Abstract
While focal fat deposition in the liver mostly occurs in typical locations related to non-portal venous supply, unusual patterns of focal fat deposition, including multi-nodular, mass-like, and perivascular patterns, mimic malignancies and cause diagnostic challenges. Patients with unusual focal fat deposition often have potential underlying etiologies such as diabetes, alcohol abuse, metabolic disease, or various medications/chemotherapy. Some cases can be explained by non-portal venous supply or ischemia. Chemical-shift MRI or contrast-enhanced ultrasound (CEUS) is useful for non-invasive diagnosis of focal fat deposition. We illustrate a series of US, CT, and MR imaging features of focal fatty deposition in the liver mimicking other conditions and seek possible causes. Understanding of imaging patterns of focal fat deposition and its potential causes can help a non-invasive diagnosis by performing confirmatory imaging tests and prevent unnecessary invasive procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Fatty liver refers to a wide spectrum of conditions characterized by triglyceride accumulation within the cytoplasm of the hepatocytes. Fatty liver can be divided into macrovesicular and microvesicular steatosis. Macrovesicular steatosis, which contains large fat vacuoles and displaced nuclei in the hepatocytes, is typically associated with excessive alcohol intake, non-alcoholic fatty liver disease (NAFLD), and type II diabetes. Microvesicular steatosis, which is characterized by small intracytoplasmic fatty inclusions without the displacement of the nuclei, usually occurs with severe impairment of mitochondrial fatty acid beta-oxidation, as either a primary disorder or secondary to drug toxicity [1].
While most fatty liver diffusely involves the whole liver, focal or multi-focal fat deposition in the liver is occasionally encountered and causes a diagnostic challenge. Some regions of the liver are well known as common sites of focal fat deposition related to non-portal venous supply. These regions include the medial segment of the left lobe of the liver adjacent to the falciform ligament, the gallbladder bed, the anterior portion of segment I, and the posterior portion of segment IV [2–4]. Focal fatty sparing in the diffuse fatty also frequently occurs in the similar locations because of non-portal venous supply. However, unusual focal or multi-focal fat deposition involving atypical regions in the liver can lead to a misdiagnosis as infiltrative or multi-nodular hepatic malignancies such as metastases [5, 6]. Therefore, it is important to be familiar with the atypical imaging patterns of focal fat deposition and associated risk factors. Hence, an appropriate next diagnostic step can be suggested and unnecessary invasive procedures can be prevented.
Key imaging findings of focal fat deposition
Macroscopic focal fat deposition usually appears as a highly echoic non-spherical mass with slight posterior sound attenuation on US. Sonic attenuation is attributed to the fact that fatty tissue attenuates sound more than unaffected liver parenchyma [7]. However, accurate characterization of a hyperechoic lesion is not easy in some patients because other factors, such as concomitant liver fibrosis and poor sonic window may hinder the presence of fat. US has a limited diagnostic performance with low sensitivity and specificity for detecting microvesicular fat compared with macrovesicular fat [8].
Contrast-enhanced US is very helpful to diagnose focal fat deposition by demonstrating iso-enhancement relative to the liver during the arterial, portal, and late phases. CT usually shows a variable degree of hypoattenuation depending on the amount of fat within the lesion instead of typical fat density. However, there are several useful CT imaging features of focal fat deposition compared to hepatic malignancies, including non-spherical or geographic border, absence of mass effect, hypoattenuating lesions corresponding to hyperechoic lesions on US, and undisturbed extension of hepatic vessels within the lesion. On US and CT, recognizing specific hyperechogenicity or hypoattenuation of focal fat deposition in the liver may be unreliable in the presence of a liver fat content of less than 30%.
T1-wighted gradient-echo (GRE) MRI with hepatocyte-specific contrast agent such as gadoxetic acid (Gd-EOB-DTPA) and gadobenate dimeglumine (Gd-BOPTA) showed no significant differences in signal enhancement ratio between focal fat deposition and normal liver during all dynamic contrast-enhanced phases including hepatobiliary phase (HBP) [9, 10]. Although most focal fat deposition showed hypointense on both pre-enhanced image and HBP phase, enhancement patterns of focal fat deposition varied.
