Abstract
Objective
Fibromuscular dysplasia (FMD) is an uncommon non-inflammatory and non-atherosclerotic cause of arterial disease that may result in stenosis, tortuosity, aneurysm, or dissection. The clinical presentation depends on the vascular bed involved and ranges from asymptomatic to multisystem disease and end organ ischemia. The purpose of this article is to review the role of imaging in patients with FMD with an emphasis on renal FMD. The relevant epidemiology, histopathology, imaging techniques, and interpretation of images will be discussed.
Conclusion
Renal artery FMD requires a high index of suspicion for accurate and prompt diagnosis and implementation of appropriate therapy. The treatment will vary based on clinical presentation and distribution of involvement. Noninvasive imaging with duplex ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) are reasonable alternatives for the depiction of FMD in comparison to catheter-directed angiography (CA). Patients with FMD are often treated by multispecialty practice including the interventional radiologist.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Fibromuscular dysplasia (FMD) is an idiopathic noninflammatory, non-atherosclerotic arteriopathy that may result in stenosis, tortuosity, aneurysm, or dissection [1–3]. Currently, there are no large population-based studies investigating the prevalence of FMD in the community, or it is not known whether it varies by ethnic or racial groups [4]. Although historically considered rare, more recent data suggest FMD may be more common than initially believed. Data from asymptomatic, healthy kidney donors suggest that up to 4% may have FMD [1, 3, 5–8]. For patients with resistant hypertension, the prevalence may be even higher [9]. FMD is also increasingly being reported as an incidental finding when patients are imaged for other indications [4].
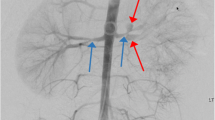
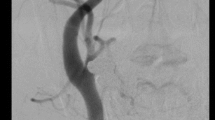
Most often, middle-aged women between their 3rd and 6th decades are affected, although FMD also affects men and has been described in all ages [1, 10]. Notably, a mean delay of 4–9 years from the time of first symptom to diagnosis of FMD has been reported [4, 11]. Although any artery in the body may be involved, the renal, carotid, and vertebral arteries are most common. In a recent multicenter registry of 447 FMD patients in the United States, the most common vascular territories involved were the renal arteries (79.7%), followed by extracranial carotid artery (74.3%), external iliac artery (60%), vertebral artery (36.6%), mesenteric arteries (26.3%), intracranial carotid artery (17%), and brachial artery (15.9%) [4]. Simultaneous involvement of multiple vascular beds is not uncommon (Figs. 1, 2) [4].
57-year-old male with history of intracranial aneurysm with FMD involvement of multiple vascular beds at MRA. Coronal MIP image (A) demonstrates mild renal artery FMD, greater on the left, without aneurysm (arrows). The kidneys were otherwise normal in size and contour. Oblique volume-rendered reformatted image (B) shows alternating aneurysms and stenoses involving the celiac artery and SMA (arrows)
78-year-old female with FMD involvement of multiple vascular beds at CTA. Oblique volume-rendered image A shows multifocal areas of alternating stenosis and aneurysm involving the right renal artery and proximal celiac artery. Dissection resulting in long segment high-grade stenosis or occlusion of the SMA (arrow) is present with collateral vessel formation. Curved planar reformat B shows FMD involvement of the left internal carotid artery (arrow)
Obtaining an accurate diagnosis of FMD is crucial to appropriate treatment planning for the predominantly young and otherwise healthy patient with FMD. In the case of renovascular FMD, the approaches to therapy and outcomes differ considerably with respect to patients with atherosclerotic renal artery disease [12–15]; in some cases, catheter-based angiogram with planned angioplasty may be indicated at the outset with the goal of improved hypertension control or even cure [2, 16]. Catheter-directed digital subtraction angiography (CA) is considered the reference standard imaging modality for renovascular FMD. Duplex ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) are increasingly being employed for the noninvasive assessment for renovascular disease with promising results. In this review, we highlight the role of clinical and histopathologic features of FMD, imaging findings, and advanced imaging techniques in the evaluation of patients with suspected FMD, with an emphasis on findings in the renal vascular territories.
Clinical features
The clinical presentation of FMD depends on the distribution of arterial involvement and may vary from asymptomatic to severe multisystem dysfunction and uncommonly, end organ ischemia. These presentations are directly related to the underlying pathology such as stenosis, tortuosity, aneurysm, or dissection [1]. Renal artery involvement most commonly presents as hypertension [1]. There is increasing awareness of patients presenting with severe headaches and hypertension, in which there is simultaneous involvement of the renal and carotid arteries. Furthermore, there is also a growing observation that patients with FMD experience spontaneous coronary artery dissection [17]. In contrast to patients with atherosclerotic renovascular disease, renal failure/insufficiency is a rare clinical manifestation of FMD in adults and was only seen in 2% of the US Registry [4, 10]. The etiology of FMD is unknown; various environmental (smoking), hormonal (exposure to endogenous or exogenous estrogens), and mechanical (repeated stretching of the renal artery) factors have been described, but the exact association remains unclear [18, 19]. Familial cases are estimated to represent 7%–11% of all FMD patients [4, 20], and recently an association with the PHACTR1 gene was identified which is also associated with spontaneous cervical dissection [21]. A 2014 study found elevated plasma levels of circulating transforming growth factors (TGF)-β1 and TGF-β2 and elevated secretions of TGF-β1 and TGF-β2 by fibroblasts in FMD patients compared to controls; however, the significance of these findings is not clear and remains to be confirmed [22]. When patients with FMD were surveyed for other heritable-connective tissue diseases, such as Ehlers-Danlos, Loeys-Dietz, Marfans, and genes associated with familial thoracic aortic aneurysm and dissection, there was no significant overlap of disease [23].
