Abstract
Purpose
The purpose of the study is to investigate the computed tomographic characteristic and clinical findings of gastric neuroendocrine carcinoma (G-NEC) to increase awareness of this disease.
Methods
Twenty-two patients with a diagnosis of G-NEC were identified through the PACS of our hospital from August 2010 to November 2014. The clinical data, computed tomography (CT) features, and pathology records were analyzed.
Results
Among the 22 patients, 21 were male (95.45%), and 1 was female (4.55%). The mean age was 63.5 years old. Positive rates of neuroendocrine markers were 77.28% for chromogranin A staining, 86.36% for synaptophysin staining. All cases were single lesions including 16 (72.73%) in the gastric fundus, 3 (13.64%) in the gastric body and 1 (4.55%) in the gastric angle. Additionally 2 (9.09%) were found in the gastric antrum. Gastric wall was local thickening in 15 cases, and mass formation in 7 cases, with the stenosis and deformation of the adjacent gastric cavity. The long-axis diameter of the lesions ranged from 1.2 to7.4 cm (mean diameter, 2.47 cm), and the long-axis diameter was <2 cm in 12 case, 2–7.4 cm in 10 cases. The radiodensity values of the lesions were homogeneous density in 15 cases ranging from 22 to 47 HU (mean 34 HU). An ulcer with an irregular base and slightly raised borders located in the stomach was seen in 19 cases. The CT images showed homogeneous enhancement in 15 cases and heterogeneous enhancement in 7 cases. Obvious enhancement was seen in two cases, moderate enhancement was seen in sixteen cases, and mildly enhancement was seen in four cases. The peak value occurred in the arterial phase in 5 cases and the peak value was seen in 17 cases in the portal phase. Eleven lesions invaded the gastric serosa, and lymphatic metastasis was observed in 21 cases, 8 of which were combined with liver metastasis. CT images revealed 2 cases of the liver metastasis had obvious enhancement.
Conclusion
The CT features regarding location, incidence rates of ulcer and enhancement pattern described in our findings are common in all malignant gastric tumors. Therefore, the diagnosis of G-NEC must be confirmed with pathological test.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Neuroendocrine neoplasms (NENs) are an extremely rare malignant tumor arising from neuroendocrine cells of the diffuse neuroendocrine system. This entity was described in 1907 by Oberndorfer et al. [1]. It occurs in 1–2 cases/1000000 persons per year and accounting for 8.7% of all gastrointestinal NENs. The increased incidence is due to the better awareness of the disease [2]. According to the World Health Organization (WHO) 2010 classification [3], gastrointestinal NENs are classified as neuroendocrine tumor G1, neuroendocrine tumor G2, neuroendocrine carcinoma (NEC), small cell type, large cell type, and mixed adenoneuro endocrine carcinoma (a new term for mixed tumors). Due to the highly malignant biological behavior, the operative planning and the prognosis of the gastric NEC (G-NEC) is quite different compared with well-differentiated gastric NENs [4, 5].Therefore, it could be very helpful to understand the G-NEC’s medical image features and make an accurate diagnosis of G-NEC before surgery.
Currently, approximately one hundred and seventy cases of G-NEC have been reported in the English medical literature. However, to the best of our knowledge, the majority of them have focused on the clinical characteristics and prognosis. Very little emphasis has been placed on the radiological presentations of this tumor [6–8].The appropriate pre-operative diagnosis and operative planning could be delayed due to a difficult early definition of the clinical and radiological presentation of this disease. The present study analyzed our experience with 22 patients with G-NEC to help radiologists recognize this tumor when making a diagnosis.
Materials and methods
Patient selection
This study was approved by the institutional review board, and the requirement for written informed consent was waived in this retrospective study. From August 2010 to November 2014, We searched pathology records and the PACS system, and identified 22 patients with G-NEC. The search terms included: (stomach) and (neuroendocrine carcinoma). All patients had undergone gastroscopic biopsies before treatment. The patients included twenty-one men and one woman ranging in age from 43 to 80 years with a mean age of 63.5 years at first diagnosis (Table 1).
