Abstract
Purpose
To identify the predictors of malignancy on CT for the evaluation of gastrointestinal stromal tumors (GIST) by correlating CT findings with the mitotic index in order to propose a “CT-based predictive model of Miettinen index.”
Methods
One radiologist and one resident in radiology with 14- and 4-year experience in oncological field reviewed the CT findings of 42 patients by consensus, with respect to lesion site, size, contour, tumor growth pattern, enhancing pattern, degree of enhancement of tumor, percentage of tumor necrosis, mesenteric fat infiltration, ulceration, calcification, regional lymphadenopathy, direct invasion to adjacent organs, and distant metastasis. All parameters were correlated with the mitotic index evaluated at histopathological analysis following surgery. Normality of variables was evaluated using Shapiro–Wilk test. Pearson’s correlation test was used to assess the interaction between variables. The diagnostic accuracy percentage of tumor necrosis was measured by receiver operating characteristic (ROC) analysis for detecting whether the number of mitosis per 50 high-power fields was > 5.
Results
A significant statistical correlation was found between percentage of tumor necrosis and the mitotic index (p < 0.005), dimension, and location of the tumor.
Conclusion
CT could be an accurate technique in the prediction of malignancy of GIST in a CT risk assessment system, based on the location of the tumor, its size, and the percentage of tumor necrosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although Gastrointestinal stromal tumors (GISTs) are uncommon abdominal neoplasia, they represent the most common mesenchymal tumors of the gastrointestinal tract (1–2% of all gastrointestinal neoplasms) [1, 2].
GISTs can originate in any site along the gastrointestinal tract (GI), although they are most frequently found inside the stomach (from 45 to 65%), followed by those found inside the small intestine (15% to 25%), colon (5% to 10%), and esophagus (5%). However, GISTs may also develop as primary tumors of the omentum, mesentery, or retroperitoneum [3]. The discovery of GISTs’ origin from Cajal’s interstitial cells and the expression of the c-kit protein (CD117), a transmembrane receptor with a tyrosine kinase activity, that is responsible for various cellular functions, prompted to consider these tumors as well-defined entities [3]. GISTs have malignant potential with varying degrees of aggressiveness closely related to their clinical manifestations and subsequent treatment and prognosis [4, 5]. In GISTs, tumor size and mitotic count, the most important features describing the neoplastic risk, can be classified with many systems, generally through surgical excision, but also with biopsy, even if less accurately. Furthermore, numerous authors observed that GISTs’ behavior vary in relation to the anatomical location: the primary GISTs of the stomach are less aggressive than tumors in other locations, especially the small intestine [6]. Miettinen and Lasota [3] proposed a risk assessment scheme that differentiated risk for gastric from intestinal GISTs. More recently Miettinen and Lasota [7] refined their risk assessment table based on follow-up information about over 1900 patients who had been affected by GIST over time; anatomic site, tumor size (maximum diameter in centimeters), and mitotic rate were suggested as the most important factors for predicting biological risk or malignant potential of GISTs [1, 3, 8]. From a diagnostic point of view, even though a recent investigation has suggested that magnetic resonance imaging (MRI) could play an important role in predicting the high malignancy potential of GISTs [9], CT is still considered the technique of choice for detecting and characterizing GISTs. It provides indeed information about tumor size, anatomic location, growth pattern, evidence of necrosis, invasion of adjacent organs, and metastasis and also to monitor response to treatment and to assess disease progression [10,11,12,13].
In particular, some authors have attempted to find the association between CT findings and pathological features, specifically biological risk of GISTs; however, these findings are still conflicting and there is no consensus [6, 9, 14]. Considering the previous statement and that the risk assessment of GISTs, according to Miettinen and Lasota, depends on the combination of mitotic rate, site, and size of tumor, the last two characteristics which can be defined properly on a CT scan, we searched for a possible CT surrogate of mitotic index which can be useful both to improve the role of CT in the pre-operative prognostic evaluation of these tumors and, in particular, to propose a “CT-based predictive model of Miettinen index.”