Histopathologic classification
FMD has historically been classified histopathologically based on the most affected arterial wall layer (intima, media, or adventitia) and the composition of the arterial lesion (fibroplasia or hyperplasia of smooth muscle cells) [24]. The classification scheme was initially described for the renal arteries, but may be applied to other vascular beds [10]. Medial FMD is the most common histological variant and is further divided into medial fibroplasia, perimedial fibroplasia, and medial hyperplasia [24]. Medial fibroplasia, which presents as the classic “string-of-beds” on angiography, accounted for 60–70% of FMD in initial reports and >90% today [4]. Perimedial fibroplasia, “beads” of which are smaller and less numerous than those seen in medial fibroplasia, previously accounted for 15–25% of FMD but now only represents <1% [4]. Medial hyperplasia is the least common variant of medial FMD, accounting for <1% of cases. Intimal fibroplasia and adventitial fibroplasia account for 1–2% and <1% of lesions, respectively, and typically present as tubular or focal stenosis [18]. Recent data from the US FMD Registry reported nearly 25% of patients in the registry experienced a dissection, and 21% experienced an aneurysm by the time of FMD diagnosis, although these numbers may underestimate the frequency of aneurysm and dissection in this disease due to a lack of systematic screening with cross-sectional imaging of patients with FMD [25]. Aneurysm and dissection are believed to represent complications of the disease and not separate histopathological entities [4, 10]. More than one histopathologic subtype can be present simultaneously in the same patient, although it occurs infrequently [4, 26]. Often, the findings at histology often correspond to the appearance at imaging.
Today, FMD is a disease that is almost exclusively diagnosed radiographically. Very few histopathologic samples are obtained for diagnostic purposes. The 2012 European Consensus proposed a simplified radiographic classification of multifocal, tubular (≥1 cm), and unifocal (<1 cm stenosis) FMD [2]. The latter two entities were further combined into a single definition of focal disease [2, 11]. This binary angiographic classification (multifocal vs. focal) was successful in distinguishing between two distinct clinical phenotypes: patients with focal FMD were younger at diagnosis, more likely to be male, more frequently smokers, and had higher blood pressure at presentation than the multifocal group [11]. The American Heart Association Scientific Statement published in 2014 also supports the use of a binary angiographic classification of multifocal or focal FMD [18]. In brief, the multifocal lesions with alternating areas of stenosis and dilatation correspond with the classic “string-of-beads” appearance of FMD. Focal disease is defined as a focal concentric or tubular stenosis that is not necessarily confined to the mid and distal portions of the artery [16]. Associated secondary findings such as aneurysm, dissection, and arterial tortuosity may be present with both phenotypes.
Imaging findings
Imaging studies play an important role in detecting or confirming the diagnosis of suspected FMD and associated complications by demonstrating the typical arterial abnormalities. Advanced cross-sectional imaging techniques also provide important information regarding renal morphology.
The right main renal artery is more commonly affected by FMD but bilateral vessel involvement can be seen in 32–54% of cases (Fig. 3) [14, 27, 28]. Distribution of disease may include the main renal artery alone or combined main renal artery and branch vessel involvement. Isolated branch vessel involvement is rare. FMD findings are most commonly identified in the mid to distal portion of the renal artery and major branches, whereas atherosclerotic renal artery disease tends to affect the ostia and proximal artery [1]. As aforementioned, the “string-of-beads” appearance of the renal arteries is the most common finding at imaging and is characteristic of multifocal FMD (Fig. 4) [29]. While the overall effect of alternating stenosis and dilation in multifocal FMD may be arterial stenosis, it is impossible to assess the degree stenosis by radiographic imaging alone—only intra-arterial assessment of the pressure gradient across the lesion is able to accurately assess the hemodynamic effect of FMD (systolic gradient of <10 mm Hg is considered normal) [16, 30, 31].
52-year-old male with bilateral renal artery FMD. CA (A) shows focal disease in the distal right renal artery (black arrow) and a 1.2 cm dissecting aneurysm of the distal left renal artery (green arrow). Diminished enhancement of the left kidney is present (not shown). The patient underwent successful PTA of both renal arteries. Coned down CA image of the right renal artery web before (B) and MRA volume-rendered reformat after (C) shows resolution of the finding (arrow). Axial phase-contrast noncontrast MRA (D) was also performed, showing the distal left renal artery aneurysm (arrow)
Complications of FMD include renal artery aneurysm and dissection and are well depicted at imaging. According to Olin et al., out of 447 cases of FMD, 5.6% had renal artery aneurysm, and 4.3% had renal artery dissection [4]. Renal artery occlusion occurs uncommonly, however when present, it may cause renal infarction (Figs. 5, 6). Renal parenchymal changes including volume loss, decreased cortical thickness, and decreased length may occur in the context of unilateral disease [10], most often with focal disease or post-arterial dissection. Kidney size asymmetry (>2 cm difference) is considered a significant surrogate of underlying renal artery stenosis, and kidney atrophy has been advocated as a parameter quantifying the effect of FMD on the renal artery, as well as a surrogate marker favoring revascularization [32, 33]. Differential diagnostic considerations for renal FMD at imaging include atherosclerotic disease, Ehlers-Danlos syndrome, Williams’ syndromes, inflammatory vasculitis, and type 1 neurofibromatosis [3].
