Abstract
Purpose
The purpose of this study was to compare quality of life (QoL) after two different transarterial therapies [transarterial chemoembolization (TACE) and transarterial radioembolization (TARE)] for patients with unresectable hepatocellular carcinoma (HCC) to assess tumor therapy in palliative situation additional to traditional aims like survival or image response.
Material and methods
QoL was evaluated with two validated questionnaires (EORTC QLQ-30 and EORTC HCC18) before and 14d after treatment in 94 initial therapies (TACE n = 67; TARE n = 27). QoL changes after treatment were analyzed. Tumor response was evaluated using RECIST/WHO/mRECIST/EASL criteria. A multivariate linear regression was undertaken to identify potential influence factors on change of QoL.
Results
Mean return rate of questionnaires was 71.3% allowing analysis of 67 therapies (TACE n = 46; TARE n = 21). Initial global health status/QoL was significantly higher in TACE (62.5%) compared to TARE with 50.8%. Absolute global health decrease was higher in TACE (− 10.5%) compared to TARE (− 4.8%, p = 0.396). Also relative global health decrease was higher in TACE (− 16.82%) compared to TARE (− 9.37%). Findings for other items were corresponding, as less impairment was found for TARE compared to TACE for physical/social functioning, fatigue and pain. Objective mRECIST response rate was 22.8% in TACE and 21.1% in TARE.
Conclusion
Neither TACE nor TARE showed a major decrease in QoL after first treatment. TACE showed a slightly but not significantly higher decrease, so this study is not clearly in favor for one treatment. But with the addition that TARE showed less decrease even in patients with higher tumor burden and lower baseline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Transarterial therapies are widely used in palliative setting to treat primary hepatic malignancy, especially when resection or other curative treatments are not possible [1,2,3,4,5].
HCC is the most frequent primary liver malignancy, and it is increasing in the western world being mainly caused by cirrhosis due to chronic HBV or HCV infection, alcohol abuse or steatohepatitis [6, 7]. Unfortunately, it is rarely diagnosed in an early stage; 70% of the HCC are not resectable at time of diagnosis [8].
According to the European Association for the Study of the Liver-European Organisation for Research and Treatment of Cancer (EASL-EORTC) Clinical Practice Guidelines, TACE is indicated for Barcelona clinic liver cancer (BCLC) intermediate stage unresectable HCC [9]. Due to the lack of positive phase 3 studies, TARE is not explicitly recommended in international guidelines but it is mentioned as a potential therapy [9, 10]. Whereas TACE is usually contraindicated in patients with portal vein thrombosis (PVT), TARE is a possible treatment.
Up to 95% of patients with advanced cancer consider a good QoL as important as a long life [11]. In contrast, the focus in cancer treatment research has been primarily on survival time without explicit note of QoL. Over the last years, a growing interest on assessment of QoL in patients with cancer could be noted and QoL is perceived as a relevant outcome parameter additional to traditional outcome parameters [8, 12].
The concept of value-based medicine further correlates patient outcome based on survival and QoL with economic costs of health-care interventions to achieve a high treatment quality and cost-effective health care [13]. Survival can easily be evaluated based on clinical studies, but validated data on QoL are still limited. Furthermore, subjective QoL has to be processed in a quantitative way for comparison of different interventions.
Most previous studies on palliative liver treatment focused on traditional goals such as local tumor response, progression-free and overall survival [5, 14,15,16]. A few studies, however, addressed QoL after TACE and TARE, but they frequently used different QoL assessment tools and an inconsistent time to follow up, so it is difficult to compare treatments in most cases [2, 4, 17,18,19,20]. Xie et al. compared QoL after liver resection with TACE, while Xing et al. only analyzed health-related QoL after drug-eluting beads (DEB) TACE [17, 21]. Wible et al. also analyzed QoL after initial TACE, but they used the Short Form-36 as QoL assessment tool [18]. This questionnaire is neither specialized for liver disease nor for tumor disease in general.