Materials and methods
Patient selection
This retrospective study was approved by the institutional review board at our hospital. The requirement for written informed consent was ignored because of the retrospective study design. We reviewed the pathological and radiological findings of 72 patients with histopathological diagnosis of GIST who had been treated at our hospital from January 2008 to December 2018. Among them, 30 patients were excluded because they had not undergone abdominal CT scans at our hospital (n = 27), no histopathological data of surgical specimens were available (n = 2), and because they had undergone neoadjuvant chemotherapy before surgery (n = 1). In order to avoid measurement bias, from the beginning we also decided to exclude GISTs smaller than 5 mm in diameter and incidentally detected at pathological examination because they had not been seen on CT images. Forty-two patients (24 males and 18 females, mean age 68 years, range 26–91 years) were finally enrolled in this study. Inclusion criteria were stromal tumor originating in the GI tract; CT examination performed within 15 days before the surgery; surgical intervention performed at our hospital; and tumor size bigger than 5 mm in diameter.
CT examination
Abdominal CT scans (CTs) were obtained using a 4-detector row configuration (LightSpeed Plus, General Electric Healthcare, Milwaukee, USA) for 18 patients, using a 16-detector row (LightSpeed 16 Pro, General Electric Healthcare, Milwaukee, USA) configuration for 12 patients and using a 64-detector row configuration (VCT, General Electric Healthcare, Milwaukee, USA) for the remaining 12 patients. In all patients, the exam was performed with a spiral technique in a cranio-caudal direction (from the base of the lungs to the pelvic brim) and supine position, using unenhanced and contrast-enhanced CTs in 36 patients, while in 6 patients the unenhanced phase was not performed. All the contrast-enhanced CTs were done in the late arterial phase (delay 45–50 s) and in the portal venous phase (delay 70–80 s) with an intravenous injection of 2 mL/kg of non-ionic contrast material (Iopamiro 370; Bracco Diagnostics, Milan, Italy), followed by 30 mL of saline solution using a peristaltic semiautomated power injector (3–4 mL/s flow rate, SIAS 757, Bologna Italy) with an 18-gauge needle in the antecubital vein. The technical parameters are reported in Table 1. An automatic current modulation tube was used to minimize radiation exposure. A standard reconstruction algorithm was used. Patients were instructed not to breath during helical imaging in order to avoid motion artifacts.
Image analysis
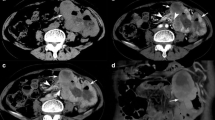
A radiologist and a resident in radiology (with 14 and 4 years’ experience in the oncologic field, respectively), who were blind to the surgical and pathological data, independently reviewed the CT images for each of the 42 GIST patients and reached a consensus regarding the final interpretation. The reviewers evaluated only the CT scans obtained during the portal venous phase for tumor evaluation and all CT scans for metastasis evaluation. Each CT scan was analyzed on a reconstruction and image interpretation console (Advantage Workstation 4.4, GE Healthcare, Milwaukee, WI, USA), adjusting the window level and width on images each time, and using a 2D multiplanar reconstruction technique (coronal, sagittal, and oblique planes). CT images were reviewed to determine the following characteristics of each lesion: site, size (diameter and area), area and percentage of necrosis of the tumor mass, contour, growth pattern, enhancing pattern, degree of enhancement, mesenteric fat infiltration, ulceration, calcification, regional lymphadenopathy, ascites, direct invasion of adjacent organs, and distant metastasis. In relation to site, lesions were classified according to the gastrointestinal segment of origin (gastric and non-gastric GIST). The size of each tumor was measured either in the greatest diameter (expressed as mm) or in the area (expressed as mm2). Tumor necrosis was considered present if low attenuation area was visually identified within the mass with corresponding Hounsfield units (HU) measuring between 0 and 30 HU in the portal venous phase and without any relevant HU increase (up to a maximum of 5 HU) between unenhanced and post-contrast images. In the 6 cases in which an unenhanced scan had not been performed, only HU values in the portal venous phase were considered. Percentage of necrosis was calculated as the ratio between the area of hypodensity and the total area of the tumor, both measured by freehand drawing 2 area of interest (ROI), in the axial plane, on the CT slice representative of tumor largest diameter (Fig. 1). Lesion contours were classified as either round or lobulated. Tumor growth patterns were classified as endoluminal, exophytic, or mixed. Endoluminal growth was defined when the tumor was confined to the bowel lumen, without invasion of the extraluminal space, whereas exophytic growth pattern was defined when the tumor was confined to the extraluminal space without bulging into the bowel lumen. Enhancement patterns (i.e., homogeneous or heterogeneous) were assessed subjectively, whereas the degree of enhancement of each tumor was obtained both as absolute and relative enhancement compared to the aorta. Absolute enhancement was defined as the difference in mean HU between the unenhanced phase and portal venous phase, placing a ROI on the lesion (including both the solid and hypodense portions, if present). Relative enhancement to the aorta (or aorta-tumor relative enhancement) was also calculated as the difference between mean tumor enhancement and aorta enhancement during the portal venous phase, placing a ROI on the lesion (including both the solid and hypodense portions, if present) and on the aorta. Mesenteric fat infiltration was considered when the margin of the mass was indistinct, while ulceration was considered present when a focal tissue defect filled with air or fluid was found in the mass lesion. The presence of calcifications was reported when high-attenuation foci were found within the mass lesion. Regional lymph nodes were considered pathological if the short-axis diameter was larger than 1 cm or if their enhancement was similar to the lesion enhancement. We finally evaluated the correlations between each CT finding and mitotic index, in order to investigate a possible CT surrogate of this histological parameter.