57-year-old patient presents with right lower quadrant pain. CTA coronal reformat (A), MIP reformat (B), and volume-rendered reformat (C) show a long segment stenosis (white arrow) of the proximal and mid right renal artery with mild wall thickening. A small 4 mm aneurysm in the mid right renal artery (green arrow) is noted. Using center line tool (D) with corresponding oblique multiplanar reformat (E), dissections with long segment stenosis (white arrow) and aneurysm (green arrow) are well delineated. Coronal reformat (F) shows segmental infarction of the right lower kidney (white arrow) due to branch vessel occlusion (not shown)
46-year-old female with FMD. Coronal HASTE MRI shows atrophic left kidney with multifocal areas of cortical scarring. Coronal oblique reformat from contrast-enhanced MRA shows left renal artery occlusion immediately distal to the ostia, most likely due to dissection, and multifocal areas of right renal artery stenosis and luminal irregularity. The right kidney was otherwise normal in size and contour
Imaging techniques
The most commonly employed imaging methods include catheter-directed angiography (CA), conventional and Doppler (Duplex) ultrasound, CT, and MRI.
Catheter angiography
Patients with suspected FMD are usually referred to an interventional radiologist to confirm the diagnosis and for therapeutic intervention. Catheter-directed angiography (CA) remains the gold standard imaging modality for the diagnosis of renovascular FMD. If properly performed, CA provides anatomic information by precisely depicting the abnormal arterial findings including the degree, nature, and extent of arterial stenosis. CA also enables quantitative hemodynamic assessment for FMD through direct pressure measurements and intravascular ultrasound (IVUS) [1]. IVUS has been shown to delineate renal artery endoluminal abnormalities including eccentric ridges, fluttering membranes, and spiraling folds even when CA was normal [30]. Furthermore, the excellent spatial resolution (<0.1 mm) of CA allows for assessment of not only the typically involved medium sized vessels, but also excellent delineation of smaller and peripheral branch vessels (Fig. 7). Disadvantages of CA include invasiveness, exposure to ionizing radiation, and iodinated contrast agents. Intra-arterial carbon dioxide is a reasonable alternative to iodinated contrast in CA for patients with renal function impairment [34].
38-year-old female with hypertension. MIP image from CTA performed for screening shows subtle irregularity of the mid right renal artery (white arrow in (A)). Given concern for FMD, catheter-directed digital subtraction angiography (B) was subsequently performed revealing multifocal areas of main renal artery luminal irregularity (white arrow) and segmental branch vessel involvement (green arrow). The segmental branch vessel involvement was better delineated at DSA
An important pitfall that the radiologist should be aware of on CA is the phenomenon of “standing waves,” which is a potential angiographic mimic of FMD. Standing waves also demonstrate a beaded appearance of an arterial segment. Standing waves are a benign phenomenon with an uncertain etiology. Various mechanisms for the development of standing waves have been proposed including vasospasm, vessel response to rapid injection of contrast material, or flow-related artifact [35]. Interestingly, standing waves have been noted to resolve with the use of a vasodilator in some reports or even upon re-injection of contrast [36, 37]. Distinction between standing waves and FMD can be suggested as standing waves typically produce regular and transient findings, whereas FMD tends to be irregular and fixed [35].
Duplex ultrasound
The advantage of duplex ultrasonography lies in the availability of various modes of noninvasive ultrasound interrogation, enabling comprehensive anatomic and hemodynamic assessment of the vasculature. In FMD, the combination of findings on Duplex modalities is key to the diagnosis. Duplex ultrasonography, which includes real-time brightness mode (2D B-mode) and color pulsed-wave Doppler, has an established high sensitivity and specificity in the diagnosis of renal artery stenosis, via direct parameters including the arterial Peak Systolic Velocity (PSV) and the Renal-to-Aortic Ratio (RAR) [38]. Proper technique is essential given the challenges to sonographic imaging of the renal vasculature, including accurate identification of the vessels, overlying bowel gas, respiratory motion, and patient cooperation.
2D B-mode interrogation is tailored to the anatomic evaluation of the renal artery vasculature (including main renal arteries and accessories arteries) and generates an image based on short ultrasound pulsed waves that are dispersed into tissue using a 2.5-3 MHz phased array or curved linear transducer. Power Doppler analysis may be of benefit when there is difficulty locating the arteries given increased sensitivity to the detection of flow [33]. 2D B-mode imaging findings suggestive of FMD include vessel tortuosity, artery dissection, or aneurysm (Fig. 8). Arterial dissection is identified on B-mode imaging as an intimal flap with separation of flow on color Doppler analysis.