Hence, several MRI techniques have been used to qualify and quantify liver fat including chemical-shift imaging, fat saturation, and fat-selective excitation methods [11].
MRI using chemical-shift gradient-echo imaging (CSI) technique with in-phase and opposed-phase acquisitions is the most commonly used method, easily applicable and confirmative imaging test to diagnose focal fat deposition. Focal fat deposition can be confidently diagnosed when there is a signal intensity drop on opposed-phase images in comparison with in-phase images, whereas signal intensity of the normal liver parenchyma is similar between two phases [12, 13]. Multiple-echo chemical shift with T2*-correction may be needed when substantial iron deposition is present to prevent underestimate focal fat detection [14]. Subtraction from out-of-phase image dataset from the in-phase image dataset with Dixon technique is also a simple and convenient method to detect focal fat deposition (Fig. 3). Fat saturation technique is also useful especially when the lesion is composed exclusively of fat because no signal drop can be detected on opposed phase in CSI. Although magnetic resonance spectroscopy (MRS) detects focal fat quantification more accurately than CSI, the acquisition and analysis of MRS data is complicated and time-consuming. Further MRS using single-voxel represents small portion of the liver instead of the entire liver. In contrast, MRI such as CSI is widely available, easy to analyze data, and able to determine the whole liver [15].
Additional imaging characteristics for focal fat deposition include geographic border, enhancement similar to the surrounding liver parenchyma on MR images with liver-specific contrast agent such as gadobenate dimeglumine or gadoxetic acid, absence of mass effect, stability in size over time, and undisturbed extension of hepatic vessels within the lesion. These characteristics are helpful to differentiate focal fat deposition from fat-containing tumors such as hepatic adenomas, hepatocellular carcinomas, or angiomyolipoma.
Morphological patterns of focal fat deposition
Small multi-nodular deposition
It is challenging to suggest the diagnosis of focal fat deposition when it manifests as multiple small nodular lesions throughout the liver. Differential diagnoses on imaging often include metastases, lymphoma, sarcoidosis, abscesses, candidiasis, and hemangiomas. The etiology of this unusual pattern is not well understood. In our experience, many of these patients have potential underlying causes of fatty liver including a history of chemotherapy due to malignancy, metabolic disease, or alcohol abuse. A suggestion of the diagnosis of focal fat deposition is even more difficult when the patient has a history of malignancy.
Homogeneous hyperechogenicity, absence of hypoechoic halo, and non-spherical margin of individual lesions are helpful suggestive findings of focal fat deposition on ultrasound (Fig. 1). The combination of bright hyperechogenicity and marked hypoattenuation on CT can raise a possibility of fat-containing lesions. The lesions are iso-enhancing compared to the liver on contrast-enhanced ultrasound in all phases (Fig. 2). CSI can confirm the diagnosis. Subtraction of opposed-phase images from in-phase images with Dixon technique is a simple and comprehensive imaging technique to detect fat within liver lesions (Fig. 3).
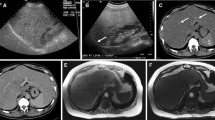
Small multi-nodular fat deposition in a 47-year-old man who received chemotherapy for lymphoma and malignant thymoma. A Transverse gray-scale ultrasound shows multiple small homogeneous hyperechoic nodules without hypoechoic halo in the liver. Individual nodules have non-spherical margins. B and C In- and opposed-phase axial T1-weighted MR images show multiple nodular lesions with dropped signal intensity (arrows) on opposed-phase image (C) compared with in-phase image (B)
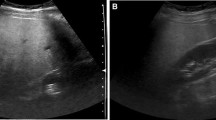
Small multi-nodular fat deposition in a 25-year-old woman. A and B Dual imaging display of contrast-enhanced ultrasound scans shows contrast only imaging on the left and gray-scale imaging on the right. There are multiple brightly echogenic nodules on gray-scale ultrasound images. The nodules show iso-enhancement relative to the liver in the arterial A and portal venous B phases
Small multi-nodular fat deposition in a 58-year-old woman with pancreatic neuroendocrine tumor on chemotherapy. A Axial contrast-enhanced CT scan shows small multiple hypoattenuating nodules (arrows) in the liver. B and C In- and opposed-phase axial T1-weighted MR images show small nodular lesions with dropped signal intensity (arrows) on opposed-phase image (C). D Subtracted image of opposed-phase from the in-phase dataset clearly shows small fat-containing nodules that are hyperintense (arrows)
Solitary mass-like deposition
Focal fat deposition may involve a focal area of the liver, forming a mass-like lesion on imaging. It shows a well-defined margin with lobulated or geographic border without mass effect. It is homogeneously hyperechoic on ultrasound mimicking a hepatic hemangioma, but it is usually more echogenic than typical hemangiomas and shows a mild posterior sonic attenuation instead of sonic enhancement which is often seen in hemangiomas. CT shows a variable degree of hypoattenuation depending on the amount of fat within the lesion. The presence of undisturbed portal or hepatic veins within the lesion can be a helpful clue to the diagnosis. CSI is necessary to confirm the diagnosis.