CT evaluation
Since all patients with suspected gastrointestinal disease will likely to have metastatic in liver. Therefore, all patients underwent a dual-phase contrast-enhanced CT examination (i.e., unenhanced and two-phase contrast-enhanced CT studies). Patient with suspected gastrointestinal disease had preparation, which included oral administration of 600–1000 mL of water to distend the stomach immediately before the CT scanning, followed by an injection of 40 mg of butyl scopolamine for decreasing bowel peristalsis and facilitating hypotonia. All CT examinations were performed with a 64 multi-detector (Discovery CT750HD, GE Healthcare, Wisconsin, USA). The imaging parameters were as follows: a tube voltage of 120 kV, a tube current of 350 mA, rotation time of 0.5 s. Additionally it was used a field of view of 500 mm, matrix of 512 mm, and section thicknesses of 0.75 mm. 70–120 mL (1.5 mL/kg) of a non-ionic contrast medium (iopromide, 370 mg/mL) was injected at a rate of 3 mL/s via the ante cubital vein by a dual-head pump injector (Medrad, Warrendale, USA). Finally, 20-mL saline flush was injected at a rate of 3 mL/s. Each of the datasets was reconstructed with 5-mm thickness. Contrast-enhanced CT scans were performed with a scanning delay of 30 s (arterial phase) and 70 s (portal venous phase) after start of intravenous (i.v.) injection of iopromide. CT dose index volume for the 3 phases was 15 mSv.
Image analyzes
The CT images were analyzed in consensus by two radiologists with 14 and 30 years’ experience in abdominal CT. All analyses were performed at AW4.4 workstation (GE Healthcare, Waukesha, Wisconsin) and radiologists were blinded to the clinical information of patients. The evaluated parameters included the tumor location (gastric fundus, gastric body, gastric angle, and gastric antrum), long-axis diameter, shape (focal thickening or mass), attenuation (measured at all enhanced phases), and characteristics of enhancement. The characteristics include enhancement pattern and degree of enhancement. The enhancement pattern of the tumor was classified as homogeneous or heterogeneous based on arterial phase. The degree of enhancement the tumor was based on dynamic CT imaging using HU attenuation, where “obvious enhancement” if ≥40 HU, “moderate enhancement” if ≥20 HU and “mildly enhancement” if <20 HU. The region of interest (ROI) was placed at the center of the mass far away from the areas of focal changes, large vessels, and prominent artifacts. All measurements were repeated three times at the three contiguous imaging levels, and average values were calculated to ensure consistency. In addition, patient’s age, sex, symptoms, and duration of symptoms, were reviewed.
Pathological evaluation
The pathological images were analyzed by two pathologists, independently. According to the 2010 WHO classification of digestive system tumors, the morphological findings of endocrine features on an HE-stained section should be combined with the neuroendocrine markers (including synaptophysin and chromogranin A) before a definitive diagnosis of G-NEC was made [9]. When the percentage of tumor cells with strong immunoreactive intensity was more than 5%, the tumor was classified as positive for marker [10]. The Ki-67 labeling index and the mitotic count were applied to evaluate the grading of the tumors, in which NEC require the cut-off value for Ki-67 labeling index as “more than 20% of 2000 cells” or for mitotic count as “more than 20 per 10 high-power field” in areas of highest nuclear labeling [9]. According to the WHO classification, the TNM staging of tumors was determined by the pathological and radiographic findings.
Results
Patient characteristics
The Clinical and pathological factors of G-NEC patients are summarized in Table 1. All of the 22 tumors were diagnosed as G-NEC, of which 17 (77.28%) tumors were positive for chromogranin A staining and 19 (86.36%) were positive for synaptophysin staining. Most of the patients presented with nonspecific symptoms including epigastric pain or discomfort (n = 15), dysphagia (n = 2), nausea or vomiting (n = 2), and hematochezia (n = 2). Other presenting symptoms included acid reflux (n = 5), haematemesis or tarry stool (n = 4). 3 (7.0%) were found at medical checkups without any clinical symptoms. None of the patients were found having carcinoid syndrome. The time from the symptoms presence or medical checkup to hospital ranged from 25 to 240 days, with a median of 60 days.