Left renal artery with FMD. The mid artery demonstrates increased velocities (PSV 243 cm/s) with color mosaicism and mixing indicative of turbulence and an area of hemodynamically significant stenosis. The waveform exhibits spectral broadening typical of nonlaminar flow. The distal artery demonstrates tortuosity, which is a nonspecific, although common, finding in FMD
Interrogation of the B-mode image with pulsed-wave Doppler using a 60-degree angle of insonation allows for optimal hemodynamic assessment. Sequential velocity measurements are obtained in all portions of the renal arteries and primary branches, including assessment for vessel tortuosity, flow turbulence, and velocity shifts. Random spot-checking of areas in the renal artery is not sufficient, and focal lesions can be potentially missed [33, 38, 39]. The diagnosis of fibromuscular dysplasia is subjective and relies on a characteristic pattern of turbulence, tortuosity, and velocity shifts in the mid or distal renal artery (Fig. 9) [33, 38, 39]. Typical renovascular FMD manifests as stenosis of the mid-distal renal arteries but focal disease may cause isolated stenosis in any portion of the renal artery and, if proximal, may be mistaken for atherosclerosis [11]. The spectral waveform distal to the stenosis may appear blunted and broadened, termed “parvus–tardus” morphology. When this is observed, the proximal artery should be assessed to identify the area of stenosis. Increased diastolic flow distal to a stenotic lesion may also be present, as the vasculature dilates in response to obstructed arterial in-flow, although in the case of increased renal cortical resistance (i.e. nephrosclerosis secondary to long-standing hypertension), a high resistant waveform may be present instead [33]. Visualization of beading is only rarely seen on duplex ultrasound, and the degree of arterial stenosis cannot be accurately determined due to multifocal areas of stenosis and dilatation. Therefore, reporting a percent stenosis by visual assessment alone is not accurate and is strongly discouraged [16]. At our institution, it is practice to describe the features of FMD when seen on duplex ultrasound as: “There is turbulence, tortuosity, and elevated velocities seen in the mid-distal renal artery. This is consistent with (or may be seen with) FMD.”
Duplex ultrasonographic findings of FMD using B-mode and Color Flow Doppler Imaging. Color images represent various times in a recorded CFDI cine loop (A) B-mode image demonstrating some irregularity of the renal artery wall. B Appearance of normal color flow in the same segment in late systole and diastole. C, D Increasing mosaicism and color mixing observed in the same region representing nonlaminar flow and turbulence in early systole
Ideally, velocity flow, color Doppler, and spectral waveform findings can be reproduced in two views (anterior and oblique) or captured using a recorded cine loop to confirm the diagnosis. An elevated ratio of the renal artery and the juxta-renal aorta PSV or “renal-to-aortic ratio” (RAR) is a validated index of proximal renal artery stenosis, although some report detecting mid-distal disease in FMD, later confirmed on angiography or IVUS, using a threshold RAR ≥2–3.5 [30, 40, 41]. This measure is inaccurate in the setting of low or high aortic peak systolic velocities (<40 and >100 cm/s respectively) and to date has not been validated for FMD [32, 33, 38].
Additional indirect measures of renal artery stenosis include the renal resistive index (RRI), acceleration time (AT), and acceleration index (AI), although only the RRI and AT have been evaluated in studies with FMD patients [30, 41, 42]. Overall, these measures are not as reliable as direct Doppler color flow analysis and velocities. The constellation of findings using gray scale and color Doppler assessment may suggest and characterize the diagnosis of FMD, and further assessment with other imaging techniques such as CA/IVUS, CTA, and MRA should be considered in more ambiguous or challenging cases. In the post-procedure setting, duplex ultrasonography is a robust imaging modality for surveillance of the renal arteries [2, 4].
CT angiography
CTA has been increasingly used for the noninvasive imaging evaluation of renovascular disease given excellent image quality, high spatial resolution, and reproducibility. CTA is also readily available and quick to perform. CTA of the neck, chest, abdomen, and pelvis can be easily performed in a single acquisition. Multiplanar reformatted images (MPR) and maximum intensity projection images (MIP) reliably demonstrate arterial FMD [34]. The spatial resolution of CTA (0.5 mm) is superior to MRA (1–2 mm), yet remains inferior to DSA (<0.1 mm). Additional advantages of CTA are the visualization of the vessel wall, vessel lumen, and renal parenchyma [30, 34]. Renal size discrepancy and cortical thinning are additional findings that may be present in unilateral FMD and are well delineated on CTA. Potential disadvantages include slightly greater radiation dose delivered for CTA and the exposure to iodinated contrast agents. With improvements and advances in CT technology, including multidetector row CT, automated dose modulation, new image reconstruction algorithms, and the use of dual energy CTA, reductions in CT radiation dose may be achieved.