Non-portal venous supply as a potential cause for mass-like focal fat deposition is occasionally identified on arterial-phase CT or MRI. Aberrant pancreatic venous supply (Fig. 4) has been suggested for one of the etiologies for focal fat deposition as the pancreatic vein has higher concentration of insulin than the rest of the portal vein. Focal ischemic change due to a procedure or an obstructing mass can be a potential cause for focal fat deposition (Fig. 5).
Solitary mass-like fat deposition in a 57-year-old man who underwent distal pancreatectomy and right hepatectomy for pancreatic neuroendocrine tumor and hepatic metastasis. A and B Two axial contrast-enhanced CT scans show a hypoattenuating mass in segment IV of the liver. An aberrant pancreatic venous supply (arrows) to the mass is visualized on axial (B) and coronal (C) CT scans. D and E In- and opposed-phase axial T1-weighted MR images show dropped signal intensity in the mass (arrow) on opposed-phase image (E) compared with in-phase image (D), confirming the diagnosis of focal fat deposition related to non-portal venous supply
Solitary mass-like fat deposition in a 55-year-old woman with endoscopic retrograde biliary drainage due to hilar cholangiocarcinoma. A Axial contrast-enhanced CT scan shows a mass-like hypoattenuating lesion in segment VIII with geographic margin and patient portal veins within the lesion. A biliary stent is seen in the central portion of the segment 8 bile duct (arrow). B and C In- and opposed-phase axial T1-weighted MR images show markedly dropped signal intensity in the mass-like lesion (arrow) on opposed-phase image (C) compared with in-phase image (B). D The lesion is hypointense relative to the liver on fat-suppressed T2-weighted MR image due to high fat content within the lesion.
Multiple large confluent masses
Focal fat deposition with the appearance of multiple large confluent masses is uncommon and the diagnosis is challenging. Bright hyperechogenicity on US, marked hypoattenuation on CT, absence of mass effect, geographic margin, and undisturbed intralesional veins are important clues to suggest the diagnosis (Fig. 6). MRI with in- and opposed-phase T1-weighted sequences can confirm the diagnosis.
Multiple large confluent mass-like fat deposition in a 69-year-old woman. A Axial contrast-enhanced CT scan shows multiple large, confluent hypoattenuating mass-like lesions with geographic margin. There are peripheral rim-like markedly hypoattenuating areas representing areas with more severe steatosis. Patent, undisturbed hepatic veins (arrows) are seen within the lesion. B and C In- and opposed-phase axial T1-weighted MR images show markedly dropped signal intensity in the mass-like lesions on opposed-phase image (C) compared with in-phase image (B)
Atrophy of the involved hepatic parenchyma in some cases may suggest the ischemic liver injury as a potential cause of the findings. This pattern of focal fat deposition is also seen in primary sclerosing cholangitis suggesting the underlying biliary strictures or cholangitis as a potential cause for focal fat deposition (Fig. 7).