CT findings
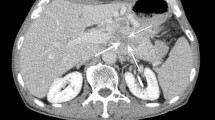
Among 22 cases of G-NEC, all the cases showed a single tumor. Sixteen (72.73%) were located in the gastric fundus (Fig. 1A), three (13.64%) in the gastric body and one (4.55%) in the gastric angle. Additionally, two (9.09%) was seen in the gastric antrum on the greater curvature aspect (Fig. 2A). The CT manifestation of this tumor included local (n = 15) thickening of gastric wall or mass formation (n = 7), with a stenosis and deformation of the adjacent gastric cavity. The long-axis diameter of the lesions was 1.2 to 7.4 cm (mean size, 2.47 cm). Twelve (54.55%) of the 22 lesions were less than 2.0 cm. The remaining ten (45.45%) were 2.0 to 7.4 cm. In our series, eleven (50%) of the 22 cases of G-NEC were identified as local thickening of the gastric wall with the long-axis diameter <2.0 cm. Six (27.27%) were classified as mass formation with the long-axis diameter of 2.0 to 7.4 cm. Fifty percent of the tumors showed invading the gastric serosa consistent with the results of pathological diagnosis. The main changes of the CT imaging features were an irregular outer layer of the gastric wall and haziness of the perigastric fat. CT findings in 18 patients were interpreted as gastric adenocarcinoma and gastric lymphoma in four.
G-NEC in 72-year-old man. A Unenhanced CT image of stomach reveals a 3.1 cm tumor of heterogeneous attenuation, with an ulcerated surface and an irregular outer layer of the gastric wall, accompanied by a liver metastasis, at the fundus of the stomach. B Contrast-enhanced CT image shows moderate enhancement of mass, with the peak value of the tumor on the portal phase. B1 Arterial phase of contrast enhancement image; B2 Portal phase of contrast enhancement image
G-NEC in 64-year-old man. A Unenhanced CT image of stomach reveals local gastric wall thickening of inhomogeneous attenuation at the angle of the stomach. B Contrast-enhanced CT image shows moderate inhomogeneous enhancement of mass, with the peak value of the tumor on the portal phase. B1 Arterial phase of contrast enhancement image; B2 Portal phase of contrast enhancement image
The tumor showed predominantly homogeneous density with the radiodensity values of solid masses ranging from 22 to 47 HU (mean 34 HU) in non-contrast phase. In 19 cases, an ulcer was demonstrated to be present in the stomach with an irregular base and slightly raised borders. Homogeneous enhancement was seen in fifteen cases (68.18%, 15/22), with the radiodensity values of arterial phase ranging from 44 to 171 HU and venous phase ranging from 61 to 143 HU. 31.82% (7/22) cases demonstrated heterogeneous enhancement due to the necrotic or cystic areas. After enhancement, obvious enhancement was seen in two cases, moderate enhancement was seen in sixteen cases (Figs. 1B, 2B), mildly enhancement was seen in four cases. The peak value of the tumor was found in 5 cases in the arterial phase and seventeen in the portal phase. Lymph node involvement was found in 21 cases, mainly fasten on retro peritoneum and flattened the tumor with definite lines (Fig. 2A–C). Eight cases had liver metastasis (Fig. 1A–C). No metastasis was observed in one case. CT images revealed 2 cases of the liver metastasis had obvious enhancement.