CTA has performed very well compared to DSA for the diagnosis of atherosclerotic renal artery stenosis [43, 44]. Few authors have investigated the accuracy of CTA compared to CA for FMD with promising results. In a study of 21 hypertensive patients with CA-proven FMD, all 40 lesions (100%) were retrospectively identified at CTA [45]. CTA made the diagnosis of renal artery FMD in 100% of patients (n = 20) with a per-lesion sensitivity of 87% when both axial and reformatted images were evaluated in a separate retrospective study [46]. These excellent results are contrary to the work of Vasbinder et al. ??In this study, 356 patients with suspected renal artery stenosis (including atherosclerotic disease and FMD) underwent prospective CTA, MRA, and CA. 26 of 72 patients with RAS were diagnosed with FMD (36%). The sensitivity and specificity of CT compared to CA were 64 and 92%, respectively. The authors note that the low detection rate may be related to suboptimal imaging technique, reader inexperience, and patient selection bias [47]. An additional consideration is that the findings at CTA may not always correspond to a pressure gradient characterized at CA. The significance of this discrepancy warrants further investigation (Fig. 10). Initial work demonstrating that CTA was less reliable for the assessment of distal and branch vessel lesions is likely to be overcome with improved CT technology, spatial resolution, and advanced three-dimensional reformats [48, 49].
54-year-old female presented with resistant hypertension. Volume-rendered image obtained from CTA A shows the typical beaded appearance of right renal artery FMD (arrow). The left renal artery was occluded (dashed arrow) with atrophy of the left kidney. The patient subsequently underwent CA (B), which confirmed the findings of right renal artery FMD (arrow) and left renal artery occlusion (dashed arrow). However, no pressure gradient in the right renal artery was identified at CA
MR angiography
MRI is a robust tool in the diagnosis of renal arterial disease and FMD given its lack of ionizing radiation, superior soft tissue contrast resolution, and multiparametric capabilities. MR angiography (MRA) may be performed with or without intravenous contrast. Contrast-enhanced MRA is considered the noninvasive reference standard for detection of renal artery stenosis in some series [50]. Noncontrast MRA techniques are also increasingly attractive in the era of NSF, for patients with impaired renal function or gadolinium contrast allergy. Prior work has shown reasonable agreement of noncontrast MRA techniques including phase contrast and time of flight with contrast-enhanced MRA. These older techniques are limited to main renal arteries with have poor assessment of distal arterial or branch vessel disease [51, 52]. Newer noncontrast MRA techniques, such as balanced steady-state free precession (Bal-SSFP) and arterial spin labeling (ASL), represent promising alternatives to contrast-enhanced MRA or CA [53, 54]. Glockner et al. showed that the intrarenal segmental arterial branches were better delineated on Bal-SSFP MRA compared to contrast-enhanced MRA [55].
The same anatomic findings, including the presence and the severity of arterial stenosis, the presence of accessory renal arteries or variant anatomy identified on Duplex US or CTA, are also present on MRA. MRA similarly demonstrates morphologic information including renal size and cortical thickness. Dissection is the most common cause of renal artery occlusion in FMD, and MRI is useful for demonstrating the dissection flap and abnormal signal intensity within the two lumens [34, 56].
A significant disadvantage of MR-based techniques is the inferior spatial resolution of MRA compared to CA or CTA, limiting the assessment of the branch vessels for involvement and for the detection of subtle irregularities in the vessel wall [34]. Assessment and delineation of small aneurysms <6 mm in size is also limited with current MR capabilities. In our experience, patient motion is a significant limitation of MRI in that motion degraded images could potentially result in findings that mimic FMD. Overall exam cost, relatively long duration of exam time, and the need for excellent patient cooperation are additional considerations and potential limitations of the widespread use of MRA for this application. Further work is necessary to evaluate new flow-based or phase-contrast techniques for quantification of pressure gradients across a vessel as these techniques could potentially replace invasive CA in the future.
Preliminary work has demonstrated excellent performance of contrast-enhanced MRA for the diagnosis of renal artery stenosis [57–59]. Contrast-enhanced MRA detected findings of FMD in 97% (35/35) of main renal arteries that were considered abnormal at CA [60]. In this study, the sensitivity was 68, 95 and 100% for detecting arterial stenosis, “string-of-pearls,” and aneurysm, respectively [60]. The authors defined aneurysm as any abnormal widening of the artery with loss of the parallelism of the arterial vascular walls [60]. Four of four FMD lesions in the main renal artery were detected with MRA in a prospective study comparing MRA, CTA, and CA in patients with suspected renovascular hypertension [61]. However, similar to the findings for CTA, the sensitivity, and specificity for FMD detection using MRA were lower in the prospective study by Vasbinder et al. (sensitivity of 62% and specificity of 84%) [47].
Overall imaging strategy
Our experience suggests that noninvasive imaging modalities including Duplex US, CTA, and MRA perform very well for the evaluation of renal artery FMD with particular advantages including accessibility, low complication rate, versatility, noninvasiveness, and in many cases, cost effectiveness. Noninvasive techniques may also play a role in directing subsequent endovascular or surgical interventions.
