Abstract
Objective
To evaluate the performance of a novel, quantitative computer-aided diagnostic (CAD) algorithm on four-phase multidetector computed tomography (MDCT) to detect peak lesion attenuation to enable differentiation of clear cell renal cell carcinoma (ccRCC) from chromophobe RCC (chRCC), papillary RCC (pRCC), oncocytoma, and fat-poor angiomyolipoma (fp-AML).
Materials and methods
We queried our clinical databases to obtain a cohort of histologically proven renal masses with preoperative MDCT with four phases [unenhanced (U), corticomedullary (CM), nephrographic (NP), and excretory (E)]. A whole lesion 3D contour was obtained in all four phases. The CAD algorithm determined a region of interest (ROI) of peak lesion attenuation within the 3D lesion contour. For comparison, a manual ROI was separately placed in the most enhancing portion of the lesion by visual inspection for a reference standard, and in uninvolved renal cortex. Relative lesion attenuation for both CAD and manual methods was obtained by normalizing the CAD peak lesion attenuation ROI (and the reference standard manually placed ROI) to uninvolved renal cortex with the formula [(peak lesion attenuation ROI − cortex ROI)/cortex ROI] × 100%. ROC analysis and area under the curve (AUC) were used to assess diagnostic performance. Bland–Altman analysis was used to compare peak ROI between CAD and manual method.
Results
The study cohort comprised 200 patients with 200 unique renal masses: 106 (53%) ccRCC, 32 (16%) oncocytomas, 18 (9%) chRCCs, 34 (17%) pRCCs, and 10 (5%) fp-AMLs. In the CM phase, CAD-derived ROI enabled characterization of ccRCC from chRCC, pRCC, oncocytoma, and fp-AML with AUCs of 0.850 (95% CI 0.732–0.968), 0.959 (95% CI 0.930–0.989), 0.792 (95% CI 0.716–0.869), and 0.825 (95% CI 0.703–0.948), respectively. On Bland–Altman analysis, there was excellent agreement of CAD and manual methods with mean differences between 14 and 26 HU in each phase.
Conclusion
A novel, quantitative CAD algorithm enabled robust peak HU lesion detection and discrimination of ccRCC from other renal lesions with similar performance compared to the manual method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Renal cell carcinoma (RCC) is among the most commonly diagnosed forms of cancer among both men and women in the United States with 2% growth in incidence [1, 2]. Up to 70% of renal neoplasms are detected incidentally at imaging, and all solid enhancing renal masses without macroscopic fat are presumed to be malignant. However, approximately 20% of clinical T1a small renal masses (<4 cm) at nephrectomy and 30% of lesions at biopsy are benign, most commonly oncocytoma and fat-poor angiomyolipoma (fp-AML). Thus, a significant percentage of patients with incidentally detected renal masses are subjected to the risks of unnecessary procedures [3–7].
While these benign lesions, and more indolent RCCs such as chromophobe RCC (chRCC) and papillary RCC (pRCC), may undergo active surveillance, clear cell RCC (ccRCC) is more likely to be treated with resection or ablation because it tends to be more aggressive with higher metastatic potential [7–10]. Although a percutaneous biopsy to confirm renal mass histology has become broadly accepted, it may have non-diagnostic rate of 10–20% and a relatively poor negative predictive value [11, 12]. Multiphasic computed tomography (CT) or magnetic resonance (MR) imaging has become the standard for characterizing masses in the liver, lung, and brain, but has not yet been established in the kidney [11, 13–15]. Thus, an imaging-based method to differentiate malignant from benign renal lesions, and also to distinguish the more aggressive ccRCC from less aggressive malignant subtypes, would be of great clinical value, particularly in older patients with comorbidities in whom a conservative approach would be favored.
