Abstract
The aims of this study are to assess any relationship between peribiliary hyperenhancement on MRI in patients with primary sclerosing cholangitis (PSC) and their Mayo risk score and to assess which timing of peribiliary hyperenhancement correlates best with the Mayo risk score. In this HIPAA-compliant, IRB-approved retrospective study, 101 patients who underwent MRI for known or suspected PSC were identified. Of those, 62 patients (mean age 48 years; 40 males) were diagnosed with PSC by a hepatologist based on findings on MRI, ERCP, and/or liver biopsy, and comprise the final cohort. Data were recorded on whether peribiliary hyperenhancement was present, the post-contrast phase and the extent of involvement. The components to calculate the Mayo risk score were recorded. Statistical analysis was performed using the student T test, Fisher’s exact test, and the Kaplan–Meier estimate. Of 62 patients, 41 (66.1%) patients had a low-Mayo risk score (<0), 14 (22.6%) had an intermediate-risk score (≤2 and >0), and 7 (11.3%) had a high-risk score (>2). On MRI, 29 (46.8%) patients demonstrated arterial peribiliary hyperenhancement. Both the presence and extent of peribiliary hyperenhancement showed significant associations with Mayo risk score (p < 0.01). Using the combined end point of liver transplantation or death, there was a statistically significant difference in survival times between those with and those without arterial peribiliary hyperenhancement (p < 0.05). The presence of arterial peribiliary hyperenhancement in patients with PSC on MRI is associated with higher Mayo risk scores and may suggest a poorer prognosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Primary sclerosing cholangitis (PSC) is a chronic fibroinflammatory disease characterized by multifocal bile duct stricturing involving the intrahepatic and/or extrahepatic bile ducts which can eventually progress to cirrhosis and liver failure [1]. While its etiology is incompletely understood, PSC is associated with inflammatory bowel disease in 70–80% of cases [1–3]. Endoscopic retrograde cholangiopancreatography (ERCP) is generally considered the gold standard for diagnosis [4, 5]; however, magnetic resonance cholangiopancreatography (MRCP) of the liver and bile ducts has emerged as a less invasive alternative with comparable sensitivity and specificity [6–9].
A number of studies have assessed the utility of MRI for the diagnosis of PSC; however, relatively sparse research has been performed regarding the ability of MRI to predict disease severity or outcome. While Petrovic et al. [10] reported that delayed (3 min post contrast) peribiliary hyperenhancement on gadolinium-enhanced MRI showed a weak correlation with survival, as predicted by the Mayo risk score, the relationship between early or arterial peribiliary hyperenhancement on magnetic resonance imaging (MRI) in PSC patients and disease outcome has not been previously evaluated.
The Mayo risk score is a validated natural history model for PSC and has been shown to demonstrate a good correlation between estimated and actual survival to liver transplantation [11]. Some authors have even advocated that the Mayo risk score be incorporated into transplant guidelines in PSC patients [12]. The Mayo risk score is calculated based on patient age, serum aspartate aminotransferase (AST), bilirubin and albumin levels, as well as a history of variceal bleeding to estimate the probability of survival for the following 4 years [11]. This model generates point estimates of survival and can be considered a surrogate marker for PSC disease outcome and survival [10].
The aims of this study are, therefore, (1) to assess whether there is any relationship between peribiliary hyperenhancement on MRI in patients with PSC and their Mayo risk score and (2) to assess if peribiliary hyperenhancement correlates with the clinical outcome.
Materials and Methods
Subjects
A retrospective review was performed of all patients with known or suspected PSC who underwent multiphasic contrast-enhanced MRI of the liver and biliary tree during a 4-year period. This retrospective study was approved by our institutional review board and was compliant with the Health Insurance Portability and Accountability Act. Patient informed consent was waived.
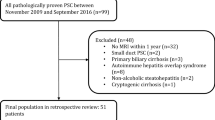
Patients were initially identified by searching a computerized database of all MRI examinations performed at our institution with any reference to PSC in the exam report indication, findings, or impression. During the study period, 253 MRI studies on 101 patients with known or suspected PSC were performed. To be included in this study, a diagnosis of PSC had to be established by a hepatologist based on the patient’s clinical, laboratory, and radiologic data including ERCP and/or MRI results, and liver biopsy when available. Sixteen of the initial 101 patients identified were excluded because the diagnosis of PSC could not be confirmed. An additional 11 patients were excluded because liver biopsy specimens were reported as potentially reflecting autoimmune pancreatitis/IgG4-related sclerosing disease. Because the Mayo risk score provides a point estimate of survival, serum samples obtained within 90 days of the MRI study date were considered to appropriately reflect the patients’ clinical condition at the time of the MRI exam. Twelve patients were excluded from this study as no contemporaneous serum samples were available at the time of MRI to calculate the Mayo risk score (Fig. 1). The final study cohort comprised 62 patients (Table 1). For any patient who had multiple MRI exams performed during the study period, only the first MRI exam where there were contemporaneous serum samples available was included in this study.