General recommendations for the screening of FMD are based on expert consensus opinion but are somewhat conflicting. One study prospectively examined 58 patients with suspected renovascular disease, showed no benefit to any one modality including Color Flow Doppler Imaging (CFDI), CTA, contrast-enhanced MRA, and CA, in the identification of FMD [61]. Investigators from the US and Europe, published recommendations in 2005 and 2012 that support initial ultrasound screening of the renal arteries in patients with suspected FMD [2, 62]. Additionally, more recent work has outlined specific guidelines on when and how to screen for FMD [1, 16]. While it is recognized that Doppler analysis in duplex ultrasonography allows for noninvasive hemodynamic assessment of the renal arteries, many groups recommend that FMD diagnosis be confirmed by CTA or Gd-MRA [38]. First line screening examinations at our institution include Duplex ultrasound and contrast-enhanced whole body CTA for anatomic screening. When turbulent flow is detected at Duplex ultrasound, patients will undergo CA for evaluation of pressure gradient across a vessel. Respiratory triggered Bal-SSFP noncontrast MRA is performed in patients at risk of NSF or having any other contraindication to gadolinium.
Management
Medical management of hypertension is first line therapy for patients with renal FMD. In patients with refractory hypertension to multiple medications, percutaneous transluminal angioplasty (PTA) may be performed in the main renal arteries or branch vessels with excellent technical and clinical success rates (Fig. 11) [63–65]. Low FMD recurrence rate post therapy has been reported, although outcomes do depend on patient age and duration of hypertension [15, 26, 65]. In some patients, such as those with small renal arteries (<4 mm), branch disease, or extensive intimal or perimedial fibroplasia, the expected outcome from surgery may be better than that expected with PTA [18]. Such patients are managed surgically, including aortorenal bypass or arterial reconstruction and autotransplantation. Currently, there is no prospective, randomized control trial comparing the different treatment options for FMD [65].
Focal FMD in an 18-year-old male with refractory hypertension. MIP-reformatted image from CTA shows irregular long segment luminal narrowing and irregularity of the right renal artery without focal aneurysm (A). Reformat using center line tool demonstrates the area to involve the mid and distal right renal artery (B). Similar findings were noted at subsequent DSA (C). Persistent hemodynamically significant pressure gradient of 65 mmHg persisted after right renal artery PTA. The patient then underwent successful right renal artery stenting with resolution of right renal artery stenosis (D)
Summary and conclusions
Despite being a relatively uncommon cause of arterial disease, FMD requires high index of suspicion and rigorous imaging evaluation. Multiple imaging modalities are available for the evaluation of renal arterial FMD, including catheter-directed angiography, Duplex US, CTA, and MRA. Appropriate treatment of this potentially treatable and reversible cause of secondary hypertension is underpinned by correct diagnosis: hence, high-quality imaging of the arterial lesions, associated complications, and renal morphologic changes is essential.
References
Olin JW, Sealove BA (2011) Diagnosis, management, and future developments of fibromuscular dysplasia. J Vasc Surg 53(3):826–836 e821
Persu A, Touze E, Mousseaux E, et al. (2012) Diagnosis and management of fibromuscular dysplasia: an expert consensus. Eur J Clin Invest 42(3):338–347
Plouin PF, Perdu J, La Batide-Alanore A, et al. (2007) Fibromuscular dysplasia. Orphanet J Rare Dis 2:28
Olin JW, Froehlich J, Gu X, et al. (2012) The United States Registry for fibromuscular dysplasia: results in the first 447 patients. Circulation 125(25):3182–3190
Cragg AH, Smith TP, Thompson BH, et al. (1989) Incidental fibromuscular dysplasia in potential renal donors: long-term clinical follow-up. Radiology 172(1):145–147
Blondin D, Lanzman R, Schellhammer F, et al. (2010) Fibromuscular dysplasia in living renal donors: still a challenge to computed tomographic angiography. Eur J Radiol 75(1):67–71
Neymark E, LaBerge JM, Hirose R, et al. (2000) Arteriographic detection of renovascular disease in potential renal donors: incidence and effect on donor surgery. Radiology 214(3):755–760
Lorenz EC, Vrtiska TJ, Lieske JC, et al. (2010) Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol 5(3):431–438
Hendricks NJ, Matsumoto AH, Angle JF, et al. (2014) Is fibromuscular dysplasia underdiagnosed? A comparison of the prevalence of FMD seen in CORAL trial participants versus a single institution population of renal donor candidates. Vasc Med 19(5):363–367
Slovut DP, Olin JW (2004) Fibromuscular dysplasia. N Engl J Med 350(18):1862–1871
Savard S, Steichen O, Azarine A, et al. (2012) Association between 2 angiographic subtypes of renal artery fibromuscular dysplasia and clinical characteristics. Circulation 126(25):3062–3069
