5.
Gaker DL, Steel BL. Radical prostatectomy with preservation of urinary continence: pathology and long-term results. J Urol. 2004 Dec;172(6 Pt 2):2549–52.
Walz J, Burnett AL, Costello AJ, Eastham JA, Graefen M, Guillonneau B, et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010 Feb;57(2):179–92.
Hernandez D, Salas D, Giménez D, Buitrago P, Esquena S, Palou J, et al. Pelvic MRI findings in relapsed prostate cancer after radical prostatectomy. Radiat Oncol. 2015 Dec 24;10(1):262.
Liauw SL, Pitroda SP, Eggener SE, Stadler WM, Pelizzari CA, Vannier MW, et al. Evaluation of the prostate bed for local recurrence after radical prostatectomy using endorectal magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2013 Feb 1;85(2):378–84.
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017;71(4):618–29.
Froemming A, Verma S, Eberhardt SC, Oto A, Alexander L, Allen B, et al. ACR Appropriateness Criteria Post-Treatment Follow-up of Prostate Cancer [Internet]. American College of Radiology; 2017 [cited 2019 Nov 18]. Available from: https://acsearch.acr.org/docs/69369/Narrative/
Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol. 2019 Mar 18;
Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012 Apr;22(4):746–57.
Nguyen DP, Giannarini G, Seiler R, Schiller R, Thoeny HC, Thalmann GN, et al. Local recurrence after retropubic radical prostatectomy for prostate cancer does not exclusively occur at the anastomotic site. BJU Int. 2013 Aug;112(4):E243-249.
Croke J, Malone S, Roustan Delatour N, Belanger E, Avruch L, Morash C, et al. Postoperative radiotherapy in prostate cancer: the case of the missing target. Int J Radiat Oncol Biol Phys. 2012 Jul 15;83(4):1160–8.
Wang J, Kudchadker R, Choi S, Pettaway CA, Choi H, Hobbs BD, et al. Local recurrence map to guide target volume delineation after radical prostatectomy. Pract Radiat Oncol. 2014 Nov 1;4(6):e239–46.
Hövels AM, Heesakkers RAM, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008 Apr 1;63(4):387–95.
Magnetta MJ, Catania R, Girometti R, Westphalen AC, Borhani AA, Furlan A. Prostate MRI: staging and decision-making. Abdom Radiol N Y. 2020 Feb 11;
Nanni C, Schiavina R, Brunocilla E, Boschi S, Borghesi M, Zanoni L, et al. 18F-Fluciclovine PET/CT for the Detection of Prostate Cancer Relapse: A Comparison to 11C-Choline PET/CT. Clin Nucl Med. 2015 Aug;40(8):e386-391.
Fuccio C, Castellucci P, Schiavina R, Guidalotti PL, Gavaruzzi G, Montini GC, et al. Role of 11C-choline PET/CT in the re-staging of prostate cancer patients with biochemical relapse and negative results at bone scintigraphy. Eur J Radiol. 2012 Aug;81(8):e893-896.
Evangelista L, Zattoni F, Guttilla A, Saladini G, Zattoni F, Colletti PM, et al. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med. 2013 May;38(5):305–14.
Fanti S, Minozzi S, Castellucci P, Balduzzi S, Herrmann K, Krause BJ, et al. PET/CT with (11)C-choline for evaluation of prostate cancer patients with biochemical recurrence: meta-analysis and critical review of available data. Eur J Nucl Med Mol Imaging. 2016 Jan;43(1):55–69.
Giovacchini G, Picchio M, Coradeschi E, Bettinardi V, Gianolli L, Scattoni V, et al. Predictive factors of [(11)C]choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2010 Feb;37(2):301–9.
Kitajima K, Murphy RC, Nathan MA, Froemming AT, Hagen CE, Takahashi N, et al. Detection of recurrent prostate cancer after radical prostatectomy: comparison of 11C-choline PET/CT with pelvic multiparametric MR imaging with endorectal coil. J Nucl Med Off Publ Soc Nucl Med. 2014 Feb;55(2):223–32.
Mitchell CR, Lowe VJ, Rangel LJ, Hung JC, Kwon ED, Karnes RJ. Operational characteristics of (11)c-choline positron emission tomography/computerized tomography for prostate cancer with biochemical recurrence after initial treatment. J Urol. 2013 Apr;189(4):1308–13.
Nanni C, Zanoni L, Pultrone C, Schiavina R, Brunocilla E, Lodi F, et al. (18)F-FACBC (anti1-amino-3-(18)F-fluorocyclobutane-1-carboxylic acid) versus (11)C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging. 2016 Aug;43(9):1601–10.