Histology
The tumor samples were fixed in 10% buffered formalin and margins were inked in order to allow the most accurate assessment of their status. An accurate macroscopic description was done including tumor size, macroscopic ulceration, necrotic or haemorrhagic areas, tumor rupture, and extraparietal extension. The samples were included in paraffin, sectioned at 4 micron, and colored with Hematoxylin–Eosin. Immunohistochemical evaluations with primary antibodies CD117, CD34, DOG1, desmin, smooth actin, S100, and Ki67 were performed. At microscopic examination, GISTs were classified in spindle cell, epithelioid and mixed subtypes. Mitotic figures were counted in 50 fields (at 400 ×) by searching the most mitotically active areas. Additional parameters were evaluated in all cases: nuclear atypia, coagulative necrosis, ulceration, mucosal invasion, level of parietal diffusion, status of the margins. The pathology report included the size, the mitotic count, the anatomic location, the status of the margins, the immunophenotype, and the proliferation rate evaluated by Ki67 antibody. The risk assessment was reported according to Miettinen and Lasota [3].
Statistical analysis
Cohen’s weighted kappa with bootstrapped confidence interval was run to determine if there was agreement between CT and histological ordinal variables. Normality of variables was evaluated using Shapiro–Wilk test. Wilcoxon–Mann–Whitney test was used to compare “≤ 5 mitosis” and “> 5 mitosis” groups on continuous or interval distributed variables, while Fisher’s exact test was used on categorical variables. Spearman’s test was run to test correlation between high mitotic index (> 5 mitotic figures per HPF) and CT variables. A ROC analysis was performed to choose a cut-off of CT percentage of hypodensity suitable to replace the histological mitotic index in a possible “CT-based predictive model of Miettinen index” and in particular to search for a cut-off of CT percentage of hypodensity useful to define if the number of mitosis per 50 high-power fields was > 5. Pearson’s phi correlation test was used to evaluate the interaction between the presence of metastasis and other categorical variables. Statistical tests were two-tailed. A p value of less than 0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed with Stata software (version 12.1, StataCorp, Texas).
Results
GIST site, size, and histology
All analyzed GISTs had positive expression of c-kit protein (CD117) at immunohistochemical analysis. Tumors were located in the stomach (gastric GISTs) in 31 out of 42 (74%) cases and in the other portions of gastrointestinal tract (non-gastric GISTs) in the remaining 11 (26%) cases (duodenum, n = 4, 10%; jejunum, n = 4, 10%; ileum, n = 2, 4%; and omentum, n = 1, 2%). There was a complete concordance regarding the lesion site between CT and pathology. There was a high concordance between CT and pathology also regarding lesion size category (≤ 2 cm, 2-5 cm, 5–10 cm, > 10 cm) (Cohen’s weighted k = 0.95, 95% CI 0.86–1.00). In particular, lesions’ size varied between 15 and 313 mm, with an average size of 97 mm for CT and between 15 and 380 mm with an average size of 94.7 mm for pathology. Thirty out of 42 (71%) GISTs presented a mitotic index ≤ 5, whereas 12 (29%) out of 42 GISTs presented a mitotic index > 5. The correlations between each CT finding and mitotic index are reported in Table 2.