Several studies have attempted to characterize renal masses on imaging by correlating unique multiphasic patterns of contrast attenuation with the histology of RCC subtypes and benign lesions on CT and MR. These patterns are identified by visual assessment of peak lesion attenuation and manual placement of a region of interest (ROI) within the most enhancing portion of the lesion and background cortex over multiple phases, often without the use of a standardized four-phase renal protocol [16–28]. This technique is inherently operator dependent and subjective, with variable results reported. A reproducible and robust method to differentiate renal lesions on imaging is desired to minimize subjectivity and ensure widespread applicability. The purpose of our study was to evaluate the performance of a novel, computer-aided diagnostic (CAD) algorithm that detects peak lesion attenuation relative to renal parenchyma to differentiate ccRCC from chRCC, pRCC, oncocytoma, and fp-AML on multiphasic CT and compare CAD performance with the manual method.
Materials and methods
Patients and lesions
With institutional review board approval for this 1996 Health Insurance Portability and Accountability Act compliant retrospective study and a waiver of informed consent, we queried our institution’s pathology database for histologically proven cases of RCC, oncocytoma, and fp-AML from January 2000 to 2016. We searched our institution’s medical records to identify the date of surgery or biopsy, and our image archiving system (Centricity; GE Medical Systems, Milwaukee, WI) to identify patients in which a preoperative MDCT with a four-phase renal protocol had been acquired. The importance of our study was in the context of patients for whom quantitative data would be most beneficial in assisting clinicians with diagnosis at the time of initial presentation, management during active surveillance or triaging a patient to a minimally invasive therapy. Therefore, our query was limited to patients with pathological stage T1 renal masses, as the course of treatment for a patient with a renal mass >7 cm stage T2–T4 would most likely be surgical intervention. To avoid potential clustering effects, only patients with a solitary tumor were included. Our final cohort consisted of 200 consecutive lesions in 200 unique patients (Fig. 1).
CT acquisition and four-phase renal mass protocol
All patients had a preoperative MDCT at our institution with an intravenous (i.v.) contrast-enhanced four-phase renal mass protocol. This included an unenhanced scan and contrast-enhanced acquisitions during the corticomedullary, nephrographic, and excretory phases acquired from the lung bases to the iliac crests. Preoperative MDCT was performed on multiple scanners (4-MDCT, 16-MDCT, and 64-MDCT) from January 2000 to January 2016. Acquisition parameters were similar over the multiple generations of scanners: 120 kVp, 200–400 mA based on patient size and 3-mm scan collimation with 3-mm reconstruction intervals. Patients received a power injection of non-ionic i.v. contrast (iodixanol, Omnipaque 350, GE Healthcare), 35–45g iodine dosed to weight at a rate of 3 mL/s. Patients who weighed less than 45 kg, those who weighed 45–90 kg, and those who weighed >90 kg received 100 mL (35 g iodine), 125 mL (45 g iodine), and 150 mL (54 g iodine) of contrast material, respectively. A bolus tracking system (Care Bolus VB10, Siemens Healthcare or Smart-Prep, GE Healthcare) was used to determine the onset of imaging and a ROI was placed in the thoracoabdominal aorta junction, with a trigger set to begin at 150 HU. Images were acquired 55 s (corticomedullary), 120 s (nephrographic), and 8 min (excretory) after the threshold of 150 HU was reached.
Peak lesion attenuation: CAD method
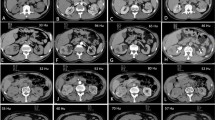
Using in-house-developed U.S. FDA 510K cleared image processing software, the entire renal mass was contoured on axial images in each of the four phases, and was manually edited to ensure the contour was limited to the target lesion and excluded adjacent perirenal fat and normal renal parenchyma. We developed a CAD algorithm to automatically place an ROI in each lesion to quantify peak lesion attenuation based on the following criteria: (1) contained within the 3D lesion contour, (2) circular ROI with a diameter of 0.5 cm, and (3) maximal attenuation ≤300 Hounsfield units (HU) to exclude regions of calcification. The CAD algorithm quantified peak lesion attenuation in each phase each by reviewing all possible circular ROIs within the lesion contour and choosing the one with the highest mean Hounsfield unit value (Fig. 2). The lesion contours and appropriateness of CAD ROI placement were confirmed by consensus by two genitourinary fellowship-trained abdominal radiologists with 19 (SSR) and 10 (MLD) years of experience (who were blinded to all patient information, including the histopathologic diagnosis).