Salem et al. published the only study comparing QoL after TACE and TARE using a validated QoL assessment tool [2]. Using the Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep) survey, they showed an increase in QoL in patients treated with TARE, but no significant difference could be seen in overall QoL related scores. The current study used other questionnaires to get a special view on HCC-related symptoms in addition to common cancer symptoms. In addition, both questionnaires are multidimensional, validated in different cultural backgrounds and self-reporting [22, 23]. Furthermore, the EORTC QLQ-C30 and the EORTC HCC18 are both prognostic for overall survival in patients with HCC [24].
The purpose of this study was to assess QoL after TACE and TARE and compare the impact of the treatment on post-interventional QoL. Furthermore, potential influence factors on QoL were analyzed.
Materials and methods
Study design
This prospective not randomized single center study at a tertiary care center was approved by the institutional ethics committee. Informed consent was obtained in every case. A tumor board made the therapy decision based on clinical factors, imaging, comorbidities and BCLC scheme. In general, smaller tumors without portal vein thrombosis were treated by TACE and larger/multinodular ones with or without portal vein thrombosis were treated by TARE. Every participant filled out the EORTC QLQ-C30 as core questionnaire for cancer disease, and in addition the QLQ-HCC18 specially developed for HCC one to three days before and two weeks after intervention. Furthermore, imaging was performed before and after treatment and a laboratory analysis containing liver enzymes, coagulation and blood cell count was obtained before intervention.
Patient population
All patients undergoing initial TACE or TARE due to HCC between November 2014 and March 2016 were asked and agreed to participate this study (n = 94; TACE 67, TARE 27). Twenty-seven patients failed to answer the questionnaire after two weeks resulting in a mean return rate of 71.3% (TACE 68.7%, TARE 77.8%). In total, QoL after 67 interventions was analyzed in this study (TACE 46, TARE 21). QoL assessment before intervention without associated QoL assessment after intervention was excluded from further analysis in this study.
The diagnosis was confirmed by imaging or histologically. Conventional TACE (cTACE) was performed in 71.7% (n = 33) and DEB TACE in 28.3% (n = 13).
Patients’ demographics and risk factors are shown in Table 1.
Treatment procedure
Standardized cTACE was performed with doxorubicin or with doxorubicin and mitomycin C in combination in 33 cases (71.7%). Chemotherapeutic dose was calculated based on body surface and adapted to liver and heart function. Lipiodol (Guerbet, Villepinte, France) was used for embolization. DEB TACE was performed with doxorubicin-loaded DC-Beads® (Biocompartibles UK Ltd., GB, 100–300/300–700 µm) in 13 cases (28.3%). In general, super selective TACE was performed to raise therapeutic effects in tumor and protect healthy liver tissue simultaneously.
For TARE, all patients received an initial angiographic work-up to identify the vascular anatomy and occlude extrahepatic collateral branches, if necessary. A 99mTc-MAA evaluation in the planned therapeutic position and a single photon emission computed tomography was performed for treatment planning and to identify the lung shunt fraction two weeks before treatment. Micron-sized embolic glass particles loaded with Yttrium-90 (Therasphere®; BTG, London, GB) were applied in the evaluated catheter position. The therapeutic dose was calculated based on tumor volume, liver function and lung shunt fraction.
Imaging
Contrast enhanced magnetic resonance imaging (MR) or computer tomography (CT) was obtained before intervention (median 42 days). Follow-up imaging was performed 2 month after treatment (median 70 days). Tumor response was evaluated using the size criteria Response Evaluation Criteria in Solid Tumors (RECIST) and World Health Organization (WHO) criteria in addition to the enhancement criteria modified RECIST (mRECIST) and EASL criteria [25].
QoL assessment tool
The EORTC QLQ-C30 and the EORTC-HCC18 are both validated for the measurement of QoL in cancer patients [23, 26]. The questionnaires consist of 48 questions divided in a global health score, functional and symptom scales/items. Each score uses a 4-point Likert-type scale with a range from not at all (1) to very much (4). According to the EORTC scoring manual, all of the scales were converted to a score with a range from 0 to 100. So every score can be calculated for quantitative QoL assessment [27]. A high score represents a high level of QoL, functioning or symptoms. A negative score after treatment indicates a decrease in QoL and functioning but a lower level of symptoms. Treatments were compared using the scores before and after treatment.