CT features and mitotic index
There were several significant differences between low mitotic index (≤ 5 mitoses) and high mitotic index (> 5 mitoses) GISTs with respect to CT features including the lesions size (area and maximum diameter) and the area and percentage of necrosis (area of hypodensity within the lesion). However, CT features that more strictly correlated with high mitotic index GISTs were the percentage of hypodensity (R = p = 0.001) and the area of hypodensity (p ≤ 0.001). The CT percentage of hypodensity was more strictly correlated with GIST’s size, and thus it was chosen as the CT feature to compare with the mitotic index in creating a possible “CT-based predictive model of Miettinen index” for evaluating the CT malignancy risk of GISTs. ROC analysis revealed both a value of 10 and 20% of CT percentage of hypodensity as an accurate cut-off. In particular, using a cut-off value of 10% all GISTs with a mitotic index > 5% were correctly classified presenting a percentage of hypodensity > 10% (12 out of 12), whereas using the cut-off value of 20% the GISTs with a mitotic index > 5 were correctly identified in 91.6% (11 out of 12) (Table 3). ROC results are presented more completely in Table 4.
Correlation between CT percentage of hypodensity and the presence of metastatic lesions
Eleven patients (36%) had metastatic lesion on CT scans: 7 patients (16%) were diagnosed with lymph-nodal metastasis, whereas 4 patients were diagnosed with both liver and lymph-nodal metastases, confirmed at surgical examination. Pearson’s phi correlation test, used to evaluate the interaction between the presence of metastasis and mitotic index > 5, percentage of hypodensity > 10% and > 20%, resulted in 0.34, 0.54, and 0.60, respectively, revealing the percentage value of hypodensity > 20% as a possible accurate CT index to predict the metastatic potential of GISTs. Seven out of 11 metastatic patients had a GIST with a mitotic number > 5. All the metastatic patients (n = 7) with a GIST mitotic index > 5 presented a CT percentage of hypodensity higher than 20% (mean value of 38.5%, ranging from 36 to 46%). Mesenteric fat infiltration was observed in 13 patients; 6 of these presented a mitotic index ≤ 5 and 12 of these patient presented a percentage value of hypodensity > 20%.
Miettinen risk assessment and “modified Miettinen CT index”
A comparison between risk stratification of GIST according to Miettinen Classification, which considers the lesion site, size, and value of mitotic index (≤ or > 5), and the CT-based predictive model of Miettinen Index, which considers the percentage of hypodensity, is reported in Table 5. The cut-off value > 20%, instead of > 10%, was chosen because it was more accurate in predicting the metastatic potential of GISTs, only with a slight decrease of the sensitivity in predicting the mitotic index (sensitivity 91% vs 100% for > 20 and > 10%, respectively) but with the same accuracy. The Cohen’s weighted k between Miettinen risk assessment and “modified Miettinen CT index” was 0.80 (95% CI 0.66–0.92). Moreover, Fisher’s Exact Test identify a good correlation between “CT-based predictive model of Miettinen index” and the presence of lymph-nodal metastasis with a p value = 0.02128. There was a 100% agreement (7 patients) in classifying GISTs between the “CT-based predictive model of Miettinen index” and “classic Miettinen index” and in particular the “CT-based predictive model of Miettinen index” correctly classifies 5 out of 7 patients with lymph-nodal metastasis in class 3 risk.
