Abstract
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive gastrointestinal malignancy with a poor 5-year survival rate. Accurate staging of PDAC is an important initial step in the development of a stage-specific treatment plan. Different staging systems/consensus statements convened by different societies and academic practices are currently used. The most recent version of the American Joint Committee on Cancer (AJCC) tumor/node/metastases (TNM) staging system for PDAC has shifted its focus from guiding management to assessing prognosis. In order to preoperatively define the resectability of PDAC and to guide management, additional classification systems have been developed. The National Comprehensive Cancer Network (NCCN) guidelines, one of the most commonly used systems, provide recommendations on the management and the determination of resectability for PDAC. The NCCN divides PDAC into three categories of resectability based on tumor-vessel relationship: ‘resectable,’ ‘borderline resectable,’ and ‘unresectable’. Among these, the borderline disease category is of special interest given its evolution over time and the resulting variations in the definition and the associated recommendations for management between different societies. It is important to be familiar with the evolving criteria, and treatment and follow-up recommendations for PDAC. In this article, the most current AJCC staging (8th edition), NCCN guidelines (version 2.2019—April 9, 2019), and challenges and controversies in borderline resectable PDAC are reviewed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most common form of pancreatic cancer and the third leading cause of cancer death in the western world, accounting for approximately 22% of the deaths from malignant gastrointestinal neoplasms. Compared to the generally improving survival for other gastrointestinal cancers, the 5-year survival of patients with PDAC remains low at a dismal 4% [1]. Accurate presurgical staging is vital to identify patients who are most likely to benefit from surgery. The American Joint Committee on Cancer (AJCC) TNM staging criteria for PDAC are used to characterize the local and systemic spread of PDAC. Recent modifications made to the AJCC staging (8th edition) for PDAC are aimed to improve its prognostic accuracy; the new staging system has been in use as of January 2018 [2, 3]. Although the AJCC staging system has improved pathologic staging, clinical classification systems for disease management [such as National Comprehensive Cancer Network (NCCN)] based on the results of presurgical imaging studies are commonly used to define resectability of PDAC [4]. The diagnosis and clinical staging of PDAC are established using computed tomography (CT) and/or magnetic resonance imaging (MRI), or biopsy/fine-needle aspiration using endoscopic ultrasound (EUS). The NCCN is one of the most used systems that has guidelines for the diagnostic workup and resection of PDAC. The NCCN classifies PDAC into three categories which are based on tumor-vessel relationships: ‘resectable,’ ‘borderline resectable,’ and ‘unresectable’ [4]. There is growing evidence to suggest that neoadjuvant therapy may be of particular benefit to patients with borderline resectable disease by increasing the likelihood of margin-negative (R0) resections. However, the imaging criteria to define borderline resectability vary between the guidelines from different societies. As a result, disease classification and the selection of treatments for this subset of patients remain a challenge. Radiologists and clinicians in other specialties need to be familiar with these evolving recommendations. In this article, AJCC staging (8th edition), NCCN guidelines (version 2.2019—April 9, 2019), and challenges and controversies in borderline resectable PDAC are reviewed. This review is a product of the dedicated efforts of the members of the Society of Abdominal Radiology’s Disease-Focused Panel for PDAC which aims to provide up-to-date information on key topics in the field of PDAC. While not exhaustively comprehensive, this article nevertheless attempts to be a sufficiently comprehensive educational resource to keep radiologists abreast of this latest information.
AJCC staging system
Over the past several years, the American Joint Committee on Cancer (AJCC) has developed the tumor/node/metastases (TNM) system for solid tumor staging. Precise staging for PDAC is critical in making treatment decisions, selecting patients for clinical trials and determining prognoses. The AJCC recently updated its TNM staging system in 2018 (8th edition) for PDAC [2, 3]. Investigators had criticized prior versions for their limited clinical applicability because of the wide variations in treatment practices in the community. Another criticism was unclear terminology, such as the lack of a clear definition for “extension beyond the pancreas” and the difficulty in making such determinations [5]. An example is the limited interobserver agreement between pathologists on histopathologic assessment for extrapancreatic extension [6]. The N-category was also criticized as it did not take into account the number of positive lymph nodes or lymph node ratio (the number of lymph nodes with positive disease divided by the total number of lymph nodes) which have been reported to have important prognostic value in patients with PDAC [7, 8].
The revised AJCC criteria for staging PDAC responded to these criticisms of the earlier versions with several changes to the T and N categories, with the current primary goal of providing information on prognosis, rather than guiding patient’s management. In the eighth edition, T stage (T1 through T4 disease) is nearly entirely based on the tumor size; extension of tumor beyond the pancreas alone is no longer considered T3 (Table 1). Subdivisions have also been added to T1 (T1a ≤ 0.5 cm, T1b 0.5–1 cm, and T1c 1–2 cm in greatest dimension). The size criteria for the T2 and T3 categories have been modified (T2 defined as > 2 and ≤ 4 cm and T3 defined as tumors > 4 cm in greatest dimension), and T4 disease has been defined as any tumor that involves the celiac axis (CA), superior mesenteric artery (SMA), or common hepatic artery (CHA), regardless of tumor size. The N-category is now stratified according to the number of involved regional lymph nodes identified at the time of surgical resection and assessment by histopathology. N1 is defined as pathologically proven metastases in one to three regional lymph nodes and N2 as proven metastasis in four or more regional lymph nodes. The criteria for M-stage [absence (M0) or presence (M1) of distant metastases] are unchanged [3]. The seventh and eighth editions of the AJCC TNM staging system are compared in Table 1.