Multiple large confluent mass-like fat deposition in a 53-year-old man with primary sclerosing cholangitis and ulcerative colitis. A Gray-scale ultrasound scan shows multiple large confluent homogeneous hyperechoic masses with non-spherical margin in the right liver. B and C In- and opposed-phase axial T1-weighted MR images show markedly dropped signal intensity in the mass-like lesions on opposed-phase image (C) compared with in-phase image (B)
Perivascular deposition
Perivascular fat deposition is rare [16] and is characterized by halos of fat surrounding the hepatic veins, the portal veins, or both hepatic and portal veins without a mass effect on the involved vessels. The imaging appearance can be tram-like or tubular when the vessels are running parallel to the imaging plane or ring-like or round when the vessels are running perpendicular to the imaging plane (Fig. 8). It may be difficult to differentiate from periportal edema and hepatic congestion caused by various etiologies. It can mimic tumorous condition such as lymphoma, neurofibromatosis, Langerhans’ cell histiocytosis on CT and ultrasound if perivascular low attenuation is located in lobar and segmental periportal area [17]. The pathophysiology of perivascular fat deposition in the liver is not well known, but perivascular deposition in the centrilobular region surrounding the hepatic veins in alcohol abuse suggests a preferential liver injury by higher concentration of hepatotoxic material [18].
Perivascular fat deposition in a 71-year-old man. A Gray-scale ultrasound scan shows irregular homogeneous hyperechoic mass-like lesions surrounding portal vein branches (arrows) with geographic margin. B and C In- and opposed-phase axial T1-weighted MR images show dropped signal intensity in the mass-like lesions on opposed-phase image (C) compared with in-phase image (B). There are thin, peripheral, rim-like areas of more severe steatosis at the margin of the lesions
Plausible causes of focal fat deposition
Alcohol abuse
The effect of alcohol abuse on the liver has been known for centuries. Hepatotoxicity of alcohol is related to its metabolism by means of the alcohol dehydrogenase and cytochrome P450 2E1 (CYP2E1) pathways. Alcohol dehydrogenase promotes fat deposition into the liver by stimulating the synthesis of fatty acids and opposing their oxidation [18]. Histologic pattern of fat deposition is usually macrovesicular or mixed microvesicular and macrovesicular. Fatty deposition due to alcoholic liver disease usually involves the entire liver, but occasionally uneven or focal distribution can occur. It begins in the centrilobular zone 3 and progresses towards the periportal zone 1, which explains perivascular fat deposition around hepatic veins in some cases. It may cause continuous fat deposition and lead to rupture of fat vacuoles, resulting in histiocytic reaction or lipogranulomas in the liver [19] which can mimic malignancy.
Insulin and diabetes
Insulin has been known to be a causative factor of hepatic steatosis. When serum insulin levels are low (i.e., fasting state), free fatty acids in the liver are oxidated to ketone bodies. Persistent high insulin levels inhibit oxidation of free fatty acids and result in more esterification of free fatty acids into triglycerides, causing abnormal accumulation of triglycerides in hepatocytes. High levels of insulin have also been related to other atypical forms of focal fat deposition [20]. The presence of an aberrant pancreaticoduodenal vein draining in a focal region of the liver supplies insulin-rich blood from the pancreas leading to focal fat deposition (Fig. 4). A peripheral fat deposition surrounding liver metastases from insulinoma represents a localized insulin effect of focal fat deposition due to insulin [21]. Subcapsular fat deposition caused by insulin added to the peritoneal dialysate in patients with renal failure and insulin-dependent diabetes also supports the explanation of focal fat deposition related to localized higher concentration of insulin (Fig. 9) [22]. Patients with type 2 diabetes have an increased chance of developing NAFLD and subsequent liver fibrosis and cirrhosis. The relationship between type 2 diabetes, insulin resistance, and NAFLD has been suggested and can be explained by increased delivery of fatty acid to the liver and accumulation as triglyceride driven by insulin resistance [23].
Subcapsular fat deposition in a 61-year-old man with renal failure caused by type 2 diabetes. The patient was on peritoneal dialysis with insulin added to the peritoneal dialysate. A Gray-scale ultrasound scan in the transverse scan shows an elongated subcapsular echogenic mass (arrows). B The subcapsular lesion (arrows) is markedly hypoattenuating on axial contrast-enhanced CT scan
Diminished perfusion
Diminished liver perfusion and tissue hypoxia have been suggested as the cause for larger areas of focal fatty infiltration at the periphery of the liver where portal venous and arterial circulation is relatively low [24]. Focal fat deposition due to ischemia can also occur by tumoral compression of adjacent liver [25]. Typical focal fat deposition in the anteromedial part of segment IV near the falciform ligament has been explained by non-portal venous supply from the systemic vein and relative lack of portal venous supply, causing an ischemic injury [26].