A 67-year-old man with a clear cell renal cell carcinoma. A The mass is heterogeneously enhancing on the corticomedullary phase. B Using an in-house-developed software, the entire lesion was segmented in the axial plane. C The CAD algorithm searched the volumetric lesion contour for the region of peak lesion attenuation contained within a circular ROI with a 0.5 cm diameter (red ROI)
Peak lesion attenuation: manual ROI method
To establish the peak lesion attenuation values that were the reference standard for this study, one circular ROI of approximately 0.5 cm diameter was manually placed on the most avidly enhancing portion of heterogeneously enhancing lesions or in the center of homogeneously enhancing lesions in each post-contrast imaging phase. In addition, one circular ROI with a diameter of 0.5 cm was manually placed in adjacent uninvolved renal cortex in each phase. The lesion ROI and cortex ROI placement was confirmed by two genitourinary fellowship-trained abdominal radiologists by consensus.
Relative lesion attenuation
To normalize for the iodine load and variations in attenuation due to individual patient and technical factors, relative lesion attenuation was obtained by normalizing the CAD peak lesion attenuation and the reference standard, manual ROI peak lesion attenuation, to uninvolved renal cortex with the formula [(peak lesion attenuation ROI − cortex ROI)/cortex ROI] × 100%.
Statistical analysis
To compare the magnitude of CAD relative lesion attenuation of ccRCCs with the other renal masses in each of the four phases, we performed t tests. P values less than 0.01 indicated statistical significance and was adjusted for multiple comparisons (Bonferroni correction). A receiver operating characteristic curve (ROC) was used to evaluate the diagnostic performance of the CAD relative attenuation measurements. The area under the curve (AUC) was used to determine the threshold with the highest accuracy in discriminating ccRCC from chRCC, pRCC, oncocytoma, and fat-poor AML. For each threshold level, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated.
Bland–Altman analysis was performed to evaluate the level of agreement between the reference measurement (manual ROI lesion attenuation) and CAD lesion attenuation. The mean difference was used for measurement of the range of differences, expressed as 95% limits of agreement between the two methods. A receiver operating characteristic curve (ROC) was used to compare the diagnostic performance of the CAD relative attenuation measurements to the manual ROI method.
All analyses were performed by using software (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.).
Results
Patients and lesions
A summary of the clinical and pathological characteristics for all patients and each lesion subtype are summarized in Table 1. Details of the study schema are illustrated in Fig. 1. Of the 200 patients in our study cohort, 130 (65%) were male, and 70 (35%) were female. The mean age at surgery or biopsy for all patients was 63 years (range 22–91 years). Of the 200 lesions analyzed, there were 106 (53%) ccRCC, 32 (16%) oncocytomas, 18 (9%) chRCCs, 34 (17%) pRCCs, and 10 (5%) fp-AMLs. For all patients, the mean time from preoperative CT to surgery or biopsy was 90 days (range 0–1061 days). Histopathology was diagnosed in 59 (29%) lesions from 20-gage percutaneous core biopsy, 103 (51%) from partial nephrectomy, 29 (15%) from total nephrectomy, and 9 (5%) from radical nephrectomy. The mean size for all lesions was 3.1 cm (range 0.8–6.9).
Diagnostic utility of CAD relative lesion attenuation in discriminating clear cell RCC from the Other lesion subtypes
The CAD relative attenuation thresholds with the highest accuracy in discriminating ccRCC from each lesion subtypes in each phase are summarized in Table 3, as well as the AUC, sensitivity, specificity, PPV, NPV. The corticomedullary phase showed the greatest utility in CAD-derived relative lesion attenuation discriminating ccRCC from the indolent RCC subtypes and benign mimics. In this phase, ccRCC had a statistically significantly higher mean CAD relative lesion attenuation with no overlap in the 95% CI (20.3%, 95% CI 12.6–28.0) compared to chRCC (−20.5%, 95% CI −42.2 to 1.1, P = 0.002), pRCC (−41.3%, 95% CI −46.8 to −35.8, P < 0.001), oncocytoma (−11.7%, 95% CI −16.2 to −7.3, P < 0.001), and fp-AML (−16.8%, 95% CI −29.6 to −3.9, P < 0.001) (Table 2; Fig. 3) and an AUC ranging from 0.792 to 0.959 (Table 3). In the nephrographic and excretory phases, CAD relative attenuation also had a statistically significantly higher (P < 0.01) mean CAD relative lesion attenuation with no overlap in the 95% CIs compared to chRCC, pRCC, and fp-AML (Table 2; Fig. 3); however, the relative attenuation profile of ccRCC and oncocytoma had considerable overlap in these phases and was discriminated with accuracies of 42% and 55%, respectively (Fig. 3; Table 3).