Statistics
Statistical analysis was performed using IBM SPSS Statistics (version 22.0, Armonk, NY, USA). Statistical significance was compared by Mann–Whitney U test for characteristics on ordinal scale and by χ2 for those on interval scale. A linear regression model was used to identify potential influence factors on QoL. Then, a multivariable linear regression with backward elimination was carried out. Significance level was p ≤ 0.05.
Results
QoL before intervention
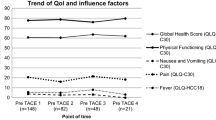
Mean pre-interventional global health status/QoL in TARE group (50.8%) was significantly lower compared to TACE group (62.5%, p = 0.029, Table 2). Except social functioning, TARE group showed significant lower scores in all functional scales. Both groups showed highest scores for insomnia, fatigue, dyspnea, pain and appetite loss in the QLQ-C30 symptom scales.
In general, TARE group showed higher scores for symptom scales in both questionnaires. Fatigue was significantly higher in TARE group compared to TACE (QLQ-C30: p = 0.002, HCC18: p = 0.010, Table 2).
Change in QoL
Mean absolute decrease in global health status/QoL was higher in TACE group (− 10.5%) compared to TARE group (− 4.8%), which was ,however, not statistically significant (p = 0.396). The highest absolute pre-/post-changes for TACE were observed in role functioning, fatigue and social functioning (Table 3).
TARE group showed highest changes in financial difficulties, role functioning and dyspnea. TACE group showed a higher but statistically insignificant decrease in the functional scales except cognitive functioning compared to TARE group. Absolute increase in fatigue after initial treatment was significantly higher in TACE (+ 19.1%) compared to TARE (+ 7.9%, p = 0.021). No statistical significance could be seen for other pre-/post-changes in EORTC QlQ-C30.
For EORTC-HCC18, the highest pre-/post-TACE changes were observed in fatigue, sexual life and body image. TARE induced highest changes in nutrition problems, abdominal swelling and pain. TACE group showed higher increase in the symptoms except nutrition problems, pain and abdominal swelling, but statistical significance was not reached (Table 3).
In general, relative changes were higher after TACE compared to TARE also (Table 4). Relative pre-/post-change in global health status was − 16.82% in TACE and only − 9.37% in TARE group. The highest relative pre-/post-changes in TACE group could be seen for nausea and vomiting (+ 350%), financial difficulties (+ 171.59%) and fever (+ 161.57%). In TARE group, the highest pre-/post-changes could be seen for financial difficulties (+ 150.10%), abdominal swelling (+ 74.96%) and nausea and vomiting (+ 71.47%).
Influence factors on QoL
Potential influence factors (gender, age, mRECIST response, MELD score) on QoL changes (global health status/QoL, physical functioning, social functioning, fatigue, nausea/vomiting, pain and fever) were analyzed in a multivariable linear regression. In TACE and TARE, gender had a significant influence on fatigue after treatment (TACE: p = 0.018; TARE: p = 0.012). Higher increase in fatigue was observed in females compared to males after TACE and TARE. Gender (p = 0.026) and age (p = 0.041) were also independently associated with significantly higher level of pain after TARE. Females developed a higher level of pain compared to males, and the older the patient, the higher the increase in pain after intervention. In addition, less fever after TACE was significantly associated with a higher mRECIST response after intervention (p = 0.049). Statistical significance was not reached for the other potential influence factors in the two groups.
Tumor response
Tumor response could be compared following 63 therapies (TACE n = 44; TARE n = 19). Objective (CR + PR) RECIST response rate was 2.3% in TACE. SD rate was highest in TACE with 63.6%. RECIST-PD was 34.1% in TACE. TARE showed a higher RECIST SD rate compared to TACE with 68.4% and PD in 31.6%. According to the WHO criteria, SD rate was highest in TACE with 52.3% (PR 6.8%, PD 40.9%). WHO rates in TARE were corresponding (PR 5.3%, SD 57.9%, PD 36.8%).
Complete mRECIST and EASL response was 6.8% for TACE. Both of them showed highest rates in SD for TACE (mRECIST PR 15.9%, SD 52.3%, PD 25%; EASL PR 18.2, SD 40.9%, PD 34.1%). SD enhancement rates were highest in TARE group also (mRECIST CR 0%, PR 21.1%, SD 52.6%, PD 26.3%; EASL CR 0%, PR 10.5%, SD 57.9%, PD 31.6%, Figure 1).