The goal of the updated AJCC staging system, as stated by the panel itself, is to determine prognosis, and not to guide management given the wide range of treatment practices in the medical community. However, various organizations/societies have developed guidelines for the clinical management of patients with PDAC. These guidelines are typically based on the findings from cross-sectional imaging studies (such as tumor-peripancreatic vessel relationship) to generally classify patients with PDAC as resectable, borderline resectable, or unresectable (locally advanced or metastatic). Such guidelines from the NCCN, and a limited comparison with those from other societies, will be discussed in ensuing sections [4, 9].
NCCN guidelines
The most current guidelines from the NCCN place patients with PDAC into one of three broad groups: resectable, borderline resectable, and unresectable disease based predominantly on imaging findings (Figs. 1, 2, 3) (Table 2) [4, 9]. The NCCN’s clinical practice guidelines for PDAC are a consensus statement to aid diagnosis and treatment. The guidelines are reviewed and updated on a continuing basis to ensure that the recommendations consider the most current evidence. This section provides an overview of the most recent version of the NCCN guidelines for PDAC (version 2.2019—April 9, 2019) [4].
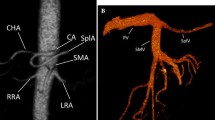
Borderline resectable PDAC. Coronal pancreatic phase CT image show a pancreatic mass encasing the CA, CHA, LGA, and SA (GDA was not involved; not shown here). Based on the NCCN guidelines, and tumor-vessel relationship (CA encasement) the disease may be classified as borderline resectable. However, if classified according to the MDACC, ACTO, and AHPBA/SSAT/SSO classification systems, the disease would be considered unresectable (SMA superior mesenteric artery, CA celiac axis, SA splenic artery, LGA left gastric artery, GDA gastroduodenal artery)
NCCN recommendations on the use of diagnostic tools: when and how-to image
The NCCN recommends multi-detector computed tomography (MDCT) of the abdomen obtained in the pancreatic and portal venous phases as the preferred imaging modality to be performed at presentation and preferably no more than 4 weeks before surgery [10]. The NCCN considers dual-phase pancreatic CT protocol (Table 3) as necessary for optimal evaluation of the primary tumor, for assessing the tumor’s relationship to adjacent arterial/venous vasculature, and for detecting liver and peritoneal metastasis (the specifics of vascular involvement will be discussed later in this article). Additional imaging of the chest and pelvis can be considered as per institutional preference. Although MRI has shown equal sensitivity in local staging of PDAC (Table 3), CT is recommended as the preferred technique owing to its wide availability, superior spatial resolution, and rapid acquisition. In addition, clinicians from various specialties (e.g., surgeons, radiation oncologists etc.) have better familiarity with CT than MRI. MRI is therefore recommended as an adjunct tool when CT findings are indeterminate (small pancreatic tumors or liver lesions), or when contrast-enhanced CT is not possible due to a life-threatening allergy to iodinated contrast agent [11].
To ensure all relevant imaging findings like evaluation of primary tumor, its relationship with arterial and venous structures and extrapancreatic extension are reported for complete assessment and staging of PDAC, the NCCN recommends the use of a radiology reporting template as defined by a multidisciplinary expert consensus group (Society of Abdominal Radiology and American Pancreatic Association) [10].
Other than CT and MRI, additional imaging techniques [endoscopic ultrasound (EUS), endoscopic retrograde cholangiopancreatography (ERCP), PET/CT, dual-energy CT (DECT) and PET/MRI] are also considered useful; some have established roles, while the roles of others are noted to be still evolving.
EUS is recommended primarily as a tool to procure tissue for diagnosis and to provide additional information to characterize pancreatic lesions which are either occult or indeterminate on staging CT/MRI. EUS is generally recommended over CT-guided FNA because of the lower risk of peritoneal seeding.
ERCP is recommended primarily as a means to treat biliary obstruction in symptomatic patients when surgical intervention is either delayed or not elected, and before neoadjuvant therapy [12]. In the diagnostic setting, MRCP is considered equivalent to EUS/ERCP.
PET/CT in conjunction with a pancreatic protocol CT exam improves the detection of metastatic disease, but its role in the staging of the PDAC is not clearly established [13, 14]. The NCCN suggests that PET/CT be considered following a dedicated pancreatic protocol CT in those patients who are at high risk for distant/disseminated disease (such as large primary tumors or large regional lymph nodes, borderline resectable disease, markedly elevated CA19-9, excessive weight loss and/or marked symptomatology such as severe abdominal pain). Newer techniques (DECT and PET/MRI) are being used in clinical practice, but their roles are not addressed in the current guidelines.
The guidelines note that diagnostic laparoscopy can be considered in patients with resectable disease on imaging but who are at high risk for distant/disseminate disease (e.g., large tumors, notably high CA 19-9, extreme weight loss, etc.) and in those patients with borderline resectable disease to evaluate for possible metastatic spread. However, the routine use of diagnostic laparoscopy is controversial [15, 16]. Positive cytology from washings alone obtained during laparoscopy or laparotomy is considered M1 disease under the current guidelines.
Role of CA 19-9
In addition to the above measures, serum CA 19-9 level (drawn following biliary decompression and with confirmation that serum bilirubin levels have normalized) and baseline standard laboratory studies are also recommended. Not all patients with PDAC have tumors that express CA 19-9, a sialylated Lewis A blood group antigen. CA 19-9 can be a good diagnostic and prognostic marker in those tumors that express it. Preoperative CA 19-9 levels have shown correlation with resectability and can provide additional information for staging [17, 18]. The NCCN recommends measurement of serum CA 19-9 levels before and after neoadjuvant treatment, before surgery, immediately prior to adjuvant treatment, and for the purposes of surveillance [4].