Bar graph represents the mean CAD relative lesion attenuation values on multiphasic multidetector CT for clear cell RCC, chromophobe RCC, papillary RCC, oncocytoma, and fat-poor AML. Error bars represent 95% CIs. CAD computer-aided diagnosis, CT computed tomography, RCC renal cell carcinoma, AML angiomyolipoma. * P value <0.01 compared to clear cell RCC relative attenuation
Agreement between manual ROI and CAD ROI lesion attenuation measurements
Bland–Altman plots for assessing agreement between manual ROI peak lesion attenuation and CAD peak lesion attenuation methods are shown in Figure 4. No notable differences were observed between the two measurements, other than a slight positive bias in the CAD attenuation measurement. The mean difference between the two measurements was 14.0 HU in the corticomedullary phase, 25.5 HU in the nephrographic phase, and 26.6 HU in the excretory phase. ROC analysis of the diagnostic utility of manual relative lesion attenuation is shown in Table 3. Discrimination of ccRCC using CAD-derived relative lesion attenuation was comparable to the manual method for each lesion subtype in each phase with equal or higher AUCs and overlap in the 95% CIs of the AUC.
Bland–Altman plots for agreement between manual ROI peak lesion attenuation. CAD-derived peak lesion attenuation measurements had a mean difference of 14 HU in the corticomedullary phase (A), 25.5 HU in the nephrographic phase (B), and 26.6 HU in the excretory phase (C). ROI region of interest, CAD computer-aided diagnosis, HU Hounsfield units
Discussion
The annual 2% increase in incidentally detected small renal masses (stage T1a) remains clinically challenging since all non-fatty solid enhancing masses are considered malignant until proven otherwise. Up to 20% of these T1a lesions are benign at surgical resection, and another 20% are less aggressive non-clear cell variants of RCC [1–7]. Unlike almost all liver, lung, and brain lesions, which are characterized routinely on imaging, histopathology on biopsy or nephrectomy remains the only accepted method for renal mass characterization [11, 13–15]. However, diagnosis by nephrectomy has several disadvantages. In addition to being costly, there are inherent intra-operative risks associated with anesthesia and surgery especially for patients with less aggressive RCC subtypes and benign tumors. There is increasing evidence that both simple and partial nephrectomy can worsen preexisting chronic kidney disease and increase cardiovascular comorbidities, especially in these often elderly patients [29, 30].
Renal mass biopsy has been an important diagnostic advance for characterization since the 1990s. Although relatively safe, biopsy has risk of bleeding and requires radiological and pathological subspecialty expertise for performance and interpretation. Characterization of renal masses from percutaneous biopsy also has variable accuracy ranging from 70% to 90% depending on tumor size, location, and physician experience and sampling technique, with a reported non-diagnostic biopsy rate between 10% and 30% depending on operator and pathological expertise [31, 32]. Retrospective studies have shown that if accurate and reliable pre-treatment knowledge of tumor histology and phenotype were available, the results would lead to an alteration from therapy (including surgery or tumor ablation) to active surveillance in up to 60% of cases [33–37]. Because 70% of renal lesions are detected incidentally on imaging, therapy is a clinical need for a robust method to obtain objective and quantitative data to assist in characterizing renal neoplasm histology on imaging.