Discussion
GISTs have a complex biological behavior which makes predicting their malignant potentially difficult. For this reason, all GISTs are virtually considered malignant regardless of their size. However, many efforts have been made over the years to design useful criteria to stratify GISTs according to risk of recurrence or metastasis. The National Institute Health Consensus Classification [15] first proposed a risk assessment classification for GISTs, based on tumor size and mitotic index. The latter correlates with the proliferative activity of tumors, suggesting a more aggressive biological behavior when mitotic index is > 5. In 2006, Miettinen and Lasota introduced a new predictive factor in addition to size and mitotic index: the location of the lesion. In particular, they proposed a new pathological classification, differentiating GISTs in four risk groups after surgery: high, intermediate, low, and very low. Subsequently, few other modifications were proposed by other authors. In 2012, Joensuu et al., evaluating a cohort of 2560 patients with operable GISTs who had not received adjuvant Imatinib, demonstrated that large tumor size, high mitotic count, non-gastric location, tumor rupture, and male sex were independent significant prognostic factors [16]. Recently, there has been an increasing interest in identifying imaging biomarkers which can predict risk of recurrence in GIST patients. In fact, mitotic count in histopathology can be subject to variations between different institutions, because of the variability in expertise of subjective assessment, or because of alteration following neoadjuvant Imatinib therapy and the possibility of non-representative biopsy samples due to tumor heterogeneity [17]. Accordingly, prediction of the risk based on pre-operative/pre-treatment imaging feature can be attractive [18, 19]. Several authors tried to find a correlation between CT features and prediction of malignancy on GIST. Lupescu et al. found that large primary tumor size, heterogeneous enhancement, and central necrosis reflected the tendency toward malignant GISTs in their series [20]. Yang et al. and Kim et al. described the correlation between high mitotic index and mucosal ulceration, mesenteric fat infiltration, metastasis, and GISTs’ size [21, 22]. In particular, they demonstrated the correlation between GISTs with maximum diameter greater than 5 cm and aggressive behavior, but they said that a differential diagnosis between benign and malignant GISTs was not possible on CT. Ulusan et al. claimed that size, location, enhancement pattern, metastasis, and cystic-necrotic component of the tumors were all associated with a high mitotic index [23]. In Yin et al. experience, the GISTs with maximum diameter larger than 50 mm, irregular shape, invasive growth, presence of cystic areas, and heterogeneous enhancement were at high risk [24]. More recently, Chen et al. claimed that CT features may be more useful than endoscopic ultrasound features for predicting tumor mitotic index [12]; however, other authors did not find statistical correlation between imaging characteristics and malignity potential [25,26,27].
In our study, according to the literature, we found a complete concordance (100%) between CT and pathology regarding the location of the lesions, moreover highlighting that the stomach is the most affected site. Regarding the size, we had a range variable from 15 mm to more than 380 mm and also for this parameter we found a good concordance between CT and pathology. Then, considering that CT is a valid imaging technique in the detection of GISTs’ features, we tried to find a correlation between the histological parameters, proposed by Miettinen and Lasota, and CT features in order to propose a “CT-based predictive model of Miettinen index.” In particular, we searched for a CT feature that correlates with the mitotic index, analyzing the CT percentage of hypodensity of the tumor mass, contours, tumor growth pattern, enhancing pattern, degree of enhancement, mesenteric fat infiltration, ulceration, calcification, regional lymphadenopathy, ascites, direct invasion into adjacent organs, and distant metastasis, according with the previous experiences in the literature.
A significant correlation (p = 0.0056) was found between high mitotic rate and high CT percentage of intralesional hypodensity that was calculated when the mass contained areas of low attenuation. In fact, using the cut-off value > 20% GISTs with a mitotic index > 5 were correctly identified in 91.6% of the cases (11 out of 12). Furthermore, using the CT percentage of intralesional hypodensity as a CT surrogate of mitotic count, we built a CT-based predictive model of Miettinen index, based on the combination of site, size, and CT percentage of hypodensity, which demonstrates a good correlation (Cohen’s weighted k of 0.80) with Miettinen risk assessment index in risk stratification of GIST patients. Finally, the Pearson’s phi correlation test, used to evaluate the interaction between the presence of metastasis and mitotic index > 5, CT percentage of hypodensity > 10% and > 20%, resulted in 0.34, 0.54, and 0.60, respectively, revealing the CT percentage value of hypodensity > 20% as a possible accurate CT index to predict the metastatic potential of GISTs.