Drug induced liver injury and comorbidity
A relatively uncommon but distinctive type of liver injury is due to the usage of drugs which include aspirin, tetracycline, amiodarone, valproic acid, and several antiviral nucleoside analogues such as fialuridine. These drugs lead to accumulation of microvesicular fat in hepatocytes and damage the mitochondria. This can further evolve into macrovesicular fatty liver [28]. Chemotherapy results in oxidative stress not only to cancer cells but also to normal hepatocyte leading to hepatic steatosis which can be diffuse or focal. Patients with colorectal cancer with metastases undergoing chemotherapy with 5-FU, platinum derivatives, taxanes, irinotecan, oxaliplatin, or cetuximab are susceptible to fatty deposition [29]. Fatty liver has also been reported with other chemotherapy agents including L-asparaginase, dactinomycin, mitomycin C, bleomycin sulfate, and methotrexate. Fatty liver in inflammatory bowel disease (Fig. 7) might be associated with several factors such as severe illness, with combined malnutrition, hypoproteinemia, and usage of corticosteroids [27].
Conclusions
Unusual patterns of focal fat deposition, including multi-nodular, mass-like, and perivascular patterns occasionally mimic malignancies and cause diagnostic challenges. It is important to be aware of their imaging patterns and to have complete relevant clinical history (i.e., plausible causative factors such as diabetes, alcohol abuse, usage of medications/chemotherapy and comorbidity). Recognition of the suggestive findings of focal fat deposition and subsequent confirmatory chemical-shift MR imaging (CSI) can lead to a non-invasive diagnosis of focal fat deposition.
References
Fishbein MH, Miner M, Mogren C, Chalekson J (2003) The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. J Pediatr Gastroenterol Nutr 36:54–61
Yoshikawa J, Matsui O, Takashima T, et al. (1987) Focal fatty change of the liver adjacent to the falciform ligament: CT and sonographic findings in five surgically confirmed cases. Am J Roentgenol 149:491–494. doi:10.2214/ajr.149.3.491
Paulson EK, Baker ME, Spritzer CE, et al. (1993) Focal fatty infiltration: a cause of nontumorous defects in the left hepatic lobe during CT arterial portography. J Comput Assist Tomogr 17:590–595
Kawamori Y, Matsui O, Takahashi S, et al. (1996) Focal hepatic fatty infiltration in the posterior edge of the medial segment associated with aberrant gastric venous drainage: CT, US, and MR findings. J Comput Assist Tomogr 20:356–359
Decarie PO, Lepanto L, Billiard JS, et al. (2011) Fatty liver deposition and sparing: a pictorial review. Insights Imaging 2:533–538. doi:10.1007/s13244-011-0112-5
Hamer OW, Aguirre DA, Casola G, et al. (2006) Fatty liver: imaging patterns and pitfalls. Radiographics 26:1637–1653. doi:10.1148/rg.266065004
Quinn SF, Gosink BB (1985) Characteristic sonographic signs of hepatic fatty infiltration. Am J Roentgenol 145:753–755
Dasarathy S, Dasarathy J, Khiyami A, et al. (2009) Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol 51:1061–1067
Yeom SK, Byun JH, Kim HJ, et al. (2013) Focal fat deposition at liver MRI with gadobenate dimeglumine and gadoxetic acid: quantitative and qualitative analysis. Magn Reson Imaging 31:911–917. doi:10.1016/j.mri.2013.02.002
Yamamoto A, Tamada T, Sone T, et al. (2010) Gd-EOB-DTPA-enhanced magnetic resonance imaging findings of nondiffuse fatty change of the liver. J Comput Assist Tomogr 34:868–873
Reeder SB, Cruite I, Hamilton G, Sirlin CB (2011) Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging 34:729–749