Discussion
NEC, also previously known as “small cell carcinoma, large cell (neuro) endocrine carcinoma, or poorly differentiated (neuro)endocrine carcinoma” [11]. This is a poorly differentiated, high-grade malignant neoplasm that is comprised small cells or large-to-intermediate cells with marked nuclear atypia, multifocal necrosis, and a high number of mitoses (>20 per 10 HPF) [11]. Previous studies have reported the neuroendocrine markers of synaptophysin and chromogranin A must be stained to make a definitive diagnosis of NEN. Moreover, it has been reported that the analysis of synaptophysin may be more sensitive compared with the distribution of chromogranin A [9].This conclusion is consistent with the findings of our study, as the positive rate of chromogranin A and synaptophysin being 77.28% and 86.36%, respectively. The tumor rarely occurs in the gastrointestinal tract, accounting for approximately 1% involved in the esophagus, 0.2% in the colon [5]. The incidence of NEC in the stomach is exceedingly rare. According to the published study, Chieko Uchiyama et al. only found 7 cases of G-NEC in 1027 patients with gastric carcinoma that underwent gastrectomy [12]. The frequency found in the present study was consistent with those in the literature. Between 08/2011 and 11/2014, 763 patients with NEC confirmed by pathology were retrospectively reviewed, only 22 cases in the stomach (0.6%). The mean age of the reported cases was 66.2 years (range 51–81) [9], which is consistent with the age of our series. Xu X et al. reported the sex distribution of male to female G-NEC patients was 2.9:1 [9]. It has been noticed that it is most common in male patients, with gender as a probable risk factor. Similarly, this apparent male predominance was also observed in some studies [8, 10, 12, 13]. G-NEC patients may present with epigastric pain or discomfort, dysphagia, nausea and vomiting, and hematochezia. Other features such as acid reflux [14], tarry stools [5] may also be present. Epigastric pain or discomfort is common because of the increased thickening of the gastric wall. A previous study has even reported a patient with the presence of hypersecretion syndrome [8]. In addition, patients of G-NEC without any clinical symptoms have also been reported. Because the clinical manifestation is not typical, the disease can stay hidden for a long time ranged from 25 to 240 days, with a median of 60 days. However, Xu X et al. reported cases of G-NEC diagnosed in 3 days after the onset of the first symptom [9].
G-NEC is exclusively located in the fundus or the body of the stomach within 8 cm in the long-axis diameter [9, 15]. In the present study, 19 (86.36%) of the 22 cases of G-NEC were identified in the gastric fundus or body on the greater or lesser curvature. The remaining three cases of G-NEC were found in atypical locations such as the angle or antrum of the stomach. Twelve (54.55%) cases of G-NEC in the present study were less than 2.0 cm in the long-axis diameter, and the remaining ten (45.45%) were 2 to 7.4 cm. The long-axis diameters and locations of the cases of G-NEC in our series were similar to those in previous reports [9, 15].
Most of the tumors were identified as a local thickening or bulky mass of the gastric wall with enlarged lymph nodes around the stomach. Similarly to other gastric malignant tumors, this feature suggests the high-grade malignant nature of this tumor. The findings are consistent with the histopathological examination which showed that 11 (50%) of the 22 cases of G-NEC invaded the serosal layer. The other three cases of G-NEC invaded the submucosal layer or muscular layer. The result is also according with the Karakoyun et al. [16] who confirmed that thin-slice hydro-multi-detector row CT can improve the accuracy of pre-operative T and N staging of gastric cancer and contribute to treatment strategies for patients with advanced stage gastric cancer. Tumors with ulcer were frequently seen on CT images; therefore, this finding may be valuable for the differential diagnosis among gastric tumors. In present study, nine (75%, 9/12) cases of G-NEC with less than 2.0 cm in diameter were identified with ulcer, and the remaining ten cases with the long-axis diameter of 2.0 to 7.4 cm were 100%. Relatively few studies have been performed to investigate the CT contrast enhancement features of G-NEC [8]. In present study, about 68.18% of the lesions showed homogeneous enhancement during the arterial phase, only 31.82% (7/22) cases demonstrated heterogeneous enhancement due to the necrotic or cystic areas. These findings are consistent with the findings of the study by Wang D et al. [8]. Most G-NEC have been referred to as rich blood supply lesions. The obvious enhancement ratio, moderate enhancement ratio, and mildly enhancement ratio was 73.91, 17.39, and 8.7%, respectively [8]. However, in our study, nearly three-fourth of the cases (16/22, 72.73%) considered as moderate enhancement, which is slightly higher than the result of the above study [8]. During the portal phase the contrast, G-NEC demonstrated about 77.27% (17/22) cases have further enhancement. This phenomenon is in accord with Wang D’s finding in the gastrointestinal NEC [8]. He found that 86.4% cases with a peak enhancement in portal phase imaging [8].
In addition, after analyzing the CT images of liver metastasis, we identified that 2 cases had an obvious enhancement. This enhanced pattern is partly due to these lesions’ histopathological features are similar to the G-NEC and partly due to its abundant blood supply feature [8].
