Abstract
Purpose
To assess the performance of the updated Prostate Imaging Reporting and Data System (PI-RADSv2) and the apparent diffusion coefficient (ADC) for predicting confirmatory biopsy results in patients considered for active surveillance of prostate cancer (PCA).
Methods
IRB-approved, retrospective study of 371 consecutive men with clinically low-risk PCA (initial biopsy Gleason score ≤6, prostate-specific antigen <10 ng/ml, clinical stage ≤T2a) who underwent 3T-prostate MRI before confirmatory biopsy. Two independent radiologists recorded the PI-RADSv2 scores and measured the corresponding ADC values in each patient. A composite score was generated to assess the performance of combining PI-RADSv2 + ADC.
Results
PCA was upgraded on confirmatory biopsy in 107/371 (29%) patients. Inter-reader agreement was substantial (PI-RADSv2: k = 0.73; 95% CI [0.66–0.80]; ADC: r = 0.74; 95% CI [0.69–0.79]). Accuracies, sensitivities, specificities, positive predicted value and negative predicted value of PI-RADSv2 were 85, 89, 83, 68, 95 and 78, 82, 76, 58, 91% for ADC. PI-RADSv2 accuracy was significantly higher than that of ADC for predicting biopsy upgrade (p = 0.014). The combined PI-RADSv2 + ADC composite score did not perform better than PI-RADSv2 alone. Obviating biopsy in patients with PI-RADSv2 score ≤3 would have missed Gleason Score upgrade in 12/232 (5%) of patients.
Conclusion
PI-RADSv2 was superior to ADC measurements for predicting PCA upgrading on confirmatory biopsy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Active surveillance has become a firmly established strategy for the management of patients with low-risk prostate cancer (PCA). However, its widespread adoption is still hampered by the potential risk of misclassification of patients who harbor intermediate and high-risk disease, but their tumors are missed or undersampled at non-targeted prostate biopsy [1–3]. Studies have shown that 20–40% of patients who are considered to have low-risk disease based on a random prostate biopsy are subsequently upstaged if a second (confirmatory) biopsy is performed [3–13]. This is not surprising considering reports showing that a standard prostate biopsy only samples approximately 1% of prostate tissue [14–16]. Studies have also shown that a clinical workup strategy, which includes prostate magnetic resonance imaging (MRI), aids the prediction of the results of confirmatory biopsy findings; however, the results published so far demonstrate wide space for improvement in the use of MRI for this purpose [7, 17–20].
Some of the initial reports on the ability of MRI to predict confirmatory biopsy results were based solely on anatomic T1- and T2-weighted images (T2WI) [18, 21]. The current standard for prostate MRI dictates a multiparametric approach with combines these anatomic sequences with functional techniques such as diffusion-weighted (DW) and dynamic contrast-enhanced (DCE) MRI. While the benefits of this multiparametric approach for PCA detection and staging have been widely reported [22–26], the complexity of the imaging interpretation has also increased. Until recently, prostate MRI was interpreted using loosely defined criteria through qualitative “Likert” suspicion scores; however, guidelines aiming to standardize prostate MRI acquisition and interpretation have now been published, including the Prostate Imaging Reporting and data system (PI-RADS), with an updated PI-RADS version 2 recently released [27–29]. PI-RADSv2 addressed the issue of combining different suspicion scores for the same lesion on different mpMRI sequences; however, it is acknowledged that the proposed integration scheme requires further validation in different clinical settings. Furthermore, the contribution and potential incremental value of quantitative metrics derived from the diffusion-weighted imaging component of mpMRI to the PI-RADSv2 assessment is unknown. The aim of this study was to assess the performance of PI-RADSv2 and the Apparent Diffusion Coefficient (ADC) for predicting confirmatory biopsy results in patients considered for active surveillance of PCA.
Materials and methods
The institutional review board approved this retrospective study and waived the informed consent requirement. The study was compliant with the Health Insurance Portability and Accountability Act of 1996.