Tegtmeyer CJ, Selby JB, Hartwell GD, Ayers C, Tegtmeyer V (1991) Results and complications of angioplasty in fibromuscular disease. Circulation 83(2 Suppl):I155–I161
Alhadad A, Mattiasson I, Ivancev K, Gottsater A, Lindblad B (2005) Revascularisation of renal artery stenosis caused by fibromuscular dysplasia: effects on blood pressure during 7-year follow-up are influenced by duration of hypertension and branch artery stenosis. J Hum Hypertens 19(10):761–767
Davies MG, Saad WE, Peden EK, et al. (2008) The long-term outcomes of percutaneous therapy for renal artery fibromuscular dysplasia. J Vasc Surg 48(4):865–871
Trinquart L, Mounier-Vehier C, Sapoval M, Gagnon N, Plouin PF (2010) Efficacy of revascularization for renal artery stenosis caused by fibromuscular dysplasia: a systematic review and meta-analysis. Hypertension 56(3):525–532
Olin JWGH, Bacharach MJ, Biller J, Fine LJ, et al. (2014) Fibromuscular dysplasia: state of the science and critical unanswered questions. Circulation 129:1048–1078
Saw J, Ricci D, Starovoytov A, Fox R, Buller CE (2013) Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 6(1):44–52
Olin JW, Gornik HL, Bacharach JM, et al. (2014) Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation 129(9):1048–1078
Savard S, Azarine A, Jeunemaitre X, et al. (2013) Association of smoking with phenotype at diagnosis and vascular interventions in patients with renal artery fibromuscular dysplasia. Hypertension 61(6):1227–1232
Perdu J, Boutouyrie P, Bourgain C, et al. (2007) Inheritance of arterial lesions in renal fibromuscular dysplasia. J Hum Hypertens 21(5):393–400
Kiando SR, Tucker TN, Katz A, et al. (2015) Genetic study identifies common variation in PHACTR1 to associate with fibromuscular dysplasia. Circulation 132:A15370
Ganesh SK, Morissette R, Xu Z, et al. (2014) Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF-beta expression and connective tissue features. FASEB J 28(8):3313–3324
Poloskey SL, Kim E, Sanghani R, et al. (2012) Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 17(6):371–378
Harrison EGML (1971) Pathologic classification of renal arterial disease in renovascular hypertension. Mayo Clin Proc 46:161–167
Kadian-Dodov D, Gornik H, Gu X, et al. (2014) Aneurysm and dissection in fibromuscular dysplasia: findings from the United States registry for FMD. J Am Coll Cardiol 63(12_S):A2030
Meuse MA, Turba UC, Sabri SS, et al. (2010) Treatment of renal artery fibromuscular dysplasia. Tech Vasc Interv Radiol 13(2):126–133
Pannier-Moreau I, Grimbert P, Fiquet-Kempf B, et al. (1997) Possible familial origin of multifocal renal artery fibromuscular dysplasia. J Hypertens 15(12 Pt 2):1797–1801
McKenzie GA, Oderich GS, Kawashima A, Misra S (2013) Renal artery fibromuscular dysplasia in 2,640 renal donor subjects: a CT angiography analysis. J Vasc Interv Radiol 24(10):1477–1480
Lassiter FD (1998) The string-of-beads sign. Radiology 206(2):437–438
Gowda MS, Loeb AL, Crouse LJ, Kramer PH (2003) Complementary roles of color-flow duplex imaging and intravascular ultrasound in the diagnosis of renal artery fibromuscular dysplasia: should renal arteriography serve as the “gold standard”? J Am Coll Cardiol 41(8):1305–1311
Prasad A, Zafar N, Mahmud E (2009) Assessment of renal artery fibromuscular dysplasia: angiography, intravascular ultrasound (with virtual histology), and pressure wire measurements. Catheter Cardiovasc Interv 74(2):260–264
Lau JF (2012) Clinical evaluation of renal artery disease. In: Creager Mark A, Loscalzo Joseph (eds) Vascular medicine: a companion to Braunwald’s heart disease, 2nd edn. Philedelphia: Elsevier Saunders, Inc., pp 296–306
Zierler REOK (2010) Renal duplex scanning. In: Zierler RE (ed) Strandness’s duplex scanning in vascular disorders, 4th edn. Philadelphia: Lippincott Williams & Wilkins, pp 283–310
Das CJ, Neyaz Z, Thapa P, Sharma S, Vashist S (2007) Fibromuscular dysplasia of the renal arteries: a radiological review. Int Urol Nephrol 39(1):233–238
Sharma AM, Gornik HL (2012) Standing arterial waves is NOT fibromuscular dysplasia. Circ Cardiovasc Interv 5(1):e9–e11
Lehrer H (1967) The physiology of angiographic arterial waves. Radiology 89(1):11–19
Jacobsen JC, Beierholm U, Mikkelsen R, et al. (2002) “Sausage-string” appearance of arteries and arterioles can be caused by an instability of the blood vessel wall. Am J Physiol 283(5):R1118–R1130
Olin JWPM, Young JR, DeAnna S, Grubb M, Childs MB (1995) The utility of duplex ultrasound scanning of the renal arteries for diagnosing significant renal artery stenosis. Ann Intern Med 11(122):833–838
Jw O (1992) Role of duplex ultrasonography in screening for significant renal artery disease. Urol Clin North Am 2(21):215–226
Mousa AY, Campbell JE, Stone PA, et al. (2012) Short- and long-term outcomes of percutaneous transluminal angioplasty/stenting of renal fibromuscular dysplasia over a ten-year period. J Vasc Surg 55(2):421–427