Some of the G-NEC cases reported in the literature, and that involved the gastrointestinal tract were initially misdiagnosed as gastrointestinal adenocarcinoma [13, 14]. Similarly, our eighteen cases were misdiagnosed as gastric adenocarcinoma. Even though the biopsy through gastroscopy and colonoscopy are helpful to make a definitive diagnosis of G-NEC, a misdiagnosing is frequently observed [5, 8, 13, 14]. This diagnosis problem is due to the rapid develop of adenocarcinoma in the submucosal and deeper layers as a precursor cell clones partly transformed into G-NEC [5]. The same author reported a case in which were included two elements at the histological level: an adenocarcinoma component in the superficial portion of the mucous membrane layer and NEC in the submucosal and muscularis propria layers. It is hypothesized that misdiagnosis could be due to an incorrect biopsy of the lesions and small samples size.
The CT differential diagnosis for G-NEC usually includes gastric adenocarcinoma, gastric lymphoma, and gastric stromal tumor. Adenocarcinoma is the most common pathology type of gastric neoplasms. It is preferentially situated in gastric antrum, seldomly in gastric body and gastric fundus. The incidence of gastric adenocarcinoma is high among male with a median age of 67 years [17]. The most common CT pattern of gastric adenocarcinoma included local, extensive thickening of gastric wall or mass formation. Previous studies have demonstrated gastric ulcer disease is positively associated with the risk of developing gastric cancer [18]. Therefore, it has been suggested that gastric ulcer disease arose or became remarkably more prevalent in gastric cancer patients. Gastric adenocarcinoma usually presented with metastases to the liver and others organs or distant structures such as lymph nodes located in retropancreatic area, paraaortic and mesenteric nodes. Gastric lymphoma is also an extensive thickening of gastric wall or mass formation on CT images. The localization of lesions in the stomach was more involved in gastric fundus, body and antrum, seldomly in gastric cardia compared with the gastric cancer patients [19]. Furthermore, gastric lymphoma is inclined to involve more than one region of the stomach than that of the gastric cancer patients [19]. In addition, the most common CT appearance of gastric lymphoma was uniform soft-tissue mass with attenuation similar to or slightly lower than that of normal gastric wall [20]. Due to necrosis, hemorrhage, submucosal edema, or infarction, the lymphomatous gastric wall may have the heterogeneity on CT [20]. Gastric lymphoma originates as a submucosal process and gastric mucosa was usually intact in the early stage to interrupt or ulcer formation in the later. After injection of the contrast, homogeneous and slightly enhancement occurred in most gastric lymphoma at delayed-phase. It frequently metastases to the pancreas, spleen, transverse mesocolon, and pleura can also be involved [20]. Distant structures such as lymph nodes located in the mesentery, retroperitoneum, or elsewhere in the abdomen may suggest the diagnosis of gastric lymphoma [20]. Gastric stromal tumors, although rare, may occasionally have characteristics of a gastric submucosal protrusive mass at CT. It is preferentially located in gastric antrum and body, seldomly in gastric fundus, and gastric angle, etc. It is most common in male patients, with the sex distribution of male to female 3:1. The median age of patients presenting with the tumor is between 40 and 60 years. The mass grow in an exophytic pattern in 30–40%, intraluminal pattern in 29–44%, endoluminal pattern in 18–22%, and a mixed pattern in 16–20% [21]. Gastric stromal tumors rarely have enlarged regional lymph nodes and metastase to the liver and others organs.
Limitations of this research included that this is a study of small series which may potentially impact results. In addition, the retrospective design introduces selection bias and may affect whether the results seen are generalizable.
In conclusion, although G-NEC is quite rare, it should be considered in differential diagnosis of neoplasms in the stomach. Our study showed that G-NEC has variable clinical patterns and radiological features that are generally nonspecific for the diagnosis. Therefore, we think that pathological test should be performed prior to the surgical resection.