Eligibility criteria and patient characteristics (Figs. 1, 2)
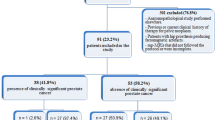
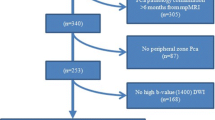
Using computerized searches of our institutional database, we identified 694 patients with an initial and confirmatory biopsy performed between October 1, 2007 and February 28, 2014. The inclusion criteria were as follows: Gleason 6 prostate cancer identified on initial biopsy, PSA < 10 ng/ml, clinical stage ≤ T2a, confirmatory prostate biopsy performed within 12 months of the initial prostate biopsy and 3T prostate MRI performed within the 6 months prior to the confirmatory biopsy, using pelvic and endorectal coils and including multiplanar T2-weighted images, multi b-value DW-MRI, and DCE-MRI (Fig. 1). As detailed in Table 1 and Fig. 2, our final study population consisted of 371 patients (mean age: 60 years, range [41–81]).
MRI acquisition
All MRI studies were performed using 3-Tesla whole-body units (Signa HDx or MR750, GE Healthcare, Waukesha, WI, USA) using a dedicated prostate protocol in line with literature recommendations [28]. Patients were examined in the supine position, with use of a body coil for excitation and a phased-array pelvic coil (GE Medical Systems) combined with a commercially available balloon-covered expandable endorectal coil (Medrad, eCoil, Warrendale, Pa) filled with air for signal reception. The anatomic images were obtained using transverse T1-weighted (repetition time msec/echo time msec, 400–650/MIN; section thickness, 5 mm; intersection gap, 1 mm; field of view, 28–36 cm; matrix, 256 × 192) and transverse, coronal, and sagittal T2-weighted fast spin-echo sequences (repetition time msec/echo time msec, 3500–5000/120; echo train length, 23; section thickness, 3–4 mm; no intersection gap; field of view, 16–20 cm; matrix, 256 × 192). DW-MRI was obtained in the transverse plane with orientation and location identical to those prescribed for the transverse T2-weighted MR imaging using a spin-echo echo-planar imaging sequence with ramp sampling using a pair of rectangular gradient pulses along with three orthogonal axes (repetition time msec/effective echo time msec, 4000–8000/min; field of view, 16–20 cm; section thickness, 3–4 mm; no intersection gap; in-plane resolution, 1.9 × 1.9 mm; b values, 0 and 400, 700, 1000 s/mm2). For DCE-MRI, gadopentetate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, Montville, NJ, USA) was administered (0.1 mmol of per kilogram of body weight at a rate of 2 ml/s) using an automatic injector (Medrad, Indianola, IA, USA); (TR/TE = 3.6–4.9/1.3–1.7 ms; slice thickness: 5 mm, no interslice gap; field of view: 24 × 24 cm; matrix: 256 × 128–160, mean temporal resolution: 10 s).
MRI interpretation
Two radiologists (JG and SN) independently reviewed all MRIs. First, PI-RADSv2 scores were assigned. If more than one lesion was identified, only the one with the highest score was recorded. Interpretation was performed on commercially available PACS workstations (GE Healthcare, USA). The same two radiologists independently measured the ADC value by placing a region-of-interest (ROI) over the lesion with the highest PI-RADSv2 score in each patient (Fig. 3). If no lesion was identified on mpMRI, the ADC was recorded from an ROI placed in a representative area of normal appearing peripheral zone on MRI.
T2-weighted image (A, D), ADC map (B, E) and fused T2-weighted image and ADC map (C, F) demonstrate a PI-RADSv2 5 lesion (white arrow) within the right transition zone (A, B, C) and left mid-gland peripheral zone (D, E, F). T2-weighted image (G), ADC map (H) and fused T2-weighted image and ADC map (I) demonstrate a vague T2 (white arrow, G) non measurable lesion with mild diffusion restriction (white arrow, H, I) classified as PI-RADSv2 2 lesion. White circle on C, F and I demonstrate ADC ROI
Lesion locations (i.e., transition vs peripheral zone) were taken in account in tumor assessment (n = 25 in transition zone). Lesions were scored according to the prostatic zone where they were located as recommended by PI-RADS [28]. In case of discrepancies between the readers regarding PI-RADSv2 scores, a third radiologist (AV) adjudicated the findings.
Histopathological analysis and image correlation
For all patients initial biopsy was performed either at an outside institution (349/371) or at our center (22/371). All confirmatory biopsies were performed at our institution. The confirmatory biopsy included a standard 12-core biopsy (medial and lateral aspects of the base, middle and apical portions of the prostate bilaterally) and two transition zone biopsies for a total of 14 cores. At the discretion of the urologist performing the procedure, samples were also obtained from suspicious lesions identified on digital rectal examination, transrectal ultrasound or MRI. All biopsy specimens were reviewed at our institution by a dedicated genitourinary pathologist. No dedicated ultrasound/fusion system was used.