Discussion
Up to 95% of advanced cancer patients consider a good QoL as important as survival time, but remarkably only a few studies analyzing the changes in QoL after palliative transarterial treatment have been published [11]. In addition, just a few of these analyze QoL across different transarterial therapies. In this study, the changes in QoL of patients with unresectable HCC receiving TACE or TARE were compared.
Patients undergoing TARE had a lower initial global health status and a higher tumor burden, but subsequently less impairment after intervention. This might suggest that TARE is better tolerated than TACE. In this study, TACE, as a more selective therapy compared to TARE, induced a higher drop in the QoL even in patient with a better baseline.
Furthermore, both groups showed highest rates in fatigue, insomnia, appetite loss and pain before intervention. This is comparable with the observation that most of the HCC patients are poor in body symptoms when diagnosed [28]. So after diagnosis, psychic symptoms are of more importance than the unspecific physical symptoms. Depressive symptoms are associated with chronic liver disease for unknown reason [29]; those symptoms are paramount in both groups before intervention.
TACE decreased QoL compared to TARE, but there is no standardized way to put the changes into a clinical relation yet. Chie et al. postulate that a change of 10.5% or more in a scale with a range from 0 to 100% could be seen as “clinical meaningful,” whereas Osoba et al. divide the changes in “little (5–10%), moderate (10–20%) and very much (≥ 20%)” [4, 30]. According to these criteria, global health decrease in TACE (− 10.5%) is clinical meaningful ,whereas there is not even a little change in TARE (− 4.8%) without clinical meaningfulness. A previous study showed comparable results after first TACE [31].
Chie et al. observed a higher global health decrease (− 19%) after first TACE using the same QoL assessment presumably due to a different TACE technique [4]. In this collective, a greater number of patients received conventional TACE with lipiodol as an embolic agent, which might result in reduced post-embolization syndrome compared to more effective embolic agents. However, a study by Hartrumpf et al. showed no statistically significant difference in QoL between conventional and DEB TACE, although lower systemic doxorubicin blood levels could be seen after DEB TACE [32].
The only study comparing health-related QoL in patients with HCC after TACE and TARE by Salem et al. showed a positive influence of TARE but without significant difference in overall QoL scores [2]. In this study, a positive influence of TARE could only be seen related to insomnia and jaundice. So this study cannot confirm that TARE increases the QoL. This might be caused by the different QoL assessment tools rather than by the treatment itself. Nevertheless, we can support the fact that TARE showed a lower but not significant decrease in the QoL compared to TACE. TARE is better tolerated in both studies, so it seems to be possible to compare both studies with regard to general changes in QoL, but with the addition that a comparison between the single items is not permissible as different QoL assessment tools were used.
Abdominal pain, fever, ascites, diarrhea and/or jaundice are well-known symptoms of advanced HCC specifically mentioned in the EORTC-HCC18 [31]. TARE resulted in a higher increase in pain and abdominal swelling, whereas deterioration of jaundice and fever was higher in TACE. Statistical significance for all of this changes in symptoms was not reached, and no change was “clinical meaningful” based on the categorization by Osoba et al. [30]. The results are not clearly in favor of one treatment regarding the HCC-specific symptoms. Nevertheless, this analysis revealed that TARE does not significantly impair QoL even in HCC patients with higher tumor burden.
TACE and TARE as treatments for hypervascular tumors (e.g., HCC) induce necrosis in the tumor largely due to the embolization or radiation effect, in most cases without a volume reduction. mRECIST criteria are therefore more appropriate to measure therapeutic effect in these tumors and predict long-term survival [25]. TACE showed slightly better mRECIST response rates compared to TARE.
mRECIST response rates vary widely in other studies analyzing TACE [31, 33]. mRECIST criteria are also suitable to describe the therapeutic effects after TARE as they showed a higher response rate compared to RECIST criteria and an acceptable inter- and intraobserver reproducibility [34]. Bhangoo et al. [28] summarized mRECIST CR, PR and SD rate as a “clinical benefit” (48% of the patients) in a study analyzing TARE for patients with unresectable HCC. This study showed a “clinical benefit” in 74% of the patients undergoing TARE for the same indication. In addition, a hypervascular border area could be seen as a response after TARE.