In this study, we showed that a novel, quantitative in-house-developed CAD algorithm reliably detected peak lesion HU in a standard ROI on multiphasic MDCT and mimicked the standard manual method of ROI placement for detection of peak lesion HU in turn enabling robust renal lesion characterization by providing multiphasic objective measurement of relative lesion enhancement in comparison to normal enhancing renal cortex. Previous studies using manual ROI placement have validated the correlation between attenuation on CT and histology, and the value of a uniform four-phase renal CT protocol by showing each renal mass has a distinct attenuation profile across the corticomedullary, nephrographic, and excretory phases. However, the variability in the lesion classification results and lack of consensus as to which phase can best distinguish ccRCC from the other lesions is likely due to small sample sizes and because their quantitative data were obtained with subjective manual placement of a ROI of variable sizes in the most enhancing portion of the lesion [19, 22, 25–28].
Additionally, our results show that when the automated CAD algorithm was used to quantify maximal lesion attenuation relative to the cortex, ccRCC was the only lesion to have positive value in the corticomedullary and excretory phases (Fig. 3; Table 2), and we were able to achieve greater differentiation with less spread in the 95% CIs than previous studies [19, 22, 25–28] which utilized subjective manual placement of a ROI of varying sizes to obtain their quantitative data. We also confirmed our results in our dataset by comparing the performance of our CAD algorithm in detecting the highest region of peak lesion attenuation to the reference standard, subjective manual placement by a radiologist in the most enhancing portion of the lesion. Bland–Altman analysis showed excellent agreement between the CAD and manual ROI peak HU measurements, with slight positive bias of the CAD measurements in each phase (Fig. 4). Because the CAD algorithm was designed to find the region of peak attenuation within the lesion contour, this confirmed our hypothesis that CAD detected the ROI with the highest attenuation likely not readily visually discernable for manual placement. Additionally, prior work has shown that a small ROI with a cross-sectional area of 0.5 cm2 has the highest accuracy in discriminating malignant renal lesions on multiphasic CT [38]; therefore, we were able to automate placement of a ROI of this size in an attempt to reduce the inherent inter- and intra-reader variation associated with repeated attempts to manually place a ROI of the same size on CT images.
Our CAD method showed the highest utility with regard to differentiation of ccRCC from its benign mimics, oncocytoma, and fp-AML, where there was no overlap in the 95% CIs of the mean relative attenuation in the corticomedullary phase. (Table 2; Fig. 3). The ROC analysis showed CAD-derived relative attenuation in the corticomedullary phase discriminated ccRCC from oncocytoma with an AUC of 0.792 (vs. 0.725 using manual method) and fp-AML with an AUC of 0.825 (vs. 0.621 manual method) (Table 3). The nephrographic and excretory phases also showed a high utility in discriminating the RCC subtypes and the benign mimics, particularly fp-AML with an AUCs of 0.886 (vs. 0.742 manual method) in the nephrographic phase and 0.876 (vs. 0.629 manual method) in the excretory phase. To our knowledge, this CAD algorithm used on multiphasic CT is the most robust non-invasive and quantitative method reported to date for discriminating ccRCC from malignant RCC subtypes and benign mimics
Several CAD algorithms on imaging have been integrated into clinical practice and assist radiologists with diagnosing and characterizing lesions in the lung, breast, liver, brain, and bowel; however to our knowledge, none has been published for renal mass characterization based on differential enhancement [39, 40]. Previous studies evaluated the utility of CAD algorithms for renal mass detection on imaging based on morphology and texture features, but did not characterize individual histological phenotypes or stratify patients with malignancies [41–45]. Other limitations of these studies include CT scans performed without a uniform four-phase renal protocol and lack of pathologically proven lesion histology. Additional studies [46, 47] utilized a CAD algorithm to classify renal tumors as either RCC or benign; however, classifying all RCC lesions together is of limited clinical value since knowledge of lesion subtype is critical for prognosis. In addition, the inherently low attenuation of pRCC skews the mean attenuation value when all RCC lesions are grouped together as ccRCC and chRCC have greater peak lesion attenuation profiles on MDCT, making their classification schema less clinically applicable.