Our study reveals an interesting result regarding lymph-nodal metastasis: 7 patients out of 42 (16%) had lymph-nodal metastasis and 4 patients were diagnosed with both liver and lymph-nodal metastases, confirmed at surgical examination. However, it is known that lymph-nodal metastasis is not a common feature in patients with GISTs, and our results were supported by several articles in the literature. Wada et al. [28] reported a series of “wild type” GISTs with frequent lymph-nodal metastasis (in our case series 1 out of 7 patients has this type of GIST). Moreover, in the literature a lymph-nodal metastasis frequency ranging from 9.8% to 20.7% was reported [29, 30], as shown also by our results that highlight a 16% of patients with lymph-nodal metastasis. In accordance with Gong et al. [30], our group of patients with lymph-nodal metastasis tend to be of older ages. Furthermore, there was a 100% agreement (7 patients) in classifying GISTs between the “CT-based predictive model of Miettinen index” and “classic Miettinen index” and in particular the “CT-based predictive model of Miettinen index” correctly classifies 5 out of 7 patients with lymph-nodal metastasis in class 3 risk. Because lymphatic metastasis rarely occurs in patients with GIST [31,32,33], nodal dissection was not routinely performed. In our case series, all patients with any suspicion of nodal metastasis underwent nodal dissection with proven synchronous nodal metastasis. Probably the lack of routine nodal dissection creates a bias in metastatic nodal status evaluation in the literature. In fact, many case reports depict the presence of positive nodal metastasis in patients with GISTs [34, 35]; however, these results should be supported by further study with larger sample size.
Some limitations of our study should be noted. First, it is a retrospective study, although all CT examinations were re-evaluated in a prospective setting; second, the small size of case population and the disproportion of comparative cohorts (12 GISTs with high mitotic index versus 30 GISTs with low mitotic index); third, in the era of advanced CT imaging, there are no CT examinations performed by using dual-energy CT (DECT) or CT-perfusion (CTp) that could be attractive for a better evaluation and/or detection of CT intralesional hypodensity and enhancement of GISTs [36,37,38,39,40,41].
In conclusion, our “CT-based predictive model of Miettinen index” seems to be effective for the pre-operative/pre-treatment prediction of risk stratifications of GISTs. If the results are confirmed in a larger case series, this model, in addition to other important risk factors such as patient age and comorbidity, could be helpful for a better management of GIST patients, to address them to the best tailored treatment at the time of diagnosis.
References
T.M. Parab, M.J. DeRogati, A.M. Boaz, et al. (2019) Gastrointestinal stromal tumors: a comprehensive review. J Gastrointest Oncol 10:144–154.
A.D. Levy, H.E. Remotti, W.M. Thompson, L.H. Sobin, M. Miettinen (2003) From the Archives of the AFIP. Gastrointestinal Stromal Tumors: radiologic Features with Pathologic Correlation. Radiographics 23:283–304.
M. Miettinen, J. Lasota (2003) Gastrointestinal Stromal Tumors (GISTs): Definition, Occurrence, Pathology, Differential Diagnosis and Molecular Genetics. Pol J Pathol 54:3–24.
M. Bosoteanu, C. Bosoteanu, M. Deacu, M. Aschie (2011) Differential diagnosis of gastric stromal tumor: case report and literature review. Rom J Morphol Embryol 52:1361–1368.
M.A. Mazzei, G. Bagnacci, F. Gentili, et al. (2018) Gastric Cancer Maximum Tumour Diameter Reduction Rate at CT Examination as a Radiological Index for Predicting Histopathological Regression after Neoadjuvant Treatment: A Multicentre GIRCG Study. Gastroenterol Res Pract 2018:1794524. https://doi.org/10.1155/2018/1794524
H.C. Kim, J.M. Lee, S.H. Kim, et al. (2004) Primary Gastrointestinal Stromal Tumors in the Omentum and Mesentery: CT Findings and Pathologic Correlation. AJR 182:1463–1467.
M. Miettinen, J. Lasota (2006) Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 130:1466–1478.
N. Iorio, R.A. Sawaya, F.K. Friedenberg (2014) Review article: the biology, diagnosis and management of gastrointestinal stromal tumours. Aliment Pharmacol Ther 39:1376–1386.
A.D. Levy, H.E. Remotti, W.M. Thompson, L.H. Sobin, M. Miettinen (2003) Anorectal Gastrointestinal Stromal Tumors: CT and MR Imaging Features with Clinical and Pathologic Correlation. AJR 180:1607–1612.