Diagnosis of PSC was established based on typical ERCP findings in 37 (59.7%) patients and/or typical MRI findings (multifocal intrahepatic and/or extrahepatic biliary strictures and dilatation with a beaded appearance) [13] in 56 (90.3%) patients. In addition, 14 (22.6%) patients had liver biopsies with histopathologic confirmation of PSC.
MRI Technique
All MRI studies except one were performed on a 1.5-Tesla magnet (Espree or Symphony, Siemens Medical Solutions, Iselin, NJ; or Excite Twin speed, GE Medical Systems, Waukesha, Wis). The remaining study was performed on a 3-Tesla magnet (Signa, GE Medical Systems, Waukesha, Wis). All studies were performed with the patient supine using a surface phased array coil and included pre-contrast T1- and T2-weighted images of the abdomen (not used for the purpose of this investigation) as well as a multiphasic examination with an axial three-dimensional frequency-selective fat-saturated T1-weighted spoiled gradient-echo (SPGR) acquisition (repetition time msec/echo time msec, 3.8–4.5/1.8–2.0; flip angle 12°; pre-interpolation slice thickness 3–4 mm; matrix 128–256 × 256–512; bandwidth ±62 kHz; field of view, 35–45 cm) obtained prior to and after the intravenous administration of 0.1 mmol/kg of a gadolinium-based contrast agent (59 patients received gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Wayne, NJ, USA), three patients received gadolinium-BOPTA (MultiHance; Bracco diagnostics, Princeton, NJ, USA)). Post-contrast images were obtained during arterial, portal venous (45 s after initiation of the arterial phase imaging) and delayed venous (90 s after initiation of the arterial phase imaging) phases. The initiation of the first post-contrast acquisition was timed to the late arterial phase based on a previously reported timing bolus technique using 2 ml of the gadolinium-based contrast agent [14]; 5 patients (8.1%) included an additional fourth delayed post-contrast phase obtained 135 s after initiation of arterial phase imaging. Sequences were obtained in suspended end expiration lasting 20–24 s.
Image Analysis
All images were reviewed on a commercial picture archiving and communication system (PACS, Centricity, GE, Waukesha, WI). Two body MRI fellowship-trained abdominal imaging radiologists (**, *** with 9 and 6 years of experience interpreting abdominal MRI, respectively) reviewed the multiphasic portion of the MRI studies independently and documented the presence or absence of peribiliary hyperenhancement on each of the post-contrast image acquisitions (i.e., arterial, portal, venous, equilibrium). Peribiliary hyperenhancement was defined as linear hyperenhancement within the hepatic parenchyma adjacent to the bile ducts including the bile duct wall [10]. In addition, the first post-contrast image sequence which demonstrated peribiliary hyperenhancement was documented if present. The degree of involvement of peribiliary hyperenhancement was classified as localized (less than 50% of a segment), segmental (involving at least 50% of a segment but still confined to a single segment), or diffuse (involving multiple segments) (Fig. 2). The segments involved were also recorded. Any disagreements between the two readers were settled by consensus review of the imaging.
Localized arterial peribiliary hyperenhancement a involving a single segment VII duct (arrow). Segmental peribiliary hyperenhancement, b involving multiple ducts in segment II of the liver on portal venous phase imaging (arrows). Diffuse arterial peribiliary hyperenhancement, c involving multiple ducts in multiple segments (arrows)
Mayo Risk Score Calculation
The Mayo risk score was calculated using the following equation [11]:
Points for variceal bleeding were awarded as follows: 0 point if there was no history of variceal bleeding, and 1 point if there was a history of variceal bleeding. The calculated score ranged from −2 to 4 and was categorized as previously published [11] into low-risk (values ≤0), intermediate-risk (>0 and ≤2), and high-risk (>2) groups. A unit increase in the Mayo risk score is associated with a 2.5-fold decrease in the probability of 4-year survival [11, 15].