Although there are exact indications for TACE and TARE, both therapies are often competing to treat HCC. With regard to a value-based approach, QoL has to be evaluated supplementary to overall survival and cost efficiency.
A potential limitation of the current study may be the short follow-up period. This short period was chosen to avoid confounding effects due to disease progression and to report acute toxicity due to the treatment. The study is also limited by the small number of participants treated in only one center and the fact that the first assessment was performed with a medical expert, while the second assessment was only answered by the patient himself. In addition, the study is not randomized as treatment decision was made by a tumor board based on clinical factors, imaging and comorbidities. The aim was to identify the best treatment for every single patient which leads to incongruent size of the treatment groups. It is acknowledged that this may cause selection bias. In this study, TACE group had smaller lesions and a significantly better QoL before intervention.
Also this study did not assess QoL in correlation with the therapeutic effect. However, this was not the focus of the analysis.
Finally, the study focused its analysis on the initial intervention and did not assess the QoL over a course of treatments, which is also most likely confounded by disease progression.
Conclusion
In summary, neither TACE nor TARE showed a major decrease in QoL after first treatment, but this study suggests that TARE results in a slightly but not significantly better QoL outcome after treatment compared to TACE. This can be even seen in patients with lower baseline and a higher tumor burden. All in all this study shows that TARE as a lobar manner is better tolerated than TACE as a (super-) selective therapy, but with the addition that the work is not clearly in favor for one treatment as statistical evidence cannot be produced.
Standardized QoL assessment has the potential for individual treatment stratification in the future. Furthermore, in times of value-based medicine QoL as an important outcome parameter gains significance to ensure cost-effective use of health care.
References
Poyanli A, Rozanes I, Acunas B, Sencer S (2001) Palliative treatment of hepatocellular carcinoma by chemoembolization. Acta Radiol 42(6):602–607
Salem R, Gilbertsen M, Butt Z, et al. (2013) Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol 11(10):1358e.1–1365.e1
Mancini R, Carpanese L, Sciuto R, et al. (2006) A multicentric phase II clinical trial on intra-arterial hepatic radiotherapy with 90yttrium SIR-spheres in unresectable, colorectal liver metastases refractory to i.v. chemotherapy: preliminary results on toxicity and response rates. In Vivo 20(6):711–714
Chie W, Yu F, Li M, et al. (2015) Quality of life changes in patients undergoing treatment for hepatocellular carcinoma. Qual Life Res 24(10):2499–2506
Llovet J, Bruix J (2003) Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37(2):429–442
El-Serag HB (2011) Hepatocellular carcinoma. N Engl J Med 365:1118–1127
El-Serag HB (2012) Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142(6):1264e.1–1273.e1
Wang Y, Chen M, Yan K, et al. (2007) Quality of life after radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinoma: comparison with transcatheter arterial chemoembolization alone. Qual Life Res 16(3):389–397
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer (2012) EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56(4):908–943
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53(3):1020–1022
Meropol NJ, Weinfurt KP, Burnett CB, et al. (2003) Perceptions of patients and physicians regarding phase I cancer clinical trials: implications for physician-patient communication. J Clin Oncol 13:2589–2596
Moinpour CM (1994) Measuring quality of life: an emerging science. Semin Oncol 5(10):48–60
Brown MM, Brown CC (2013) Update on value-based medicine. Curr Opin Ophthalmol 24(3):183–189
Wang JH, Wang CC, Hung CH, Chen CL, Lu SN (2012) Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol 56(2):412–418
Sangro B, Carpanese L, Cianni R, et al. (2011) Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across barcelona clinic liver cancer stages: a European evaluation. Hepatology. 54(3):868–878
Mao Y, Luo Z, Li B, Hu T (2012) Prospective study on the survival of HCC patients treated with transcatheter arterial lipiodol chemoembolization. Asian Pac J Cancer Prev 13(3):1039–1042