Summary of NCCN guidelines for clinical management of resectable and borderline resectable PDAC
Surgical resection
Surgical resection is currently the only potentially curative option for PDAC. The median survival of resected patients after adjuvant therapy ranges from 20.1 to 28.0 months even in optimal clinical trial conditions [19,20,21]. Patient selection should be based on the probability of achieving an R0 resection; R0 means a negative resection margin based on assessment with microscopy. Small tumor size, R0 margin, and N0 (node-negative) status are the strongest predictors of long-term patient survival [22, 23]. The guidelines advise that patient performance status, symptom burden, and comorbidity profile also be utilized to identify those patients who can undergo major surgery. The panel supports, as a central principle, multidisciplinary collaboration for the formulation of treatment plans and for determining management of PDAC. The NCCN recommends that pancreatic resections be performed at institutions that perform a large number of pancreatic tumor resections annually (at least 15–20).
For clearly resectable disease (Fig. 1 and Table 2), upfront surgical resection of the pancreatic tumor and regional lymph nodes is recommended. When dealing with a potentially resectable PDAC, the goal, as noted by the NCCN, is a prompt and cost-efficient initial workup to determine suitability for upfront surgery. While there is increasing enthusiasm for the use of neoadjuvant chemotherapy and/or chemoradiation, the NCCN did not recommend neoadjuvant therapy for tumors that are clearly resectable in patients without high-risk features, except in the context of a clinical trial. It is also important to note that tissue diagnosis is not required under the NCCN guidelines for the resection of clearly resectable disease, unless neoadjuvant therapy is considered, or the patient is enrolled in a clinical trial [4].
Unlike patients with clearly resectable disease, patients with borderline resectable disease (Fig. 2 and Table 2) are at high risk for a positive surgical margin and recurrence in the setting of upfront surgery. For patients with borderline resectable disease, the aim of neoadjuvant therapy is to sufficiently treat the tumor so that a negative resection margin can be achieved even though such a change may not be apparent at imaging. Treatment and imaging features of borderline resectable disease will be discussed later in this article.
In the setting of a suspected tumor that has the appearance of borderline resectable disease, but in which a cancer diagnosis could not be confirmed even after repeated biopsy [EUS guided (preferred) or percutaneously], intraoperative biopsies should be obtained according to the NCCN panel. If during that operation, tumor is identified and is found to be resectable, the recommendation is for tumor resection followed by adjuvant therapy. If a tumor is identified to be unresectable, patients should be managed as locally advanced or metastatic disease (depending on the findings) [4].
The nature, extent, and the type of particular surgical procedure required (e.g., Whipple procedure or distal pancreatectomy or total pancreatectomy) should be based on the size, extent, and location of the pancreatic tumor. The need for vascular resection and reconstruction is dependent on the tumor-vessel relationships in a given case. Although it is clear that patients with distant metastatic disease (liver, peritoneal and distant lymph nodes) derive no benefit from resection, institutions differ in their approaches regarding locoregional disease involvement (pancreatic and peripancreatic lymph nodes). Data from randomized control trials have not shown a survival advantage for performing an extended regional lymphadenectomy in addition to a standard pancreaticoduodenectomy. Overall, the panel recommends that an extended regional lymphadenectomy not be considered outside of a clinical trial [24, 25].
Neoadjuvant (preoperative) therapy
In patients presenting with resectable disease, surgery followed by adjuvant chemotherapy has been the standard of care. However, patients with borderline resectable disease pose a unique challenge as they are at higher risk for R1 resections (which is a microscopically positive margin) [4]. Neoadjuvant therapy in this setting has several potential benefits: (1) increases the likelihood of R0 resection by downsizing the tumor and sterilizing the field/vessel margins, (2) effectively selecting for patients with disease that is either stable or responsive to treatment, (3) treating micrometastases at an early stage, (4) decreasing the incidence of pancreatic fistula, and (5) avoiding the risk of post-operative delays in starting adjuvant therapy or inability for adjuvant therapy because of post-operative morbidity when an upfront approach is utilized. Many clinical trials utilizing neoadjuvant therapy for borderline resectable disease have demonstrated its clinical feasibility, effectiveness, and acceptability by patients [26,27,28]. Neoadjuvant therapy (preferably given at a high-volume center or coordinated through such a center) as opposed to immediate surgery is being increasingly used and is the preferred approach at most NCCN member institutions. As of the 2016 version of the NCCN guidelines, upfront resection in patients with borderline resectable disease was no longer recommended. The panel does note that no randomized phase III trials have compared neoadjuvant therapy versus upfront surgery in borderline resectable disease. The NCCN panel also determined that it is currently not clear what are the best neoadjuvant regimen, or regimens, to use in the setting of borderline resectable disease.
Several studies have also evaluated the use of neoadjuvant treatment in patients with resectable disease. Although current limited evidence suggests a better chance of a margin-negative resection when preoperative therapy is administered, the panel indicates that more conclusive evidence is needed from randomized clinical trials. Currently, the NCCN does not recommend neoadjuvant therapy for clearly resectable patients without high-risk features, except in a clinical trial [29]. For patients that have imaging findings consistent with clearly resectable disease, but have clinical features suggestive of a poor prognosis (e.g., markedly elevated CA 19-9 levels, large primary tumor, large regional lymph nodes, excessive weight loss, extreme pain), the panel states that neoadjuvant therapy could be considered after pathologic confirmation of the presence of tumor.
Practices vary regarding neoadjuvant chemotherapy and chemoradiation regimens for resectable/borderline resectable disease. The panel includes acceptable regimens as FOLFIRINOX/modified FOLFIRINOX, gemcitabine/albumin-bound paclitaxel, and gemcitabine/cisplatin (for patients with BRCA1/2 or other DNA repair mutations). Radiation after chemotherapy can be included in the neoadjuvant setting. If preoperative therapy is administered, a restaging evaluation after completion of treatment and just prior to anticipated surgery is recommended [4].