Statistical Analysis
Statistical analysis was performed using SPSS for Windows v18 and STATA IC v13 for Windows. The interobserver variability for the presence of arterial peribiliary hyperenhancement and for any peribiliary hyperenhancement was assessed using Cohen’s Kappa statistic. Categorical variables were compared using the Chi-square or Fisher’s exact test. Continuous variables were compared using a student T test. Time-to-event analysis was performed using the Kaplan–Meier estimate and log-rank test. Multivariate analysis was performed using logistic regression to assess the presence of peribiliary hyperenhancement as a predictor of prognosis independent of the Mayo risk score. Statistical significance was defined as a p < 0.05.
Results
Subjects
Of the 62 patients in the final study cohort, 40 (64.5%) were male and 22 (35.5%) female. The mean age overall was 48.3 years (range 21–81 years) with no significant difference in age detected between men and women (p = 0.94). The mean time interval between MRI and obtaining serum samples was 15.3 days (range 0–67 days). The calculated Mayo risk scores ranged in value from −2 to 3, with a mean value of −0.172. Forty-one (66.1%) patients were in the low-Mayo risk group, 14 (22.6%) were in the intermediate-risk group, and 7 (11.3%) were in the high-risk group. No significant difference was identified between Mayo risk scores when stratified by gender (p = 0.28).
The precise date of diagnosis of PSC was not always evident; however, a review of the electronic medical record did allow an estimate of the diagnosis date. The mean interval between the diagnosis of PSC and the MRI study in question was 1342 days (3.7 years). Only 9 (14.5%) of the 62 MR studies included in this study group were initial studies; the remainder were follow-up studies in patients with known PSC. There was no statistically significant difference in the presence of arterial phase hyperenhancement or any phase hyperenhancement between the initial studies and follow-up studies (p = 0.722 and p = 0.577, respectively). There were few too cases to perform any additional or multivariate analysis.
MRI Studies
The interobserver agreement for the presence of arterial peribiliary hyperenhancement was very good (κ = 0.708) and excellent for the presence of peribiliary hyperenhancement on any imaging phase post contrast (κ = 0.919). Disagreement between the two readers with regard to arterial peribiliary hyperenhancement was noted for 9/62 (14.5%) cases. In 4 cases, reader 1 assigned peribiliary hyperenhancement as occurring during the first post-contrast phase sequence, while reader 2 categorized these cases as having peribiliary hyperenhancement during the second post-contrast phase sequence. In 4 additional cases, reader 2 assigned peribiliary hyperenhancement during the first post-contrast phase sequence, whereas reader 1 categorized the peribiliary hyperenhancement as occurring during the second post-contrast phase sequence. On consensus review, all 8 of these cases were felt to reflect arterial peribiliary hyperenhancement as the timing of the contrast bolus was slightly suboptimal, leading to the first post-contrast phase acquisition occurring in an early arterial phase, and the second post-contrast phase sequence actually occurring during a late arterial phase, rather than a portal venous phase. In the remaining case, reader 1 assigned arterial peribiliary hyperenhancement as being present, while reader 2 did not. Upon review, the readers agreed that a very focal region of arterial peribiliary hyperenhancement involving a single segmental bile duct was present. There was only fair correlation between the two readers for the extent of peribiliary hyperenhancement (localized, segmental or diffuse), κ = 0.494.
The consensus data were then used for further analysis. Peribiliary hyperenhancement on any imaging phase post contrast was seen in 46 (74%) of 62 MRI exams. Arterial peribiliary hyperenhancement was seen in 29 (46.7%) MRI studies.
Relationship Between Peribiliary Hyperenhancement and the Mayo Risk Score
Arterial peribiliary hyperenhancement was present in 71.4% of the high-risk group (Mayo risk score ≥2), 73.3% of the intermediate-risk group (Mayo risk score >0 and <2), and 31.7% of the low-risk group (Mayo risk score ≤0). There was a statistically significant difference in the prevalence of arterial hyperenhancement in the intermediate-risk group compared to that in the low-risk group (p < 0.05). The difference in prevalence of arterial hyperenhancement in the high-risk group compared to that in the low-risk group approached significance (p = 0.059). There was no significant difference was detected between the high-risk and intermediate-risk groups (p > 0.05). The breakdown of phase of peribiliary hyperenhancement on MRI stratified by Mayo risk score group is shown in Table 2. There was no statistically significant association seen for second, third, or fourth phase post-contrast peribiliary hyperenhancement (p > 0.05). In fact, every patient in the intermediate- and high-risk groups demonstrated peribiliary hyperenhancement on at least one imaging phase post contrast. The extent of peribiliary hyperenhancement (none, localized, segmental, or diffuse) did show a significant association with the Mayo risk group (p < 0.01), with greater extent of peribiliary hyperenhancement associated with higher Mayo risk groups.