Abbreviations
- CT:
-
Computed tomography
- G-NEC:
-
Gastric neuroendocrine carcinoma
- NENs:
-
Neuroendocrine neoplasms
- NEC:
-
Neuroendocrine carcinoma
References
Oberndorfer S (1907) Karzinoide Tumoren des Dünndarms. Frankf Z Pathol 1:425–432
Li TT, Qiu F, Qian ZR, et al. (2014) Classification, clinicopathologic features and treatment of gastric neuroendocrine tumors. World J Gastroenterol 20(1):118–125
Scoazec JY, Couvelard A (2011) The new WHO classification of digestive neuroendocrine tumors. Ann Pathol 31(2):88–92
Jiang SX, Mikami T, Umezawa A, et al. (2006) Gastric large cell neuroendocrine carcinomas: a distinct clinicopathologic entity. Am J Surg Pathol 30(8):945–953
Miguchi M, Iseki M, Shimatani K (2012) Advanced gastric neuroendocrine carcinoma with an adenocarcinoma component. Case Rep Gastroenterol 6(1):52–57
Makis W, Ciarallo A, Hickeson M, et al. (2013) Gastric neuroendocrine carcinoma staged and followed with (18)F-FDG PET/CT–a report of 3 cases. Clin Nucl Med. 38(6):447–450
Watanabe N, Kato H, Shimizu M, et al. (2006) F-18 FDG PET imaging in gastric neuroendocrine carcinoma. Clin Nucl Med 31(6):345–346
Wang D, Zhang GB, Yan L, et al. (2012) CT and enhanced CT in diagnosis of gastrointestinal neuroendocrine carcinomas. Abdom Imaging 37(5):738–745
Xu X, Li J, Han X, et al. (2014) Clinical characteristics and prognostic factors of patients with gastric neuroendocrine carcinoma treated with radical surgery. Chin Med J (Engl) 127(13):2419–2422
Namikawa T, Oki T, Kitagawa H, et al. (2013) Neuroendocrine carcinoma of the stomach: clinicopathological and immunohistochemical evaluation. Med Mol Morphol. 46(1):34–40
Endo S, Dousei T, Yoshikawa Y, et al. (2012) Gastric neuroendocrine tumors in our institutions according to the WHO 2010 classification. Int Surg 97(4):335–339
Uchiyama C, Tamura S, Nakatsuka S, et al. (2012) Immunohistochemical consistency between primary tumors and lymph node metastases of gastric neuroendocrine carcinoma. World J Surg Oncol 10:115
Ishida M, Sekine S, Fukagawa T, et al. (2013) Neuroendocrine carcinoma of the stomach: morphologic and immunohistochemical characteristics and prognosis. Am J Surg Pathol 37(7):949–959
Kang SH, Kim KH, Seo SH, et al. (2014) Neuroendocrine carcinoma of the stomach: a case report. World J Gastrointest Surg 6(4):77–79
DelleFave G, Kwekkeboom DJ, Van Cutsem E, et al. (2012) ENETS consensus guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology 95(2):74–87
Karakoyun R, Demirci E, Karakoyun M, et al. (2014) Reliability of MDCT, with MPR and hydro-CT technique, in resectability and lymphnode staging of gastric cancer. Minerva Chir 69(3):129–140
Cidón EU, Cuenca IJ (2009) Gastric adenocarcinoma: is computed tomography (CT) useful in preoperative staging? Clin Med Oncol 3:91–97
Huang WL, Li YG, Lv YC, et al. (2014) Use of lectin microarray to differentiate gastric cancer from gastric ulcer. World J Gastroenterol 20(18):5474–5482
Wu J, Zhu H, Li K, et al. (2014) 18F-fluorodeoxyglucose positron emission tomography/computed tomography findings of gastric lymphoma: comparisons with gastric cancer. Oncol Lett 8(4):1757–1764
Gossios K, Katsimbri P, Tsianos E (2000) CT features of gastric lymphoma. Eur Radiol 10(3):425–430
Ovali GY, Tarhan S, Serter S, et al. (2005) Gastric stromal tumor. Diagn Interv Radiol 11(2):102–104
Acknowledgment
This work was supported the National Natural Science Foundations of China (81271573).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
We have no disclosures.
Rights and permissions
About this article
Cite this article
Liang, P., Wang, YX., Ren, XC. et al. Neuroendocrine carcinoma of the stomach: clinical features and CT findings. Abdom Radiol 41, 19–24 (2016). https://doi.org/10.1007/s00261-015-0593-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-015-0593-7