Statistical assessment
Data are expressed as mean ± standard deviation (SD) for continuous variables and as numbers and percentages for categorical variables. For analysis, PI-RADSv2 scores were dichotomized as positive (score ≥ 4) or negative (score ≤ 3). Agreement between readers for PI-RADSv2 was calculated using Cohen’s simple kappa statistic. Agreement between readers for ADC value measurement was calculated using the Pearson test. The agreement was interpreted as follows: 0.00–0.20, slight; 0.21–0.40, fair; 0.40–0.60, moderate; 0.61–0.80, substantial; and 0.81–1.00, almost perfect. For subsequent analysis, the adjudicated PI-RADSv2 scores and ADC values from reader 1 were used. The relationships between PI-RADSv2 score and confirmatory biopsy results were established with Chi square test. The thresholds of ADC value predicting biopsy upgrade were assessed using receiver operating characteristics curves (ROC). A linear regression line between ADC threshold classification and PI-RADSv2 score was generated. The thresholds of the linear regression equation for predicting biopsy upgrade was assessed by using receiver operatic characteristics. This equation takes in account “grey zone” data where there is discrepancy between the PI-RADS and ADC assessments (PI-RADS v2 score ≤ 3 with an ADC < 1.1275 and PI-RADS v2 ≥ 4–5 with an ADC > 1.1275). Equality of proportion test with continuity correction was used for comparison between PI-RADSv2 and ADC performance and between PI-RADSv2 combined PI-RADSv2/ADC values score.
A p value ≤ 0.05 was considered statistically significant. All analyses were performed with R software (R version 2.15.2; The R Foundation for Statistical Computing, Vienna, Austria).
Results
Pathological findings
In 107/371 (29%) patients, disease was upgraded on confirmatory biopsy (i.e., there was at least one core with Gleason score seven or greater cancer). In 79/371 patients (21%), no cancer was identified on confirmatory biopsy.
Following confirmatory biopsy, 93/371 (25%) patients underwent radical prostatectomy. In 71 of these 93 patients (76%), prostatectomy showed higher grade disease than did initial biopsy, and for 57 of these 93 patients (61%) confirmatory biopsy also showed higher grade disease than did initial biopsy.
PI-RADS v2 assessment (Table 2)
The inter-reader agreement for the PI-RADSv2 assessment was substantial (k = 0.73, 95% CI [0.66–0.8]). There was a significant association between a PI-RADSv2 score ≥ 4 and confirmatory biopsy upgrade (OR 39.6; 95% CI [20–78.3]; p < 0.0001) with an accuracy of 85%, sensitivity of 89%, specificity of 83%, positive predictive value of 68%, and negative predictive value of 95%. If biopsy was obviated in all patients with PI-RADSv2 score ≤ 3, Gleason Score upgrading would have been missed in 12/232 (5%) patients.
ADC value (Table 2)
The correlation between the ADC values for two readers was substantial (r = 0.74; 95%CI [0.69–0.79]).
The mean ADC value of PI-RADSv2 score ≥ 4 lesions (0.9 ± 0.2 × 10−3 mm2/s) was significantly lower than the mean ADC of PI-RADSv2 score ≤ 3 lesions (1.3 ± 0.3 × 10−3 mm2/s) (p < 0.0001).
An ADC value of 1.1275 × 10−3 mm2/s was chosen as the optimal cutoff value for predicting confirmatory biopsy upgrade. Using this cutoff, confirmatory biopsy upgrade was predicted with an accuracy of 78%, sensitivity of 82%, specificity of 76%, positive predictive value of 58%, and negative predictive value of 91%. If biopsy was obviated in all patients with ADC > 1.1275 × 10−3 mm2/s, Gleason Score upgrading would have been missed in 19/219 (9%) of patients.
Combination of PI-RADSv2 and ADC values
The increase in correctly identified cases (accuracy) for PI-RADS v2 (85%) compared to ADC value (78%) was significant (p = 0.014).