Additional analysis was performed by combining the high- and intermediate-risk groups (Mayo risk score >0) and comparing those patients with the low-risk group (Mayo risk score <0). Patients with higher Mayo risk scores (>0) had an increased prevalence of arterial peribiliary hyperenhancement compared to those with low-risk scores (≤0) (80 vs. 39%, p = 0.002). Additionally, in the higher-risk groups (Mayo risk score >0), significantly more patients demonstrated peribiliary hyperenhancement on any post-contrast imaging sequence when compared to patients in the low-risk group (p < 0.001).
Relationship Between Peribiliary Hyperenhancement and Survival
Using a combined end point of liver transplantation or death, a time-to-event analysis was performed based on the presence of arterial peribiliary hyperenhancement on MRI. This demonstrated a statistically significant difference in survival times between those with arterial peribiliary hyperenhancement (mean survival of 1400 days) and those without arterial peribiliary hyperenhancement (mean survival of 1746 days); median survival could not be calculated as 50% of the patients did not die or receive a liver transplant (log rank p < 0.05; Fig. 3). Multivariate analysis did not demonstrate a statistically significant association between arterial peribiliary hyperenhancement and survival independent of the individual variables of the Mayo risk score or composite Mayo risk score.
Discussion
Primary sclerosing cholangitis is an idiopathic condition characterized by chronic inflammation of the medium- and large-sized bile ducts, leading to progressive fibrosis and scarring of the hepatic parenchyma in the affected segments. PSC typically progresses slowly over the course of 10–15 years with eventual decompensation and liver failure, frequently aggravated by superimposed infections. Patients with PSC have also increased risk of developing cholangiocarcinoma, and the only known curative treatment is liver transplantation. The lack of alternative treatment options is manifested by current patient care strategies, which are limited to continuous monitoring of the liver function and management of symptoms, complications, and interventional procedures to release biliary obstruction. While efforts to develop new therapies for PSC continue [16], a challenge to develop these arises from the difficulty to monitor the progression of the disease and response to therapy. A noninvasive method to monitor peribiliary inflammation in PSC patients would be helpful in the clinical management of PSC patients as new therapeutic options arise.
Our results demonstrate that arterial peribiliary hyperenhancement in patients with PSC on MRI is significantly associated with higher Mayo risk scores, thereby suggesting poorer outcomes and decreased survival. The difference in prevalence of arterial peribiliary hyperenhancement between the low-risk group and high-risk group approached significance, and it is likely that this was due to the small number of patients in the high-risk group. While this study is not sufficient to demonstrate causality, this peribiliary hyperenhancement could intuitively be attributed to the on-going cholangitis and, therefore, a worse prognosis for those patients. Similarly, patchy arterial hepatic hyperenhancement has been correlated with acute liver inflammation and hepatocyte necrosis on biopsies in patients with chronic hepatitis whereas these histopathologic findings are rare in patients with homogeneous hepatic arterial enhancement [17]. Peribiliary hyperenhancement on any of the other phases post-contrast was not associated with higher Mayo risk scores. While these findings require further evaluation with a larger study to better evaluate the independence of this result, our findings suggest that the presence and extent of arterial peribiliary hyperenhancement should at least be reported as part of the MR studies in these patients.
Previously, Petrovic et al [10] reported a weak correlation between delayed (3 min post contrast) peribiliary hyperenhancement on MRI in patients with PSC and survival. This phenomenon is likely attributable to the accumulation of gadolinium-based contrast agents in tissues with large extracellular volumes, such as in areas of fibrosis, resulting in delayed hyperenhancement [18, 19]. We hypothesize that delayed peribiliary hyperenhancement might reflect a more chronic or fibrotic form of PSC and may not affect survival as the disease condition is more stabilized in these patients. In contrast, the presence of earlier, arterial peribiliary hyperenhancement in patients with PSC perhaps more accurately indicates active with or without chronic inflammation and, as our study suggests, may be better imaging marker of poorer disease prognosis. In addition, we postulate that more extensive involvement of the liver parenchyma might be associated with a more aggressive form of disease and consequently a worse prognosis as manifested by a higher Mayo risk score. In our study, there was a statistically significant association between the Mayo risk score and both the presence and extent of arterial phase peribiliary hyperenhancement.