Xie ZR, Luo YL, Xiao FM, Liu Q, Ma Y (2015) Health-related quality of life of patients with intermediate hepatocellular carcinoma after liver resection or transcatheter arterial chemoembolization. Asian Pac J Cancer Prev 16(10):4451–4456
Wible B, Rilling W, Drescher P, et al. (2010) Longitudinal quality of life assessment of patients with hepatocellular carcinoma after primary transarterial chemoembolization. J Vasc Interv Radiol 21(7):1024–1030
Toro A, Pulvirenti E, Palermo F, Di Carlo I (2012) Health-related quality of life in patients with hepatocellular carcinoma after hepatic resection, transcatheter arterial chemoembolization, radiofrequency ablation or no treatment. Surg Oncol 21(1):e23–e30
Eltawil K, Berry R, Abdolell M, Molinari M (2012) Quality of life and survival analysis of patients undergoing transarterial chemoembolization for primary hepatic malignancies: a prospective cohort study. HPB (Oxford) 14(5):341–350
Xing M, Webber G, Prajapati H, et al. (2015) Preservation of quality of life with doxorubicin drug-eluting bead transarterial chemoembolization for unresectable hepatocellular carcinoma: longitudinal prospective study. J Gastroenterol Hepatol 30(7):1167–1174
Blazeby J, Currie E, Zee BCY, et al. (2004) Development of a questionnaire module to supplement the EORTC QLQ-C30 to assess quality of life in patients with hepatocellular carcinoma, the EORTC QLQ-HCC18. Eur J Cancer 40(16):2439–2444
Aaronson NK, Ahmedzai S, Bergman B, et al. (1993) The european organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376
Li L, Mo FK, Chan SL, et al. (2017) Prognostic values of EORTC QLQ-C30 and QLQ-HCC18 index-scores in patients with hepatocellular carcinoma—clinical application of health-related quality-of-life data. BMC Cancer 17:8
Shim J, Lee H, Kim S, et al. (2012) Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology 262(2):708–718
Chie W, Blazeby J, Hsiao C, et al. (2012) International cross-cultural field validation of an european organization for research and treatment of cancer questionnaire module for patients with primary liver cancer, the european organization for research and treatment of cancer quality-of-life questionnaire HCC18. Hepatology 55(4):1122–1129
Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, On behalf of the EORTC Quality of Life Group (2011) The EORTC QLQ-C30 Scoring Manual, 3rd edn. Brussels: European Organisation for Research and Treatment of Cancer.
Bhangoo MS, Karnani DR, Hein PN, et al. (2015) Radioembolization with yttrium-90 microspheres for patients with unresectable hepatocellular carcinoma. J Gastrointest Oncol 6(5):469–478
Huang X, Liu X, Yu Y (2017) Depression and chronic liver diseases: are there shared underlying mechanisms? Front Mol Neurosci 10:134
Osoba D, Rodrigues G, Myles J, Zee B, Pater J (1998) Interpreting the significance of changes in health-related quality-of-life scores. JCO 16(1):139–144
Hinrichs JB, Hasdemir DB, Nordlohne M, et al. (2017) Health-related quality of life in patients with hepatocellular carcinoma treated with initial transarterial chemoembolization. Cardiovasc Intervent Radiol 40(10):1559–1566
Hartrumpf KJ, Marquardt S, Werncke T, et al. (2018) Quality of life in patients undergoing repetitive TACE for the treatment of intermediate stage HCC. J Cancer Res Clin Oncol. https://doi.org/10.1007/s00432-018-2704-7
Lammer J, Malagari K, Vogl T, et al. (2010) Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 33(1):41–52
Seyal AR, Gonzalez-Guindalini FD, Arslanoglu A, et al. (2015) Reproducibility of mRECIST in assessing response to transarterial radioembolization therapy in hepatocellular carcinoma. Hepatology 62(4):1111–1121
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not supported by any funding.
Conflict of interest
The authors declared that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. Consent for publication was obtained for every individual person’s data included in the study.
Rights and permissions
About this article
Cite this article
Kirchner, T., Marquardt, S., Werncke, T. et al. Comparison of health-related quality of life after transarterial chemoembolization and transarterial radioembolization in patients with unresectable hepatocellular carcinoma. Abdom Radiol 44, 1554–1561 (2019). https://doi.org/10.1007/s00261-018-1802-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1802-y