Other studies have shown promise in developing a diagnostic biomarker on imaging [48–50]; however, these techniques require definitive knowledge that the patient has a renal mass, and require an additional CT examination. This is not only an added expense, but often requires specialized expertise and equipment, subjecting the patient to additional radiation, risk of injection, and exposure to additional contrast material. This may not be feasible in elderly patients, patients with renal dysfunction or other comorbidities. However, if prospectively validated, segmentation of the lesion and our CAD algorithm can be combined with texture features and morphological analysis to construct a predictive model using a multiparametric approach to classify lesions [51] when the initial diagnostic CT is obtained and the renal mass is first detected.
Our study had some potential limitations. First, due to the retrospective nature of the study, there was variability in CT equipment and timing of multiple phases over a 16-year data acquisition period. Second, the segmentation of renal lesions for this study required approximately 5 min of post-processing time, but is comparable to the time necessary for a radiologist to load the DICOM images and review each scan to determine the most enhancing portion of the lesion in each phase. In addition, segmentation of the lesion provides an ROI of the entire lesion and is the first step toward the development of a quantitative multiparametric approach of renal lesion classification. Third, we had to manual place the ROI in the cortex, but we hope to automate these features in future development. Fourth, the low incidence of chRCC and fp-AML in the general population precluded a large number of these lesion types being including in our analysis and thus may be a source of selection bias.
In conclusion, we have shown that a novel CAD algorithm enabled objective and robust quantification of peak renal lesion attenuation relative to uninvolved enhancing renal parenchyma enabling robust non-invasive discrimination of ccRCC from other malignant and benign solid renal masses mimicking results from manual methods. Given our findings, this CAD algorithm may be a preliminary step in the development of a multiparametric decision model which may serve as an adjunct in clinical decision making to guide appropriate management and triage patients with incidentally detected T1 renal lesions to active surveillance, preoperative biopsy, or ablative and surgical therapies. However, our study is in the proof of concept phase and our preliminary CAD results should be validated in a prospective study and confirmed in a larger and independent cohort.
References
Tsili AC, Argyropoulou MI (2015) Advances of multidetector computed tomography in the characterization and staging of renal cell carcinoma. World J Radiol 7(6):110–127.
American Cancer Society (2016) Cancer Facts & Figures 2016, American Cancer Society, Atlanta.
Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H (2003) Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 170(6 Pt 1):2217–2220.
Silverman SG, Israel GM, Herts BR, Richie JP (2008) Management of the incidental renal mass. Radiology 249:16–31.
Kutikov A, Fossett LK, Ramchandani P, et al. (2006) Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology 68:737–740.
Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M (2014) Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging 39(3):558–604.
Gill IS, Aron M, Gervais DA, et al. (2010) Small renal mass. N Engl J Med 362:624–634.
Low G, Huang G, Fu W, Moloo Z, Girgis S (2016) Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol 8(5):484–500.
Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML (2003) Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 27:612–624.
Leone AR, Diorio GJ, Spiess PE, Gilbert SM (2016) Contemporary issues surrounding small renal masses: evaluation, diagnostic biopsy, nephron sparing, and novel treatment modalities. Oncology 30(6):507–514.
Patel H, Iyoha E, Pierorazio P, et al. (2016) A systematic review of research gaps in the evaluation and management of localized renal masses. Urology. doi:10.1016/j.urology.2016.08.013.
Richard PO, Jewett MA, Tanguay S, et al. (2016) Safety, reliability and accuracy of small renal tumor biopsies: results of a multi-institution registry. BJU. doi:10.1111/bju.13630.
Gould MK (2014) Clinical practice: lung-cancer screening with low-dose computed tomography. N Engl J Med 37(19):1813–1820.
Bota S, Piscaglia F, Marinelli S, Pecorelli A, Terzi E, Bolondi L (2012) Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma. Liver Cancer 1(3–4):190–200.
Runge VM (2013) Current technological advances in magnetic resonance with critical impact for clinical diagnosis and therapy. Invest Radiol 48(12):869–877.