A potential advantage of the peribiliary hyperenhancement over the Mayo score for the assessment of disease progression is the ability to assess disease heterogeneity within a given patient. While some of our patients presented with diffuse hepatic disease, the MRI abnormalities were more focal (i.e. segmental) in others. As new therapeutic options are tested in clinical trials and hopefully in clinical practice, it will be advantageous to have the ability to detect early changes related to active inflammation before they are reflected in changes in the Mayo risk score
There are a number of limitations to this study. The Mayo risk score is a surrogate marker of progression rather than a true assessment of outcome. This has been used previously in this context, however, and it is a well-validated prognostic indicator. In addition, some authors have suggested incorporating the Mayo risk score into transplantation recommendation for patients with PSC [12]. Our inference is supported by the additional finding that there was a significant association between survival and early phase peribiliary hyperenhancement. However, our results did not demonstrate independence of this association, when included in a multivariate analysis with the components of the Mayo risk score. This may in part be due to the small sample size, and further research with a larger cohort of patients may be helpful to clarify this finding.
There were relatively few patients with high Mayo risk scores (>2) in this study (7 patients). This is likely due to patients with higher scores being too unwell for the more demanding MRI studies and likely defaulted to CT imaging for follow-up. We allowed an interval of 3 months between the MRI study and serum samples. While the mean interval was only 15.3 days, it is possible that there could be an alteration in the clinical picture of the patient in this interval, particularly as some clinicians may have used the report findings on the MRI to make treatment decisions. Another limitation of this study is that peribiliary hyperenhancement was the only imaging feature that was evaluated, and we did not evaluate for the presence of cirrhosis or the extent of biliary stricturing related to PSC. Prior studies have shown additional potential imaging biomarkers which have a significant correlation with decompensation and progression of disease, including altered liver stiffness as measured by magnetic resonance elastography [20, 21], splenomegaly [22], and other signs of portal hypertension and parenchymal heterogeneity [23]. Potentially these factors may also have prognostic impact and would be an interesting area for further research.
Finally, it should be noted that in clinical practice, there is an overlap in the imaging and laboratory findings between PSC and IgG4-related sclerosing disease. We attempted to address this issue by excluding any cases where the possibility of autoimmune disease or an overlap syndrome was raised by either a hepatologist or on the liver biopsy. We also reviewed the IgG and IgG4 levels; however, assessment of these parameters was variable among this patient cohort.
In conclusion, we found that arterial phase peribiliary hyperenhancement at MRI does correlate with worse prognosis as indicated by higher Mayo risk scores. The extent of peribiliary hyperenhancement also showed a significant association with the Mayo Risk score. As such, this information should be highlighted when reporting these studies.
References
Lee YM, Kaplan MM (1995) Primary sclerosing cholangitis. N Engl J Med 332(14):924–933. doi:10.1056/NEJM199504063321406
Sherlock S (1991) Pathogenesis of sclerosing cholangitis: the role of nonimmune factors. Semin Liver Dis 11(1):5–10. doi:10.1055/s-2008-1040416
Razumilava N, Gores GJ, Lindor KD (2011) Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology 54(5):1842–1852. doi:10.1002/hep.24570
Lee YM, Kaplan MM (2002) Management of primary sclerosing cholangitis. Am J Gastroenterol 97(3):528–534. doi:10.1111/j.1572-0241.2002.05585.x
Majoie CB, Reeders JW, Sanders JB, Huibregtse K, Jansen PL (1991) Primary sclerosing cholangitis: a modified classification of cholangiographic findings. AJR Am J Roentgenol 157(3):495–497
Bader TR, Beavers KL, Semelka RC (2003) MR imaging features of primary sclerosing cholangitis: patterns of cirrhosis in relationship to clinical severity of disease. Radiology 226(3):675–685. doi:10.1148/radiol.2263011623