G. De Manzoni, D. Marrelli, G.L. Baiocchi, et al. (2017) The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer 20:20–30.
R. Grassi, A. Pinto, L. Mannelli, D. Marin, M.A. Mazzei (2016) New Imaging in Gastrointestinal Tract. Gastroenterol Res Pract 2016:5785871. https://doi.org/10.1155/2016/5785871
T. Chen, L. Xu, X. Dong, Y. Li, J. Yu, W. Xiong, G. Li (2019) The roles of CT and EUS in the preoperative evaluation of gastric gastrointestinal stromal tumors larger than 2 cm. Eur Radiol. https://doi.org/10.1007/s00330-018-5945-6. [Epub ahead of print].
M. Liu, L. Liu, E. Jin (2018) Gastric sub-epithelial tumors: identification of gastrointestinal stromal tumors using CT with a practical scoring method. Gastric Cancer. https://doi.org/10.1007/s10120-018-00908-6. [Epub ahead of print].
G.L. Pelandré, M.C. Djahjah, E.L. Gasparetto, M.S. Nacif, E. Marchiori, E.L.R. de Mello (2013) Tomographic findings of gastric gastrointestinal stromal tumor and correlation with the mitotic index. Arq Gastroenterol 50:244–250.
C.D. Fletcher, J.J. Berman, C. Corless, et al. (2002) Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 33:459–465.
H. Joensuu, A. Vehtari, J. Riihimäki J, et al. (2012) Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 13:265–274.
A.C. O’Neill, A.B. Shinagare, V. Kurra, et al. (2016) Assessment of metastatic risk of gastric GIST based on treatment –naive CT features. Eur J Surg Oncol 42:1222–1228.
M.R. Ambrosio, B.J. Rocca, M.G. Mastrogiulio, et al. (2014) Cystic gastrointestinal stromal tumors of the pancreas simulating cystoadenocarcinoma. Report of three cases and short review of the literature. Histol Histopathol 29:1583–1591.
M.A. Mazzei, V. Nardone, L. Di Giacomo, et al. (2018) The role of delta radiomics in gastric cancer. Quant Imaging Med Surg. 8:719–721.
I.G. Lupescu, M. Grasu, M. Boros, et al. (2007) Gastrointestinal Stromal Tumors: Retrospective Analysis of the Computer-Tomographic Aspects. J Gastrointestin Liver Dis 16:147–151.
T.H. Yang, J.I. Hwang, M.S. Yang, S.W. Hung, S.W. Chan, Jwang, Y.S. Tyan (2007) Gastrointestinal Stromal Tumors: Computed Tomographic Features and Prediction of Malignant Risk from Computed Tomographic Imaging. J Chin Med Assoc 70:367–373.
H.C. Kim, J.M. Lee, K. W. Kim, et al. (2004) Gastrointestinal Stromal Tumors of the Stomach: CT findings and Prediction of Malignancy. AJR 183:893–898.
S. Ulusan, Z. Koc, F. Kayaselcuk (2008) Gastrointestinal stromal tumours: CT findings. Br J Radiol 81:618–623.
S.H. Yin, C.M. Xie, Y.X. Mio, et al. (2009) Correlation of multi-slice spiral CT features to clinicopathologic manifestations of gastrointestinal stromal tumor: a report of 49 cases. Chinese Journal of Cancer 28:1–7.
T. Da Ronch, A. Modesto, M Bazzocchi (2006) Gastrointestinal stromal tumour: spiral computed tomography features and pathologic correlation. Radiol med 111:661–673.
T.V. Bartolotta, A. Taibbi, M. Galia, et al. (2006) Gastrointestinal stromal tumour: 40-row multislice computed tomography findings. Radiol med 111:651–660.
A. Rimondini, M. Belgrano, G. Favretto, A. Spivach, A. Sartori, F. Zanconati (2007) Contribution of CT to treatment planning in patients with GIST. Radiol med 112:691–702.
R. Wada, H. Arai, S. Kure, W.X. Peng, Z. Naito (2016) "Wild type" GIST: Clinicopathological features and clinical practice. Pathol Int. 66:431–437. https://doi.org/10.1111/pin.12431.Review.