As stated in the methods, a linear regression line between ADC threshold and PI-RADSv2 score was generated: 3.05 + 3.16 × [PI-RADS v2 (4–5)] + 0.78 × (ADC < 1.1275). Using the equation generated the optimal cutoff value in the composite PI-RADSv2/ADC score for identifying biopsy upgrade was −1.0834. This cutoff offered an accuracy of 85%, sensitivity of 89%, specificity of 83%, positive predictive value of 68%, and negative predictive value of 95% (Table 2). No difference between this composite score and PI-RADS v2 alone was seen (p = 1). If biopsy was obviated in all patients with a composite score up to −1.0834, Gleason Score upgrading would have been missed in 12/232(5%) patients.
Discussion
In this study, we evaluated the performance of PI-RADSv2, quantitative ADC measurements, and the combination of these two variables for predicting confirmatory biopsy results in patients with clinically low-risk prostate cancer. We found that all three interpretation approaches were significantly associated with Gleason Score upgrading on confirmatory biopsy; however, PI-RADSv2 performed significantly better than ADC values. Furthermore, the combination of PI-RADSv2 and ADC through the calculation of a “composite score” did not perform better than PI-RADSv2 alone. The use of PI-RADSv2 has the potential to increase the yield of significant prostate cancer detection and the identification of a subgroup of patients who would not require confirmatory biopsy. Among patients initially diagnosed with clinically low-risk prostate cancer, those with PI-RADSv2 scores ≤ 3 were significantly more likely to demonstrate low-risk features on confirmatory biopsy, while patients with PI-RADSv2 ≥ 4 were significantly more likely to have higher risk cancer. It is worth emphasizing although obviating biopsy in patients with a PI-RADSv2 score ≤ 3 on MRI would have prevented 220 biopsies in our cohort, Gleason Score upgrading would have been missed in 5% of patients. Although no diagnostic test is perfect, and there is obvious space for improvement in performance in the results shown, the tolerable risk of “missed” tumors on MRI may vary according to patient and physician preference. Nonetheless, this risk should be part of the patient physician leading to the management decision-making process.
Prostate biopsy remains the most accepted criteria to trigger treatment for men on active surveillance. Most published reports advocate confirmatory prostate biopsy within one year of initiating active surveillance based on studies suggesting 23–38% of men with low-risk prostate cancer may harbor high-grade tumors detected on second biopsy [30]. However, prostate biopsy is associated with known risks, including infectious complications, hematuria, hematospermia, and pain. Therefore, a risk-adapted approach to guide active surveillance follow-up based on patient, pathologic, and imaging characteristics can reduce the number of unnecessary prostate biopsies. During the past decade, multiparametric MRI has emerged as a promising tool to diagnose prostate cancer by helping guide prostate biopsies. Most studies report improvement in the detection rate of higher Gleason grade cancer using MRI-targeted biopsy techniques compared to systematic biopsy. In a study by Vargas et al. [18], the sensitivity and specificity to predict confirmatory biopsy results were 0.89 and 0.70, respectively. However, a recent meta-analysis [31] including the data of seven studies comprising 1028 patients demonstrated that mpMRI has a moderate diagnostic accuracy as a significant predictor of disease reclassification among AS candidates. Indeed, the pooled estimates of MRI on disease reclassification among AS candidates were as follows: sensitivity, 0.69; specificity, 0.78; positive likelihood ratio, 3.1; negative likelihood ratio, 0.40; and diagnostic odds ratio, 8. The high NPV and specificity for the prediction of biopsy reclassification upon clinical follow-up suggested that negative prostate MRI findings may support a patient remaining under AS.
In our study, low ADC values were associated with biopsy upgrade at univariate analysis. Those results are concordant with previous study [32], however in our study, PI-RADSv2 score showed superiority.
A reclassification rate of 10–30% has been reported during the follow-up of patients on active surveillance [3, 12, 16]. This is thought to be mainly due to under sampling of significant tumors at initial biopsy rather than progression of low-risk disease. Applying PI-RADS v2 approach could help identify those men incorrectly classified as low risk, subsequent to sampling error. Our study suggests that patients with a PI-RADSv2 ≥ 4 should be referred for confirmatory or MR-guided biopsy. Conversely, most patients with a PI-RADSv2 score ≤ 3 should be considered eligible for continued active surveillance, obviating the need for a repeat, confirmatory biopsy with its associated risks. Our results are also concordant with recent literature, where PI-RADSv2 approach has been shown to improve the diagnostic accuracy to detect PCA and standardize the interpretation of mpMRI [33, 34].