Adjuvant (post-operative) therapy
Several clinical trials have shown that adjuvant therapy improves outcome over observation alone following resection [19, 30]. Even with an R0 resection, recurrence rates are very high in this disease. Therefore, the guidelines recommend additional therapy for all patients with resected PDAC. In the adjuvant setting, treatment with chemotherapy is recommended; the role of radiation therapy is being evaluated. Based on the available data, no standard approach to adjuvant therapy has been established. The NCCN therefore strongly recommends enrollment into a clinical trial. Adjuvant chemotherapy regimens listed by the NCCN include gemcitabine alone or with capecitabine (category 1), 5-FU with leucovorin (category 1), and continuous infusion 5-FU and capecitabine (category 2B). Recently, modified FOLFIRINOX (category 1) was added. Other suggested regimens are chemotherapy (gemcitabine, 5-FU/leucovorin, or continuous infusion 5-FU) followed by chemoradiation (gemcitabine or fluoropyrimidine based), with subsequent chemotherapy being an option [4]. However, the majority of studies comparing chemotherapy and chemoradiation have not shown a survival advantage by adding radiation in the adjuvant setting [31, 32]. Patients who have received neoadjuvant chemotherapy or chemoradiation may also be candidates for adjuvant therapy depending on the response to neoadjuvant therapy, clinical considerations, and multidisciplinary review. The NCCN recommends initiating adjuvant therapy within 12 weeks of surgical resection, assuming complete recovery. Before beginning treatment, baseline contrast-enhanced CT (chest, abdomen, and pelvis) and serum CA 19-9 level are required as some patients will develop recurrence within just weeks following resection. If chemotherapy precedes chemoradiation, additional restaging with imaging should be done before commencing the next treatment period [4].
Imaging surveillance
For surveillance of resected disease, the NCCN recommends a history and physical examination for symptom assessment every 3 to 6 months for 2 years, then every 6 to 12 months as clinically indicated. CA 19-9 level testing and follow-up contrast-enhanced CT scans every 3 to 6 months for 2 years after surgical resection are suggested [4].
Management: locally advanced/metastatic disease
With metastatic disease and locally advanced disease (except for a small number of patients that show favorable treatment response in latter subset), the primary goals of treatment are palliation and lengthening survival. The NCCN has indicated that even with combination chemotherapy and radiation treatment, surgical resection of locally advanced disease is very unlikely. Moreover, the clinical course is often complicated by difficult-to-control issues such as tumor-related pain, biliary obstruction, gastroparesis, and gastric outlet obstruction for which palliative interventions are often needed.
The treatment strategies for patients with locally advanced disease are similar to those for patients with metastatic disease. In patients with good performance status, combination systemic therapy is recommended (such as FOLFIRINOX/modified FOLFIRINOX, gemcitabine with albumin-bound paclitaxel, and gemcitabine with cisplatin) [29]. For patients who have progressed on first-line therapy but have maintained good performance status, a second-line therapy can be considered. Those with poor performance status are best treated with single-agent chemotherapy (gemcitabine or capecitabine or continuous infusion 5-FU), but palliative measures and measures that emphasize patient comfort are also important. In locally advanced disease, chemoradiation, or stereotactic body radiation therapy (SBRT) can be used selectively, primarily in those with stable disease during initial chemotherapy, with chemo-limiting toxicity, or with issues related to local disease (such as obstruction) that are anticipated to emerge or have emerged during treatment. The goal of the addition of radiotherapy is to prevent/delay local progression. In patients with poorly controlled pain, local gastrointestinal obstructive symptoms, or gastrointestinal bleeding secondary to tumor, upfront chemoradiation or SBRT are an option. SBRT should be avoided if direct invasion of the bowel is evident on imaging. When radiation therapy is incorporated into the treatment regimen, fluoropyrimidine-based chemoradiation is generally preferred (due to a slightly better outcome) over gemcitabine-based chemoradiation [33, 34]. The panel notes that radiotherapy generally does not have a role in patients with metastatic disease. Although surgical resection of locally advanced disease is very unlikely even after chemotherapy and radiation therapy, an opportunity for curative resection may occasionally arise. In the setting of marked radiographic improvement or stability, and marked clinical improvement or decline in CA 19-9, the guidelines recommend that such patients be referred to high-volume centers for further evaluation. In some studies (limited data), patients with locally advanced disease who demonstrated radiographic and clinical improvement were shown to have survival rates similar to those initially considered to be resectable [35,36,37].
Summary of NCCN guidelines on PDAC
In summary, imaging is the most important component in the initial diagnosis and staging of PDAC. For the purpose of management, patients are divided into three groups: resectable, borderline resectable, and unresectable disease. For clearly resectable disease, upfront surgical resection of the pancreatic tumor and regional lymph nodes is recommended. Patients with borderline resectable tumors, and select patients with resectable tumors, can undergo neoadjuvant therapy in order to increase the chances for an R0 resection. Patients with locally advanced unresectable disease and good performance status at presentation can be considered for chemotherapy, chemoradiation, or SBRT; second-line therapy may be considered after progression if the patient maintains good performance status. Though an R0 resection of locally advanced disease is very unlikely, curative resection after chemotherapy and radiation therapy is occasionally possible if stability or marked radiographic improvement is shown. Patients with metastatic disease and good performance status can undergo chemotherapy; second-line therapy is considered after progression if performance status is maintained. When locally advanced disease is complicated by biliary or gastric obstruction, severe abdominal pain, or other tumor-associated symptoms, specific palliative measures are the best options. In all of these circumstances, imaging plays a central role in determining the extent of disease, and in aiding the assessment of treatment response. The current NCCN guidelines provide comprehensive recommendations regarding imaging techniques and for the reporting of imaging findings.