Martin J, Puig J, Falco J, et al. (1998) Hyperechoic liver nodules: characterization with proton fat-water chemical shift MR imaging. Radiology 207:325–330. doi:10.1148/radiology.207.2.9577476
Venkataraman S, Braga L, Semelka RC (2002) Imaging the fatty liver. Magn Reson Imaging Clin N Am 10:93–103
Lee SS, Lee Y, Kim N, et al. (2011) Hepatic fat quantification using chemical shift MR imaging and MR spectroscopy in the presence of hepatic iron deposition: validation in phantoms and in patients with chronic liver disease. J Magn Reson Imaging 33:1390–1398
Lee SS, Park SH (2014) Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol : WJG 20:7392–7402. doi:10.3748/wjg.v20.i23.7392
Hamer OW, Aguirre DA, Casola G, Sirlin CB (2005) Imaging features of perivascular fatty infiltration of the liver: initial observations 1. Radiology 237:159–169
Karcaaltincaba M, Haliloglu M, Akpinar E, et al. (2007) Multidetector CT and MRI findings in periportal space pathologies. Eur J Radiol 61:3–10
Lieber CS (2004) Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 34:9–19. doi:10.1016/j.alcohol.2004.07.008
Sakhuja P (2014) Pathology of alcoholic liver disease, can it be differentiated from nonalcoholic steatohepatitis? World J Gastroenterol WJG 20:16474–16479. doi:10.3748/wjg.v20.i44.16474
Sohn J, Siegelman E, Osiason A (2001) Unusual patterns of hepatic steatosis caused by the local effect of insulin revealed on chemical shift MR imaging. AJR Am J Roentgenol 176:471–474. doi:10.2214/ajr.176.2.1760471
Kroncke TJ, Taupitz M, Kivelitz D, et al. (2000) Multifocal nodular fatty infiltration of the liver mimicking metastatic disease on CT: imaging findings and diagnosis using MR imaging. Eur Radiol 10:1095–1100. doi:10.1007/s003300000360
Khalili K, Lan FP, Hanbidge AE, et al. (2003) Hepatic subcapsular steatosis in response to intraperitoneal insulin delivery: CT findings and prevalence. AJR Am J Roentgenol 180:1601–1604. doi:10.2214/ajr.180.6.1801601
Firneisz G (2014) Non-alcoholic fatty liver disease and type 2 diabetes mellitus: The liver disease of our age? World J Gastroenterol WJG 20:9072–9089. doi:10.3748/wjg.v20.i27.9072
Brawer MK, Austin GE, Lewin KJ (1980) Focal fatty change of the liver, a hitherto poorly recognized entity. Gastroenterology 78:247–252
Eisenberg LB, Warshauer DM, Woosley JT, et al. (1995) CT and MRI of hepatic focal nodular hyperplasia with peripheral steatosis. J Comput Assist Tomogr 19:498–500
Itai Y, Matsui O (1999) ‘Nonportal’ splanchnic venous supply to the liver: abnormal findings on CT, US and MRI. Eur Radiol 9:237–243. doi:10.1007/s003300050661
McGowan CE, Jones P, Long MD, Barritt AS (2012) Changing shape of disease: nonalcoholic fatty liver disease in Crohn’s disease—a case series and review of the literature. Inflamm Bowel Dis 18:49–54
Bissell DM, Gores GJ, Laskin DL, Hoofnagle JH (2001) Drug-induced liver injury: mechanisms and test systems. Hepatology 33:1009–1013. doi:10.1053/jhep.2001.23505
Tirumani SH, Kim KW, Nishino M, et al. (2014) Update on the role of imaging in management of metastatic colorectal cancer. Radiographics 34:1908–1928. doi:10.1148/rg.347130090
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors
Informed consent
Statement of informed consent was not applicable since the manuscript does not contain any patient data.
Rights and permissions
About this article
Cite this article
Jang, J.K., Jang, HJ., Kim, J.S. et al. Focal fat deposition in the liver: diagnostic challenges on imaging. Abdom Radiol 42, 1667–1678 (2017). https://doi.org/10.1007/s00261-017-1049-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1049-z