Our study has several limitations. First, it is retrospective, and uses confirmatory biopsy as the reference standard, rather than long-term outcome measures. Second, we did not evaluate fusion biopsies correlation results as its systematic use began after the patients included in this study were treated at our institution. Third, the results are reported at a per-patient basis, as there is no direct pathologic correlation for each abnormality identified on MRI. However, we show how the MRI findings could potentially impact patient management, which is probably more relevant than reporting on management of individual lesions. Forth, we dichotomized PI-RADSv2 score for assessment (1–3 vs. 4–5). Although this is the cutoff recommended by the PI-RADSv2 guideline as representing clinically significant prostate cancer likely or definitely present, we did not test the effects of utilizing different cutoffs on our results. However, the cutoff we employed is also supported by recent literature suggesting that PI-RADS score three lesions are associated with low risk of clinically significant cancer [35] Finally, other diffusion-weighted MRI methods, including higher/different b value combinations, high-resolution DWI, diffusion-tensor imaging, and intravoxel incoherent motion (IVIM) techniques, may provide added value and should be evaluated in future studies.
In summary, the success of active surveillance as a management strategy for prostate cancer relies primarily on the accurate identification of patients with low-risk disease. PI-RADSv2 showed to be predictive of upgrading on confirmatory prostate biopsy, suggesting that prostate mpMRI, and more specifically diffusion-weighted imaging, may contribute to the complex process of assessing patient eligibility for active surveillance.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- PCA:
-
Prostate cancer
- T2WI:
-
T2-weighted images
- MRI:
-
Magnetic resonance imaging
- DW:
-
Diffusion-weighted
- DCE:
-
Dynamic contrast-enhanced
- PI-RADS:
-
Prostate imaging reporting and data system
- SD:
-
Standard deviation
References
Adamy A, Yee DS, Matsushita K, et al. (2011) Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J Urol 185:477–482
Wong LM, Alibhai SM, Trottier G, et al. (2014) A negative confirmatory biopsy among men on active surveillance for prostate cancer does not protect them from histologic grade progression. Eur Urol 66:406–413
Wong LM, Ferrara S, Alibhai SM, et al. (2015) Diagnostic prostate biopsy performed in a non-academic center increases the risk of re-classification at confirmatory biopsy for men considering active surveillance for prostate cancer. Prostate Cancer Prostatic Dis 18:69–74
Dall’Era MA, Albertsen PC, Bangma C, et al. (2012) Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol 62:976–983
Porten SP, Whitson JM, Cowan JE, et al. (2011) Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol 29:2795–2800
Porten SP, Whitson JM, Cowan JE, et al. (2011) Changes in cancer volume in serial biopsies of men on active surveillance for early stage prostate cancer. J Urol 186:1825–1829
Recabal P, Ehdaie B (2015) The role of MRI in active surveillance for men with localized prostate cancer. Curr Opin Urol 25:504–509
Klotz L (2015) Active surveillance for low-risk prostate cancer. Curr Urol Rep 16:24
Klotz L (2015) Active surveillance for prostate cancer: debate over the application, not the concept. Eur Urol 67:1006–1008
Loeb S, Bruinsma SM, Nicholson J, et al. (2015) Active surveillance for prostate cancer: a systematic review of clinicopathologic variables and biomarkers for risk stratification. Eur Urol 67:619–626
Tosoian JJ, Sundi D, Trock BJ, et al. (2015) Pathologic outcomes in favorable-risk prostate cancer: comparative analysis of men electing active surveillance and immediate surgery. Eur Urol. doi:10.1016/j.eururo.2015.09.032
Bruinsma SM, Bokhorst LP, Roobol MJ, Bangma CH (2015) How often is biopsy necessary in patients with prostate cancer on active surveillance? J Urol. doi:10.1016/j.juro.2015.10.061
Bosco C, Cozzi G, Kinsella J, et al. (2016) Confirmatory biopsy for the assessment of prostate cancer in men considering active surveillance: reference centre experience. Ecancermedicalscience 10:633
Partin AW (2000) Does sextant prostate biopsy provide adequate sampling for early detection of prostate cancer? Curr Urol Rep 1:245
Patel A (2007) Finding a balanced strategy in prostate cancer diagnosis for case detection by prostate needle biopsy at first presentation: “all for more cores and more cores for all” or individualised sampling regimens? Which way forward? Eur Urol 52:313–315