Reske SN, Blumstein NM, Glatting G. [11C]choline PET/CT imaging in occult local relapse of prostate cancer after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2008 Jan;35(1):9–17.
Scattoni V, Picchio M, Suardi N, Messa C, Freschi M, Roscigno M, et al. Detection of lymph-node metastases with integrated [11C]choline PET/CT in patients with PSA failure after radical retropubic prostatectomy: results confirmed by open pelvic-retroperitoneal lymphadenectomy. Eur Urol. 2007 Aug;52(2):423–9.
Umbehr MH, Müntener M, Hany T, Sulser T, Bachmann LM. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol. 2013 Jul;64(1):106–17.
Odewole OA, Tade FI, Nieh PT, Savir-Baruch B, Jani AB, Master VA, et al. Recurrent prostate cancer detection with anti-3-[(18)F]FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging. 2016 Sep;43(10):1773–83.
Schuster DM, Nieh PT, Jani AB, Amzat R, Bowman FD, Halkar RK, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014 May;191(5):1446–53.
Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med Off Publ Soc Nucl Med. 2006 Feb;47(2):287–97.
Langsteger W, Balogova S, Huchet V, Beheshti M, Paycha F, Egrot C, et al. Fluorocholine (18F) and sodium fluoride (18F) PET/CT in the detection of prostate cancer: prospective comparison of diagnostic performance determined by masked reading. Q J Nucl Med Mol Imaging Off Publ Ital Assoc Nucl Med AIMN Int Assoc Radiopharmacol IAR Sect Soc Of. 2011 Aug;55(4):448–57.
Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. Radiogr Rev Publ Radiol Soc N Am Inc. 2018 Feb;38(1):200–17.
Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, Specificity, and Predictors of Positive 68 Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2016;70(6):926–37.
Calais J, Ceci F, Eiber M, Hope TA, Hofman MS, Rischpler C, et al. 18F-fluciclovine PET-CT and 68 Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019 Sep;20(9):1286–94.
Leventis AK, Shariat SF, Slawin KM. Local recurrence after radical prostatectomy: correlation of US features with prostatic fossa biopsy findings. Radiology. 2001 May;219(2):432–9.
Rouvière O, Vitry T, Lyonnet D. Imaging of prostate cancer local recurrences: why and how? Eur Radiol. 2010 May 1;20(5):1254–66.
Naito S. Evaluation and management of prostate-specific antigen recurrence after radical prostatectomy for localized prostate cancer. Jpn J Clin Oncol. 2005 Jul;35(7):365–74.
Scattoni V, Montorsi F, Picchio M, Roscigno M, Salonia A, Rigatti P, et al. Diagnosis of local recurrence after radical prostatectomy. BJU Int. 2004 Mar;93(5):680–8.
Deliveliotis C, Manousakas T, Chrisofos M, Skolarikos A, Delis A, Dimopoulos C. Diagnostic efficacy of transrectal ultrasound-guided biopsy of the prostatic fossa in patients with rising PSA following radical prostatectomy. World J Urol. 2007 Jun;25(3):309–13.
Wasserman NF, Kapoor DA, Hildebrandt WC, Zhang G, Born KM, Eppel SM, et al. Transrectal US in evaluation of patients after radical prostatectomy. Part I. Normal postoperative anatomy. Radiology. 1992 Nov 1;185(2):361–6.
Connolly JA, Shinohara K, Presti JC, Carroll PR. Local recurrence after radical prostatectomy: characteristics in size, location, and relationship to prostate-specific antigen and surgical margins. Urology. 1996 Feb;47(2):225–31.
Saleem MD, Sanders H, Abu El Naser M, El-Galley R. Factors predicting cancer detection in biopsy of the prostatic fossa after radical prostatectomy. Urology. 1998 Feb;51(2):283–6.
Krämer S, Görich J, Gottfried HW, Riska P, Aschoff AJ, Rilinger N, et al. Sensitivity of computed tomography in detecting local recurrence of prostatic carcinoma following radical prostatectomy. Br J Radiol. 1997 Oct;70(838):995–9.
Kane CJ, Amling CL, Johnstone PAS, Pak N, Lance RS, Thrasher JB, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003 Mar 1;61(3):607–11.
Funding
None Applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no financial or other disclosures relationship with any commercial organization that may have a direct or indirect interest in this manuscript. There is no conflict of interest to declare for this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Magnetta, M.J., Casalino, D. & Heller, M.T. Imaging assessment of local recurrence of prostate cancer after radical prostatectomy. Abdom Radiol 45, 4073–4083 (2020). https://doi.org/10.1007/s00261-020-02505-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02505-7