Berstad AE, Aabakken L, Smith HJ, et al. (2006) Diagnostic accuracy of magnetic resonance and endoscopic retrograde cholangiography in primary sclerosing cholangitis. Clin Gastroenterol Hepatol 4(4):514–520. doi:10.1016/j.cgh.2005.10.007
Elsayes KM, Oliveira EP, Narra VR, et al. (2006) MR and MRCP in the evaluation of primary sclerosing cholangitis: current applications and imaging findings. J Comput Assist Tomogr 30(3):398–404
Weber C, Kuhlencordt R, Grotelueschen R, et al. (2008) Magnetic resonance cholangiopancreatography in the diagnosis of primary sclerosing cholangitis. Endoscopy 40(9):739–745. doi:10.1055/s-2008-1077509
Petrovic BD, Nikolaidis P, Hammond NA, et al. (2007) Correlation between findings on MRCP and gadolinium-enhanced MR of the liver and a survival model for primary sclerosing cholangitis. Dig Dis Sci 52(12):3499–3506. doi:10.1007/s10620-006-9720-1
Kim WR, Therneau TM, Wiesner RH, et al. (2000) A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc 75(7):688–694. doi:10.4065/75.7.688
Wiesner RH, Porayko MK, Hay JE, et al. (1996) Liver transplantation for primary sclerosing cholangitis: impact of risk factors on outcome. Liver Transplant Surg Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc 2(5 Suppl 1):99–108
Vitellas KM, Keogan MT, Freed KS, Enns RA, Spritzer CE, Baillie JM, Nelson RC (2000) Radiologic manifestations of sclerosing cholangitis with emphasis on MR cholangiopancreatography. Radiogr Rev Publ Radiol Soc N Am Inc 20(4):959–975; quiz 1108-1109, 1112. doi:10.1148/radiographics.20.4.g00jl04959
Earls JP, Rofsky NM, DeCorato DR, Krinsky GA, Weinreb JC (1997) Hepatic arterial-phase dynamic gadolinium-enhanced MR imaging: optimization with a test examination and a power injector. Radiology 202(1):268–273. doi:10.1148/radiology.202.1.8988222
Kim WR, Poterucha JJ, Wiesner RH, et al. (1999) The relative role of the Child-Pugh classification and the Mayo natural history model in the assessment of survival in patients with primary sclerosing cholangitis. Hepatology 29(6):1643–1648. doi:10.1002/hep.510290607
Lindor KD (2011) New treatment strategies for primary sclerosing cholangitis. Dig Dis 29(1):113–116. doi:10.1159/000324145
Semelka RC, Chung JJ, Hussain SM, Marcos HB, Woosley JT (2001) Chronic hepatitis: correlation of early patchy and late linear enhancement patterns on gadolinium-enhanced MR images with histopathology initial experience. J Magn Reson Imaging JMRI 13(3):385–391
Balci NC, Semelka RC (2005) Contrast agents for MR imaging of the liver. Radiol Clin North Am 43(5):887–898, viii. doi:10.1016/j.rcl.2005.05.004
Faria SC, Ganesan K, Mwangi I, Shiehmorteza M, Viamonte B, Mazhar S, Peterson M, Kono Y, Santillan C, Casola G, Sirlin CB (2009) MR imaging of liver fibrosis: current state of the art. Radiogr Rev Publ Radiol Soc N Am Inc 29(6):1615–1635. doi:10.1148/rg.296095512
Corpechot C, Gaouar F, El Naggar A, et al. (2014) Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology 146(4):970–979 ((quiz e915-976)). doi:10.1053/j.gastro.2013.12.030
Eaton JE, Dzyubak B, Venkatesh SK, et al. (2016) Performance of magnetic resonance elastography in primary sclerosing cholangitis. J Gastroenterol Hepatol 31(6):1184–1190. doi:10.1111/jgh.13263
Ehlken H, Wroblewski R, Corpechot C, et al. (2016) Spleen size for the prediction of clinical outcome in patients with primary sclerosing cholangitis. Gut 65(7):1230–1232. doi:10.1136/gutjnl-2016-311452
Ruiz A, Lemoinne S, Carrat F, et al. (2014) Radiologic course of primary sclerosing cholangitis: assessment by three-dimensional magnetic resonance cholangiography and predictive features of progression. Hepatology 59(1):242–250. doi:10.1002/hep.26620
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jennifer M Ni Mhuircheartaigh declares she has no conflict of interest. Karen S Lee declares she has no conflict of interest. Michael P Curry declares he has no conflict of interest. Ivan Pedrosa declares he has no conflict of interest. Koenraad J Mortele declares he has no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Ni Mhuircheartaigh, J.M., Lee, K.S., Curry, M.P. et al. Early Peribiliary Hyperenhancement on MRI in Patients with Primary Sclerosing Cholangitis: Significance and Association with the Mayo Risk Score. Abdom Radiol 42, 152–158 (2017). https://doi.org/10.1007/s00261-016-0847-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0847-z