Veeratterapillay R, Ijabla R, Conaway D, Haslam P, Soomro N, Heer R (2015) Contrast-enhanced CT in 100 clear cell renal cell cancers—an analysis of attenuation, tumour size, and survival. Clin Radiol 70(12):1357–1361.
Zokalj I, Marotti M, Koaric B (2014) Pretreatment differentiation of renal cell carcinoma subtypes by CT: the influence of different tumor attenuation measurement approaches. Int Urol Nephrol 46:1089–1100.
Young JR, Margolis D, Sauk S, Pantuck AJ, Sayre J, Raman SS (2013) Clear cell renal cell carcinoma: discrimination from other renal cell carcinoma subtypes and oncocytoma at multiphasic multidetector CT. Radiology 267(2):444–453.
Lee-Felker S, Felker E, Tan N, et al. (2014) Qualitative and quantitative MDCT features for differentiating clear cell renal cell carcinoma from other solid renal cortical masses. AJR Am J Roentgenol 203(5):W516–W524.
Zhang J, Lefkowitz RA, Ishill NM, et al. (2007) Solid renal cortical tumors: differentiation with CT. Radiology 244(2):494–504.
Ruppert-Kohlmayr AJ, Uggowitzer M, Meissnitzer T, Ruppert G (2004) Differentiation of renal clear cell carcinoma and renal papillary carcinoma using quantitative CT attenuation parameters. AJR Am J Roentgenol 183(5):1387–1391.
Pierorazio PM, Hyams ES, Tsai S, et al. (2013) Multiphasic attenuation patterns of small renal masses (≤4 cm) on preoperative computed tomography: utility for distinguishing subtypes of renal cell carcinoma, angiomyolipoma, and oncocytoma. Urology 81(6):1265–1271.
Ishigami K, Pakalniskis M, Leite L, et al. (2015) Characterization of renal cell carcinoma, oncocytoma, and lipid-poor angiomyolipoma by unenhanced, nephrographic, and delayed phase contrast-enhanced computed tomography. Clin Imaging 39:76–84.
Kim JK, Park SY, Shon JE, Cho KS (2004) Angiomyolipoma with minimal fat: differentiation from renal cell carcinoma at biphasic helical CT. Radiology 230:677–684.
Bird V, Kanagarajah P, Morillo G, et al. (2011) Differentiation of oncocytoma and renal cell carcinoma in small renal masses (<4 cm): the role of 4-phase computerized tomography. World J Urol 29:787–792.
Bata P, Gyebnar J, Tarnoki D, et al. (2013) Clear cell renal cell carcinoma and papillary renal cell carcinoma: differentiation of distinct histological types with multiphase CT. Diagn Interv Radiol 19:387–392.
Gakis G, Kramer U, Schilling D, Kruck S, Stenzl A, Schlemmer H (2011) Small renal oncocytomas: differentiation with multiphase. CT Eur J Radiol 80:274–278.
Choi S, Jeon S, Chang S (2012) Characterization of small renal masses less than 4 cm with quadriphasic multidetector helical computed tomography: differentiation of benign and malignant lesions. Korean J Urol 53(3):159–164.
Mashni JW, Assel M, Maschino A, et al. (2015) New chronic kidney disease and overall survival after nephrectomy for small renal cortical tumors. Urology 86(6):1137–1143.
Zini L, Perrotte P, Capitanio U, et al. (2009) Radical vs partial nephrectomy: effect on overall and noncancer mortality. Cancer 115:1465–1471.
Samplaski MK, Zhou M, Lane BR, et al. (2011) Renal mass sampling: an enlightened perspective. Int J Urol 18:5–19.
Leveridge M, Finelli A, Kachura J, et al. (2011) Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol 60:578–584.
Maturen KE, Nghiem HV, Caoili EM, et al. (2007) Renal mass core biopsy: accuracy and impact on clinical management. Am J Roentgenol 188:563–570.
Veltri A, Garetto I, Tosetti I, et al. (2011) Diagnostic accuracy and clinical impact of imaging-guided needle biopsy of renal masses. Retrospective analysis on 150 cases. Eur Radiol 21(2):393–401.