Rodrigues A, Freitas R, Nogueira-Silva P, Jeronimo C, Henrique R (2014) Biopsy sampling and histopathological markers for diagnosis of prostate cancer. Expert Rev Anticancer Ther 14:1323–1336
Vilanova JC, Comet J, Capdevila A, et al. (2001) The value of endorectal MR imaging to predict positive biopsies in clinically intermediate-risk prostate cancer patients. Eur Radiol 11:229–235
Vargas HA, Akin O, Afaq A, et al. (2012) Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol 188:1732–1738
Fascelli M, George AK, Frye T, et al. (2015) The role of MRI in active surveillance for prostate cancer. Curr Urol Rep 16:42
Pessoa RR, Viana PC, Mattedi RL, et al. (2016) Value of 3-Tesla multiparametric magnetic resonance imaging and targeted biopsy for improved risk stratification in patients considered for active surveillance. BJU Int. doi:10.1111/bju.13624
Vargas HA, Akin O, Shukla-Dave A, et al. (2012) Performance characteristics of MR imaging in the evaluation of clinically low-risk prostate cancer: a prospective study. Radiology 265:478–487
Bratan F, Melodelima C, Souchon R, et al. (2015) How accurate is multiparametric MR imaging in evaluation of prostate cancer volume? Radiology 275:144–154
Donati OF, Jung SI, Vargas HA, et al. (2013) Multiparametric prostate MR imaging with T2-weighted, diffusion-weighted, and dynamic contrast-enhanced sequences: are all pulse sequences necessary to detect locally recurrent prostate cancer after radiation therapy? Radiology 268:440–450
Hoeks CM, Barentsz JO, Hambrock T, et al. (2011) Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology 261:46–66
Kim JY, Kim SH, Kim YH, et al. (2014) Low-risk prostate cancer: the accuracy of multiparametric MR imaging for detection. Radiology 271:435–444
Peng Y, Jiang Y, Antic T, et al. (2014) Validation of quantitative analysis of multiparametric prostate MR images for prostate cancer detection and aggressiveness assessment: a cross-imager study. Radiology 271:461–471
Kayat Bittencourt L, Litjens G, Hulsbergen-van de Kaa CA, et al. (2015) Prostate cancer: the european society of urogenital radiology prostate imaging reporting and data system criteria for predicting extraprostatic extension by using 3-t multiparametric MR imaging. Radiology. doi:10.1148/radiol.15141412:141412
Vargas HA, Hotker AM, Goldman DA, et al. (2015) Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol. doi:10.1007/s00330-015-4015-6
Weinreb JC, Barentsz JO, Choyke PL, et al. (2016) PI-RADS prostate imaging—reporting and data system: 2015, Version 2. Eur Urol 69:16–40
Ross AE, Loeb S, Landis P, et al. (2010) Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol 28:2810–2816
Guo R, Cai L, Fan Y, et al. (2015) Magnetic resonance imaging on disease reclassification among active surveillance candidates with low-risk prostate cancer: a diagnostic meta-analysis. Prostate Cancer Prostatic Dis 18:221–228
van As NJ, de Souza NM, Riches SF, et al. (2009) A study of diffusion-weighted magnetic resonance imaging in men with untreated localised prostate cancer on active surveillance. Eur Urol 56:981–987
Zhao C, Gao G, Fang D, et al. (2016) The efficiency of multiparametric magnetic resonance imaging (mpMRI) using PI-RADS Version 2 in the diagnosis of clinically significant prostate cancer. Clin Imaging 40:885–888
Vargas HA, Hotker AM, Goldman DA, et al. (2016) Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol 26:1606–1612
Liddell H, Jyoti R, Haxhimolla HZ (2015) mp-MRI prostate characterised PIRADS 3 lesions are associated with a low risk of clinically significant prostate cancer—a retrospective review of 92 biopsied PIRADS 3 lesions. Curr Urol 8:96–100
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was partially funded by NIH grant P30 (CA008748) (Evis Sala, Hedvik Hricak, Alberto Vargas).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
The institutional review board approved this retrospective study and waived the informed consent requirement.
Rights and permissions
About this article
Cite this article
Nougaret, S., Robertson, N., Golia Pernicka, J. et al. The performance of PI-RADSv2 and quantitative apparent diffusion coefficient for predicting confirmatory prostate biopsy findings in patients considered for active surveillance of prostate cancer. Abdom Radiol 42, 1968–1974 (2017). https://doi.org/10.1007/s00261-017-1086-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1086-7