A. Gaitanidis, M. El Lakis, M. Alevizakos, A. Tsaroucha, M. Pitiakoudis (2018) Predictors of lymph node metastasis in patients with gastrointestinal stromal tumors (GISTs). Langenbecks Arch Surg. 403:599–606. https://doi.org/10.1007/s00423-018-1683-0.
N. Gong, C.S. Wong, Y.C. Chu (2011) Is lymph node metastasis a common feature of gastrointestinal stromal tumor? PET/CT correlation. Clin Nucl Med. 36:678–682. https://doi.org/10.1097/rlu.0b013e318219ad31. Erratum in: Clin Nucl Med. 2011; 36:833. Jie, Gong Nan [corrected to Gong, Nanjie]; Sing, Wong Chun [corrected to Wong, Chun Sing]; Ching, Chu Yiu [corrected to Chu, Yiu Ching]; Tiffany [removed].
T. Lehnert (1998) Gastrointestinal sarcoma (GIST): a review of surgical management. Ann Chir Gynaecol. 87:297–305.
T. Tashiro, T. Hasegawa, M. Omatsu, S. Sekine, T. Shimoda, H. Katai (2005) Gastrointestinal stromal tumor of the stomach showing lymph node metastases. Histopathology. 47:438–439. https://doi.org/10.1111/j.1365-2559.2005.02133.x.
S.M. Zwan Van der, R.P. De Matteo (2005) Gastrointestinal stromal tumor. 5 years later. Cancer. 104:1781–1788. https://doi.org/10.1002/cncr.21419.
A.E. Canda, Y. Ozsoy, O.A. Nalbant, O. Sagol (2008) Gastrointestinal stromal tumor of the stomach with lymph node metastasis. World J Surg Oncol. 6:97. https://doi.org/10.1186/1477-7819-6-97.
A. Shafizad, M. Mohammadianpanah, H. Nasrolahi, M. Mokhtari, S.A. Mousavi (2014) Lymph Node Metastasis in Gastrointestinal Stromal Tumor (GIST): to Report a Case. Iran J Cancer Prev. 7:171–174.
M.A. Mazzei, F. Gentili, L. Volterrani (2019) Dual-Energy CT Iodine Mapping and 40-keV Monoenergetic Applications in the Diagnosis of Acute Bowel Ischemia: A Necessary Clarification. AJR Am J Roentgenol. 212:W93–W94.
P. Apfaltrer, M. Meyer, C. Meier, et al. (2012) Contrast-enhanced dual-energy CT of gastrointestinal stromal tumors: is iodine-related attenuation a potential indicator of tumor response?” Invest Radiol 47:65–70.
F.G. Mazzei, L. Volterrani, S. Guerrini, et al. (2014) Reduced time CT perfusion acquisitions are sufficient to measure the permeability surface area product with a deconvolution method. Biomed Res Int 2014:573268. https://doi.org/10.1155/2014/573268.
F.G. Mazzei, M.A. Mazzei, N. Cioffi Squitieri, et al. (2014) CT perfusion in the characterisation of renal lesions: an added value to multiphasic CT. Biomed Res Int 2014:135013. https://doi.org/10.1155/2014/135013.
M.A. Mazzei, N. C. Squitieri, E. Sani, et al. (2013) Differences in perfusion CT parameter values with commercial software upgrades: a preliminary report about algorithm consistency and stability. Acta Radiol 54:805–811.
M.A. Mazzei, L. Preda, A. Cianfoni, L. Volterrani (2015) CT perfusion: technical developments and current and future applications”. Biomed Res Int 2015:397521. https://doi.org/10.1155/2015/397521.
Acknowledgements
A preliminary research with the title “Gastrointestinal stromal tumors of the stomach: CT percentage of tumor necrosis and prediction of malignancy” was presented as conference abstract at ECR 2015, Book of Abstracts—B—Scientific Sessions and Late-Breaking Clinical Trials.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the local institutional review board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mazzei, M.A., Cioffi Squitieri, N., Vindigni, C. et al. Gastrointestinal stromal tumors (GIST): a proposal of a “CT-based predictive model of Miettinen index” in predicting the risk of malignancy. Abdom Radiol 45, 2989–2996 (2020). https://doi.org/10.1007/s00261-019-02209-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02209-7