Definitions, imaging features, and reporting of borderline resectable PDAC: current challenges and controversies
Definitions of borderline resectable disease based on imaging
Surgical resection remains the only potentially curative procedure for PDAC. Guidelines have been developed by various societies, including the NCCN, primarily based on imaging, to aid in the identification of those patients who would most benefit from surgery. As highlighted before, these typically divide patients into resectable, borderline resectable, and unresectable disease based on imaging features, which will be discussed now. Criteria defining clearly resectable (no tumor contact with vessels) and unresectable disease (typically clearly greater than 180° of involvement of critical arterial structures such as the celiac and superior mesenteric arteries) are generally agreed upon between the various societies. In contrast, the definitions and treatment recommendations for borderline resectable disease notably vary between societies. The NCCN recommendations for management of borderline resectable disease have been discussed previously, and the details of the NCCN described features for borderline resectable disease are presented in detail in Table 2. This section will review the imaging features that define borderline resectable disease, noting the most significant differences between the recommendations of various societies.
Initially described as “marginally resectable”, the term borderline resectable PDAC was first described in 2006 by the Pancreas Cancer Group (a multidisciplinary clinical group) at MD Anderson Cancer Center in Houston, Texas [38, 39]. Since then, several societies have described features that classify PDAC into the borderline category based on vascular involvement [4].
The current NCCN criteria for borderline resectable disease are based on cross-sectional imaging features for arterial involvement. Borderline resectable disease is defined as (1) solid tumor contact with the common hepatic artery (CHA) without extension to the celiac axis (CA) or hepatic artery (HA) bifurcation, (2) ≤ 180° involvement of the SMA and/or celiac axis (CA), and (3) solid tumor contact with variant arterial anatomy (Fig. 2 and Tables 2, 4). The NCCN makes an exception for greater than 180° involvement of the CA if the aorta and gastroduodenal artery (GDA) are uninvolved, and the surgeons are able to perform an arterial anastomosis (modified Appleby procedure) [4]. The modified Appleby involves resection of the mass and the celiac axis en bloc. The arterial supply to the liver is maintained through the SMA in a retrograde fashion via the GDA to the proper hepatic artery. The ability to maintain adequate blood flow via this path postoperatively may require preoperative embolization of the common hepatic artery or anastomosis of the common hepatic artery to the aorta [40]. In contrast, current guidelines of other major societies [MDACC (MD Anderson Cancer Center), ACTO (Alliance for Clinical Trials in Oncology), and AHPBA/SSAT/SSO (American Hepato-Pancreato-Biliary Association/Society for Surgery of the Alimentary Tract/Society for Surgical Oncology)] consider celiac axis encasement to be unresectable (Fig. 3 and Table 4) [4, 9, 41,42,43,44,45].
The NCCN criteria for borderline resectable disease based on venous involvement include > 180° solid tumor contact of the SMV or PV, or ≤ 180° contact with SMV or PV if the vein has contour abnormality or occlusion that can be reconstructed. Solid tumor contact with the IVC is also considered borderline resectable disease [4, 46] (Tables 2 and 4). The clinical benefit for resection of the portal or superior mesenteric vein when the vein is involved by tumor remains controversial. It has been reported that when venous involvement does not exceed 2 cm in length, resection of the tumor and the involved vein followed by venous reconstruction will improve survival [47]. However, in patients with more extensive venous involvement, resection with venous grafting does not correlate with improved survival; it has been speculated that the presence of extensive venous involvement may indicate an aggressive tumor biology [48].
As noted previously, one of the challenges in applying the term “borderline” resectable disease to managing patients is that the definition of “borderline” varies between societies. A potentially even greater challenge is the variability in treatment practices across the country. Different surgeons and radiologists view the significance of vessel involvement differently given variable experience and comfort in performing operations involving vascular reconstructions and grafts [41].
Controversies in the use of radiotherapy in borderline resectable disease and the interpretation of imaging findings in this setting
An area of controversy is the use and regimen of preoperative radiotherapy with the NCCN guidelines as the data are limited to support the role of radiotherapy in the setting of neoadjuvant treatment. Several trials have evaluated the effectiveness of preoperative chemotherapy alone or in combination with radiation [49]. While the majority of these trials appear to demonstrate benefit from neoadjuvant chemotherapy and radiation, the optimal treatment regimens to best improve overall survival and/or progression-free survival have not been determined [44]. In general, these trials have shown that the primary benefit of chemotherapy is for treating systemic disease, while radiotherapy treats the primary tumor and involved vessels.
Another associated controversy is the role of preoperative therapy in “downstaging” tumor based on imaging. Recent studies have shown that imaging may not be reliable in determining resectability in patients who have undergone preoperative therapy [8]. In a study of 129 patients treated with neoadjuvant therapy, only 1 patient was downstaged to resectable disease (no tumor involvement of notable vessels) based on cross-sectional imaging findings. However, 81 out of 101 patients in the same group that eventually had surgery achieved an R0 resection [42]. Hence, distinguishing tumor fibrosis from viable tumor on imaging remains a challenge, with studies showing relatively low sensitivity and specificity (71% and 58%, respectively) for cross-sectional imaging to identify viable tumor [50]. Patients with borderline resectable disease are increasingly considered surgical candidates if they do not have imaging features of disease progression or do not develop distant metastases [51]. Other important clinical features that appear to correlate with improved survival are patient’s performance status and whether during therapy he or she shows a decrease in biochemical markers such as CA 19-9. For example, in a study of 41 patients with pancreaticobiliary tumors, patients who were considered resectable after neoadjuvant therapy, 34/41 (83%) showed on average a decrease in CA 19-9 of 87% [50].