Edwards JM, Zaccardi MJ, Strandness DE Jr (1992) A preliminary study of the role of duplex scanning in defining the adequacy of treatment of patients with renal artery fibromuscular dysplasia. J Vasc Surg 15(4):604–609 (discussion 609-611)
Li JC, Yuan Y, Qin W, et al. (2007) Evaluation of the tardus-parvus pattern in patients with atherosclerotic and nonatherosclerotic renal artery stenosis. J Ultrasound Med 26(4):419–426
Kim TS, Chung JW, Park JH, et al. (1998) Renal artery evaluation: comparison of spiral CT angiography to intra-arterial DSA. J Vasc Interv Radiol 9(4):553–559
Olbricht CJ, Paul K, Prokop M, et al. (1995) Minimally invasive diagnosis of renal artery stenosis by spiral computed tomography angiography. Kidney Int 48(4):1332–1337
Sabharwal R, Vladica P, Coleman P (2007) Multidetector spiral CT renal angiography in the diagnosis of renal artery fibromuscular dysplasia. Eur J Radiol 61(3):520–527
Beregi JP, Louvegny S, Gautier C, et al. (1999) Fibromuscular dysplasia of the renal arteries: comparison of helical CT angiography and arteriography. AJR Am J Roentgenol 172(1):27–34
Vasbinder GB, Nelemans PJ, Kessels AG, et al. (2004) Accuracy of computed tomographic angiography and magnetic resonance angiography for diagnosing renal artery stenosis. Ann Intern Med 141(9):674–682 (discussion 682)
Galanski M, Prokop M, Chavan A, et al. (1993) Renal arterial stenoses: spiral CT angiography. Radiology 189(1):185–192
Rubin GD, Dake MD, Napel S, et al. (1994) Spiral CT of renal artery stenosis: comparison of three-dimensional rendering techniques. Radiology 190(1):181–189
Leiner T, Michaely H (2008) Advances in contrast-enhanced MR angiography of the renal arteries. Magn Reson Imaging Clin N Am. 16(4):561–572, vii
Loubeyre P, Trolliet P, Cahen R, et al. (1996) MR angiography of renal artery stenosis: value of the combination of three-dimensional time-of-flight and three-dimensional phase-contrast MR angiography sequences. AJR Am J Roentgenol 167(2):489–494
Silverman JM, Friedman ML, Van Allan RJ (1996) Detection of main renal artery stenosis using phase-contrast cine MR angiography. AJR Am J Roentgenol 166(5):1131–1137
Michaely HJ, Schoenberg SO, Ittrich C, et al. (2004) Renal disease: value of functional magnetic resonance imaging with flow and perfusion measurements. Invest Radiol 39(11):698–705
Potthast S, Maki JH (2008) Non-contrast-enhanced MR imaging of the renal arteries. Magn Reson Imaging Clin N Am 16(4):573–584, vii
Glockner JF, Takahashi N, Kawashima A, et al. (2010) Non-contrast renal artery MRA using an inflow inversion recovery steady state free precession technique (Inhance): comparison with 3D contrast-enhanced MRA. J Magn Reson Imaging 31(6):1411–1418
Furie DM, Tien RD (1994) Fibromuscular dysplasia of arteries of the head and neck: imaging findings. AJR Am J Roentgenol 162(5):1205–1209
Korst MB, Joosten FB, Postma CT, et al. (2000) Accuracy of normal-dose contrast-enhanced MR angiography in assessing renal artery stenosis and accessory renal arteries. AJR Am J Roentgenol 174(3):629–634
Lee VS, Rofsky NM, Krinsky GA, Stemerman DH, Weinreb JC (1999) Single-dose breath-hold gadolinium-enhanced three-dimensional MR angiography of the renal arteries. Radiology 211(1):69–78
Gilfeather M, Yoon HC, Siegelman ES, et al. (1999) Renal artery stenosis: evaluation with conventional angiography versus gadolinium-enhanced MR angiography. Radiology 210(2):367–372
Willoteaux S, Faivre-Pierret M, Moranne O, et al. (2006) Fibromuscular dysplasia of the main renal arteries: comparison of contrast-enhanced MR angiography with digital subtraction angiography. Radiology 241(3):922–929
Rountas C, Vlychou M, Vassiou K, et al. (2007) Imaging modalities for renal artery stenosis in suspected renovascular hypertension: prospective intraindividual comparison of color Doppler US, CT angiography, GD-enhanced MR angiography, and digital substraction angiography. Ren Fail 29(3):295–302
Hirsch AT, Haskal ZJ, Hertzer NR, et al. (2006) ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 113(11):e463–e654
Sos TA, Pickering TG, Sniderman K, et al. (1983) Percutaneous transluminal renal angioplasty in renovascular hypertension due to atheroma or fibromuscular dysplasia. N Engl J Med 309(5):274–279
Kim HJ, Do YS, Shin SW, et al. (2008) Percutaneous transluminal angioplasty of renal artery fibromuscular dysplasia: mid-term results. Korean J Radiol 9(1):38–44
Begelman SM, Olin JW (2000) Fibromuscular dysplasia. Curr Opin Rheumatol 12(1):41–47
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study did not receive funding.
Conflict of Interest
Dr. Lewis declares that she has no conflict of interest. Dr. Kadian-Dodov declares that she has no conflict of interest. Dr. Bansal declares that she has no conflict of interest. Dr. Lookstein is a consultant for Johnson and Johnson.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Lewis, S., Kadian-Dodov, D., Bansal, A. et al. Multimodality imaging of fibromuscular dysplasia. Abdom Radiol 41, 2048–2060 (2016). https://doi.org/10.1007/s00261-016-0778-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0778-8