Neuzillet Y, Lechevallier E, Andre M, et al. (2004) Accuracy and clinical role of fine needle percutaneous biopsy with computerized tomography guidance of small renal masses. J Urol 171:1802–1805.
Wood BJ, Khan MA, McGovern F, et al. (1999) Imaging guided biopsy of renal masses: Indications, accuracy and impact on clinical management. J Urol 161:1470–1474.
Ambani S, Morgan T, Montgomery J, et al. (2016) Predictors of delayed intervention for patients on active surveillance for small renal masses: does renal mass biopsy influence our decision? Urology. doi:10.1016/j.urology.2016.08.013.
Rosenkrantz A, Matza B, Portnoy E, et al. (2014) Impact of size of region-of-interest on differentiation of renal cell carcinoma and renal cysts on multi-phase CT: preliminary findings. Eur J Radiol 83:239–244.
Van Ginneken B, Schaefer-Prokop CM, Prokop M (2011) Computer-aided diagnosis: how to move from the laboratory to the clinic. Radiology 261:719–732.
Summers RM (2016) Progress in fully automated abdominal CT interpretation AJR 207:67–79.
Linguraru MG, Wang S, Shah F, et al. (2009) Computer-aided renal cancer quantification and classification from contrast-enhanced CT via histograms of curvature-related features. Conference Proceedings of IEEE Engineering in Medicine and Biology Society, pp 6679–6682.
Linguraru MG, Gautam R, Peterson J, et al. (2009) Renal tumor quantification and classification in triple-phase contrast-enhanced abdominal CT. Proceedings of the IEEE International Symposium on Biomedical Imaging, pp 1310–1313.
Linguraru MG, Wang S, Shah F, et al. (2011) Automated noninvasive classification of renal cancer on multiphase CT. Med Phys 38(10):5738–5746.
Kim DY, Park JW (2004) Computer-aided detection of kidney tumor on abdominal computer tomography scans. Acta Radiol 45(7):791–795.
Liu J, Wang S, Linguraru MG, Yao J, Summers RM (2015) Computer-aided detection of exophytic renal lesions on non-contrast CT images. Med Image Anal 19(1):15–29.
Lee Y, Kim JK, Shim W, et al. (2015) Does computer-aided diagnosis permit differentiation of angiomyolipoma without visible fat from renal cell carcinoma on MDCT? AJR 205:W305–W312.
Pano B, Macias N, Salvador R, et al. (2016) Usefulness of MDCT to differentiate between renal cell carcinoma and oncocytoma: development of a predictive model. AJR 206(4):764–774.
Divgi C, Uzzo R, Gatsonis C, et al. (2012) Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: results from the REDECT trial. J Clin Oncol 31(2):187–194.
Gorin M, Rowe S, Baras A, et al. (2016) Prospective evaluation of 99mTc-sestamibi SPECT/CT for the diagnosis of renal oncocytomas and hybrid oncocytic/chromophobe tumors. Eur Urol 69(3):413–416.
Rowe SP, Gorin MA, Gordetsky J, et al. (2015) Initial experience using 99mTc-MIBI SPECT/CT for the differentiation of oncocytoma from renal cell carcinoma. Clin Nucl Med 40(4):309–313.
Raman SP, Chen Y, Schroeder JL, Huang P, Fishman EK (2014) CT texture analysis of renal masses: pilot study using random forest classification for prediction of pathology. Acad Radiol 21(12):1587–1596.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflicts of interest
All authors have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
Institutional review board (IRB) approval for a waiver of informed consent was received for this retrospective study.
Rights and permissions
About this article
Cite this article
Coy, H., Young, J.R., Douek, M.L. et al. Quantitative computer-aided diagnostic algorithm for automated detection of peak lesion attenuation in differentiating clear cell from papillary and chromophobe renal cell carcinoma, oncocytoma, and fat-poor angiomyolipoma on multiphasic multidetector computed tomography. Abdom Radiol 42, 1919–1928 (2017). https://doi.org/10.1007/s00261-017-1095-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1095-6