Overall, while controversies exist regarding the definition of borderline resectable disease and the best treatment approaches, there is a broad agreement that the goal of preoperative therapy is to improve the patient’s likelihood of achieving an R0 resection and that neoadjuvant therapy is a means to doing so. Though imaging following preoperative therapy may not reliably show whether viable tumor continues to involve vessels, imaging can be helpful to identify clear signs of disease progression, such as the development of distant metastases while on therapy, which renders a given patient unresectable. It is also important to reiterate that other patient factors such as performance status and change in CA 19-9 levels are additional factors to be considered in identifying which patients have sufficiently responded to neoadjuvant therapy to benefit from attempted surgical resection.
Summary
Surgical resection with R0 margin is the only potentially curative option for PDAC. Unfortunately, only a small subset of patients present at an early enough stage that a potentially curative resection can be considered. Determining resectability and predicting prognosis for PDAC are dependent on accurate staging of the disease. The primary goal of the most recent revision of the AJCC staging system is to aid in the determination of a given patient’s prognosis. In contrast, clinical management guidelines based on clinical and radiographic examinations have been developed by several societies; one of the most notable and comprehensive being those of the NCCN. Various guidelines, including those of the NCCN, typically divide patients into the broad categories of resectable, borderline resectable and unresectable disease based on imaging findings. For clearly resectable disease, upfront resection of the pancreatic tumor and regional lymph nodes is recommended. Patients identified as having borderline resectable disease and select patients with resectable disease but with high-risk features are recommended by the NCCN to undergo neoadjuvant therapy in order to increase the chances for an R0 resection. Patients with locally advanced unresectable disease can undergo chemotherapy and chemoradiation or SBRT, and those with metastatic disease are best treated with chemotherapy. Potential palliative measures recommended by the NCCN are to be kept in mind during treatment planning. Imaging plays a central role in determining the extent of disease. Other factors such as CA 19-9 levels and patient performance status should be considered when predicting treatment response and when selecting treatment options.
References
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67(1):7-30. https://doi.org/10.3322/caac.21387
AJCC Cancer Staging Manual. Edge SB et al. New York: Springer. 2010.
AJCC Cancer Staging Manual. Eighth Edition. Amin MB et al. New York: Springer 2017.
NCCN clinical practice guidelines in oncology - Pancretic adenocarcinoma. V1.2019 - November 8, 2018. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Published Accessed on 1/20/2019.
Saka B, Balci S, Basturk O, Bagci P, Postlewait LM, Maithel S, Knight J, El-Rayes B, Kooby D, Sarmiento J, Muraki T, Oliva I, Bandyopadhyay S, Akkas G, Goodman M, Reid MD, Krasinskas A, Everett R, Adsay V. Pancreatic Ductal Adenocarcinoma is Spread to the Peripancreatic Soft Tissue in the Majority of Resected Cases, Rendering the AJCC T-Stage Protocol (7th Edition) Inapplicable and Insignificant: A Size-Based Staging System (pT1: </=2, pT2: > 2- </=4, pT3: > 4 cm) is More Valid and Clinically Relevant. Ann Surg Oncol 2016;23(6):2010-2018. https://doi.org/10.1245/s10434-016-5093-7
Adsay NV, Bagci P, Tajiri T, Oliva I, Ohike N, Balci S, Gonzalez RS, Basturk O, Jang KT, Roa JC. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol 2012;29(3):127-141. https://doi.org/10.1053/j.semdp.2012.08.010
Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakashima A, Yuasa Y, Kondo N, Ohge H, Sueda T. Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. J Am Coll Surg 2010;211(2):196-204. https://doi.org/10.1016/j.jamcollsurg.2010.03.037
Strobel O, Hinz U, Gluth A, Hank T, Hackert T, Bergmann F, Werner J, Buchler MW. Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann Surg 2015;261(5):961-969. https://doi.org/10.1097/sla.0000000000000814
Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16(7):1727-1733. https://doi.org/10.1245/s10434-009-0408-6
Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, Macari M, Megibow AJ, Miller FH, Mortele KJ, Merchant NB, Minter RM, Tamm EP, Sahani DV, Simeone DM. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014;270(1):248-260. https://doi.org/10.1148/radiol.13131184
Vachiranubhap B, Kim YH, Balci NC, Semelka RC. Magnetic resonance imaging of adenocarcinoma of the pancreas. Top Magn Reson Imaging 2009;20(1):3-9. https://doi.org/10.1097/rmr.0b013e3181b48392
Dolejs S, Zarzaur BL, Zyromski NJ, Pitt HA, Riall TS, Hall BL, Behrman SW. Does Hyperbilirubinemia Contribute to Adverse Patient Outcomes Following Pancreatoduodenectomy? J Gastrointest Surg 2017;21(4):647-656. https://doi.org/10.1007/s11605-017-3381-6
Rijkers AP, Valkema R, Duivenvoorden HJ, van Eijck CH. Usefulness of F-18-fluorodeoxyglucose positron emission tomography to confirm suspected pancreatic cancer: a meta-analysis. Eur J Surg Oncol 2014;40(7):794-804. https://doi.org/10.1016/j.ejso.2014.03.016
Wang Z, Chen JQ, Liu JL, Qin XG, Huang Y. FDG-PET in diagnosis, staging and prognosis of pancreatic carcinoma: a meta-analysis. World J Gastroenterol 2013;19(29):4808-4817. https://doi.org/10.3748/wjg.v19.i29.4808
Allen VB, Gurusamy KS, Takwoingi Y, Kalia A, Davidson BR. Diagnostic accuracy of laparoscopy following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev 2013(11):CD009323. https://doi.org/10.1002/14651858.cd009323.pub2
Ahmed SI, Bochkarev V, Oleynikov D, Sasson AR. Patients with pancreatic adenocarcinoma benefit from staging laparoscopy. J Laparoendosc Adv Surg Tech A 2006;16(5):458-463. https://doi.org/10.1089/lap.2006.16.458
Morris-Stiff G, Taylor MA. Ca19-9 and pancreatic cancer: Is it really that good? J Gastrointest Oncol 2012;3(2):88-89. https://doi.org/10.3978/j.issn.2078-6891.2012.016
Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol 2012;3(2):105-119. https://doi.org/10.3978/j.issn.2078-6891.2011.021
Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297(3):267-277. https://doi.org/10.1001/jama.297.3.267
Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML, Haddock MG, Schaefer P, Willett CG, Rich TA. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299(9):1019-1026. https://doi.org/10.1001/jama.299.9.1019
Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Buchler MW, European Study Group for Pancreatic C. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350(12):1200-1210. https://doi.org/10.1056/nejmoa032295
Bilimoria KY, Talamonti MS, Sener SF, Bilimoria MM, Stewart AK, Winchester DP, Ko CY, Bentrem DJ. Effect of hospital volume on margin status after pancreaticoduodenectomy for cancer. J Am Coll Surg 2008;207(4):510-519. https://doi.org/10.1016/j.jamcollsurg.2008.04.033
Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS, Schulick RD, Choti MA, Lillemoe KD, Yeo CJ. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg 2006;10(9):1199-1210; discussion 1210-1191. https://doi.org/10.1016/j.gassur.2006.08.018
Tol JA, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M, Andren-Sandberg A, Asbun HJ, Bockhorn M, Buchler MW, Conlon KC, Fernandez-Cruz L, Fingerhut A, Friess H, Hartwig W, Izbicki JR, Lillemoe KD, Milicevic MN, Neoptolemos JP, Shrikhande SV, Vollmer CM, Yeo CJ, Charnley RM, International Study Group on Pancreatic S. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014;156(3):591-600. https://doi.org/10.1016/j.surg.2014.06.016
Michalski CW, Kleeff J, Wente MN, Diener MK, Buchler MW, Friess H. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg 2007;94(3):265-273. https://doi.org/10.1002/bjs.5716
Esnaola NF, Chaudhary UB, O’Brien P, Garrett-Mayer E, Camp ER, Thomas MB, Cole DJ, Montero AJ, Hoffman BJ, Romagnuolo J, Orwat KP, Marshall DT. Phase 2 trial of induction gemcitabine, oxaliplatin, and cetuximab followed by selective capecitabine-based chemoradiation in patients with borderline resectable or unresectable locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2014;88(4):837-844. https://doi.org/10.1016/j.ijrobp.2013.12.030
Festa V, Andriulli A, Valvano MR, Uomo G, Perri F, Andriulli N, Corrao S, Koch M. Neoadjuvant chemo-radiotherapy for patients with borderline resectable pancreatic cancer: a meta-analytical evaluation of prospective studies. JOP 2013;14(6):618-625. https://doi.org/10.6092/1590-8577/1724
Kim EJ, Ben-Josef E, Herman JM, Bekaii-Saab T, Dawson LA, Griffith KA, Francis IR, Greenson JK, Simeone DM, Lawrence TS, Laheru D, Wolfgang CL, Williams T, Bloomston M, Moore MJ, Wei A, Zalupski MM. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer 2013;119(15):2692-2700. https://doi.org/10.1002/cncr.28117
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17(6):1471-1474. https://doi.org/10.1245/s10434-010-0985-4
Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15(6):2403-2413. https://doi.org/10.1200/jco.1997.15.6.2403
Crane CH, Ben-Josef E, Small W, Jr. Chemotherapy for pancreatic cancer. N Engl J Med 2004;350(26):2713-2715; author reply 2713-2715.
Liao WC, Chien KL, Lin YL, Wu MS, Lin JT, Wang HP, Tu YK. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysis. Lancet Oncol 2013;14(11):1095-1103. https://doi.org/10.1016/s1470-2045(13)70388-7
Crane CH, Abbruzzese JL, Evans DB, Wolff RA, Ballo MT, Delclos M, Milas L, Mason K, Charnsangavej C, Pisters PW, Lee JE, Lenzi R, Vauthey JN, Wong AB, Phan T, Nguyen Q, Janjan NA. Is the therapeutic index better with gemcitabine-based chemoradiation than with 5-fluorouracil-based chemoradiation in locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys 2002;52(5):1293-1302.
Huang J, Robertson JM, Margolis J, Balaraman S, Gustafson G, Khilanani P, Nadeau L, Jury R, McIntosh B. Long-term results of full-dose gemcitabine with radiation therapy compared to 5-fluorouracil with radiation therapy for locally advanced pancreas cancer. Radiother Oncol 2011;99(2):114-119. https://doi.org/10.1016/j.radonc.2011.05.038
Faris JE, Blaszkowsky LS, McDermott S, Guimaraes AR, Szymonifka J, Huynh MA, Ferrone CR, Wargo JA, Allen JN, Dias LE, Kwak EL, Lillemoe KD, Thayer SP, Murphy JE, Zhu AX, Sahani DV, Wo JY, Clark JW, Fernandez-del Castillo C, Ryan DP, Hong TS. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist 2013;18(5):543-548. https://doi.org/10.1634/theoncologist.2012-0435
Bickenbach KA, Gonen M, Tang LH, O’Reilly E, Goodman K, Brennan MF, D’Angelica MI, Dematteo RP, Fong Y, Jarnagin WR, Allen PJ. Downstaging in pancreatic cancer: a matched analysis of patients resected following systemic treatment of initially locally unresectable disease. Ann Surg Oncol 2012;19(5):1663-1669. https://doi.org/10.1245/s10434-011-2156-7
Chatzizacharias NA, Tsai S, Griffin M, Tolat P, Ritch P, George B, Barnes C, Aldakkak M, Khan AH, Hall W, Erickson B, Evans DB, Christians KK. Locally advanced pancreas cancer: Staging and goals of therapy. Surgery 2018;163(5):1053-1062. https://doi.org/10.1016/j.surg.2017.09.012
Mehta VK, Fisher G, Ford JA, Poen JC, Vierra MA, Oberhelman H, Niederhuber J, Bastidas JA. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg 2001;5(1):27-35.
Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, Lee JE, Pisters PW, Evans DB, Wolff RA. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13(8):1035-1046. https://doi.org/10.1245/aso.2006.08.011
Deal S, Nathan D, Rocha FG. Modified Appleby procedure for locally advanced pancreatic cancer. Am J Surg 2018;215(5):853-855. https://doi.org/10.1016/j.amjsurg.2018.01.004
Katz MH, Crane CH, Varadhachary G. Management of borderline resectable pancreatic cancer. Semin Radiat Oncol 2014;24(2):105-112. https://doi.org/10.1016/j.semradonc.2013.11.006
Katz MH, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, Wang H, Abbruzzese J, Pisters PW, Vauthey JN, Charnsangavej C, Tamm E, Crane CH, Balachandran A. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118(23):5749-5756. https://doi.org/10.1002/cncr.27636
Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, Asbun HJ, Bassi C, Buchler M, Charnley RM, Conlon K, Cruz LF, Dervenis C, Fingerhutt A, Friess H, Gouma DJ, Hartwig W, Lillemoe KD, Montorsi M, Neoptolemos JP, Shrikhande SV, Takaori K, Traverso W, Vashist YK, Vollmer C, Yeo CJ, Izbicki JR, International Study Group of Pancreatic S. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155(6):977-988. https://doi.org/10.1016/j.surg.2014.02.001
Toesca DAS, Koong AJ, Poultsides GA, Visser BC, Haraldsdottir S, Koong AC, Chang DT. Management of Borderline Resectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys 2018;100(5):1155-1174. https://doi.org/10.1016/j.ijrobp.2017.12.287
Katz MH, Marsh R, Herman JM, Shi Q, Collison E, Venook AP, Kindler HL, Alberts SR, Philip P, Lowy AM, Pisters PW, Posner MC, Berlin JD, Ahmad SA. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 2013;20(8):2787-2795. https://doi.org/10.1245/s10434-013-2886-9
Soloff EV, Zaheer A, Meier J, Zins M, Tamm EP. Staging of pancreatic cancer: resectable, borderline resectable, and unresectable disease. Abdom Radiol (NY) 2018;43(2):301-313. https://doi.org/10.1007/s00261-017-1410-2
Illuminati G, Carboni F, Lorusso R, D’Urso A, Ceccanei G, Papaspyropoulos V, Pacile MA, Santoro E. Results of a pancreatectomy with a limited venous resection for pancreatic cancer. Surg Today 2008;38(6):517-523. https://doi.org/10.1007/s00595-007-3661-y
Hamidian Jahromi A, Jafarimehr E, Dabbous HM, Chu Q, D’Agostino H, Shi R, Wellman GP, Zibari GB, Shokouh-Amiri H. Curative resection of pancreatic adenocarcinoma with major venous resection/repair is safe procedure but will not improve survival. JOP 2014;15(5):433-441. https://doi.org/10.6092/1590-8577/2430
Hoffe S, Rao N, Shridhar R. Neoadjuvant vs adjuvant therapy for resectable pancreatic cancer: the evolving role of radiation. Semin Radiat Oncol 2014;24(2):113-125. https://doi.org/10.1016/j.semradonc.2013.11.002
Donahue TR, Isacoff WH, Hines OJ, Tomlinson JS, Farrell JJ, Bhat YM, Garon E, Clerkin B, Reber HA. Downstaging chemotherapy and alteration in the classic computed tomography/magnetic resonance imaging signs of vascular involvement in patients with pancreaticobiliary malignant tumors: influence on patient selection for surgery. Arch Surg 2011;146(7):836-843. https://doi.org/10.1001/archsurg.2011.152
Dholakia AS, Hacker-Prietz A, Wild AT, Raman SP, Wood LD, Huang P, Laheru DA, Zheng L, De Jesus-Acosta A, Le DT, Schulick R, Edil B, Ellsworth S, Pawlik TM, Iacobuzio-Donahue CA, Hruban RH, Cameron JL, Fishman EK, Wolfgang CL, Herman JM. Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiographic appearance of tumor-vessel relationships. J Radiat Oncol 2013;2(4):413-425. https://doi.org/10.1007/s13566-013-0115-6
Acknowledgements
The authors wish to thank the members of Society of Abdominal Radiology’s Disease-Focused Panel for Pancreatic Cancer for their suggestions and input in selecting the topics for the white paper (Ajit Goenka, MD; Alexander Guimares MD, PhD; Arnold Friedman, MD; Atif Zaheer, MD; David Hough, MB ChB; Michael Rosenthal, MD, PhD; Namita Gandhi, MD; Ott Le, MD; Richard Do, MD, PhD; Zarine Shah, MD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kulkarni, N.M., Soloff, E.V., Tolat, P.P. et al. White paper on pancreatic ductal adenocarcinoma from society of abdominal radiology’s disease-focused panel for pancreatic ductal adenocarcinoma: Part I, AJCC staging system, NCCN guidelines, and borderline resectable disease. Abdom Radiol 45, 716–728 (2020). https://doi.org/10.1007/s00261-019-02289-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02289-5