Abstract
Objective
To establish highly specific criteria for predicting non-enhancement in T1-hyperintense non-fat-containing (T1-high) renal lesions using unenhanced fat-suppressed T1-weighted (T1-FS) images.
Materials and methods
This IRB-approved, HIPAA-compliant, retrospective study included T1-high renal lesions found between 7/1/2012 and 7/1/2014. The largest lesion diameter and heterogeneity, mean signal intensity of lesion, and adjacent renal cortex were recorded from T1-FS images. The presence/absence of lesion enhancement was determined from subtraction images. T1 signal ratio (T1-SR) was calculated as (mean SI of lesion)/(mean SI of cortex). Logistic regression with binary outcome of the presence or absence of lesion enhancement was performed. Cut-off T1-SR to maximize specificity was established from receiver operator curve analysis.
Results
There were 101 patients (58 [57.4%] male) with non-enhancing lesions and 80 patients (51 [63.8%] male) with enhancing lesions, mean ages 64.0 ± 13.3 and 62.1 ± 13.8 years, respectively. Median sizes were 11 mm (IQR 8–16) and 20.5 mm (IQR 15–29) for non-enhancing and enhancing lesions, respectively (p < 0.0001). 19/101 (18.8%) of non-enhancing and 56/80 (70.0%) of enhancing lesions were heterogeneous (p < 0.0001). T1-SR was 1.77 ± 0.6 and 1.25 ± 0.42 for non-enhancing and enhancing lesions, respectively (p < 0.0001). For each increase of 0.5 in T1-SR, odds ratio for non-enhancement was 3.3 (95% CI 1.85–5.79), adjusted for lesion size and heterogeneity. T1-SR alone had area under the curve of 0.88 (95% CI 0.78–10.89) for non-enhancement. T1-SR ≥2.15 had positive likelihood ratio of 9.5 for non-enhancement.
Conclusion
Signal ratio of lesion to cortex ≥2.15 on unenhanced T1-weighted images is a highly specific predictor for non-enhancement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Renal lesions are exceedingly common: approximately half of adults over the age of 50 have at least one renal lesion [1–3]. Over the past several decades, as clinical use of imaging tools such as CT, MR, and ultrasound to evaluate various kinds of abdominal pathology has been steadily rising, an ever increasing number of renal lesions are being discovered incidentally [2, 4], with 59.2% of renal tumors being incidentally discovered in the late 1990s as compared with 13.0% of renal tumors being incidentally discovered in the early 1980s [5]. Renal lesions incidentally found on non-contrast MR may pose a management dilemma to clinicians, as renal cysts with proteinaceous or hemorrhagic contents are often indistinguishable from RCCs on non-contrast MR imaging [1, 4]. The risk of an incidentally discovered renal lesion being an RCC is not negligible, as approximately 61% of RCCs are discovered incidentally, 85% of which are stage I or II [6]. Therefore, when a non-fat-containing renal lesion with high signal intensity on T1-weighted sequences is incidentally detected on a non-contrast MR examination, further work-up with post-gadolinium sequences is generally indicated for complete characterization [3]. This additional work-up can lead to patient inconvenience and anxiety, as well as increased medical costs. These issues can be exaggerated in patients with renal insufficiency: when gadolinium administration is prohibitive due to nephrogenic systemic fibrosis, more extensive work-up and follow-up may ensue.
In recent years, there has been increasing interest in developing criteria for characterizing renal incidentalomas detected on non-contrast cross-sectional studies. One recent study by Childs et al. found visible in-phase signal intensity loss on dual-echo gradient-echo MRI to be predictive of malignancy in solid renal lesions [7]. Pooler et al. established that an attenuation of greater than 70 HU on non-contrast CT is highly specific for a proteinaceous or hemorrhagic cyst [8]. To our knowledge, no analogous criterion for characterizing renal incidentalomas detected on non-contrast MRI has been established thus far. The purpose of our study was to establish criteria for predicting lack of enhancement in T1-hyperintense non-fat-containing renal lesions with high specificity using only unenhanced fat-suppressed T1-weighted images. We directed our attention toward producing criteria with high specificity rather than criteria with high sensitivity, since our objective was not to establish a screening test for detection of T1-hyperintense renal lesions but rather to establish highly specific criteria that, when met, would reassure the practicing radiologist that no further work-up is necessary.
Materials and methods
Patients
Hospital institutional review board approval was obtained for this HIPAA-compliant retrospective study. The need for informed consent was waived. Our institution’s electronic medical record was queried for reports of all contrast-enhanced abdominal MR imaging done at our institution between July 1, 2012 and July 1, 2014 in patients over 18 years of age. The reports containing at least one of the following word combinations were identified: (1) the words “kidney” or “renal,” “T1,” and “hyperintense” or “high” within one sentence, (2) the words “kidney” or “renal,” “solid” or “enhancing,” and “mass” or “lesion” within one sentence, or (3) the words “RCC” or “renal cell carcinoma.” The study group was identified based on MR reports and images, with the following inclusion criteria: (1) the presence of a T1-hyperintense renal lesion (T1-High lesion), (2) lack of gross fat within the lesion, and (3) presence of pre- and post-gadolinium fat-saturated T1-weighted images of diagnostic quality. Lesions containing gross fat were excluded because, in clinical practice, such lesions are presumed to represent benign angiomyolipomas and do not present the diagnostic dilemma that non-gross-fat-containing lesions do [9]. T1-High lesions were defined as visually at least as intense as the adjacent renal cortex on pre-contrast fat-suppressed (FS) T1-weighted sequences. In patients with multiple T1-High lesions, the largest such lesion on either side (defined as the lesion with the largest maximum axial diameter on either side) was considered in our analysis.
MR imaging
The studies were performed on one of 10 MR scanners: 1.5T Signa Excite Platform version 12.0 (GE Healthcare, Cleveland, Ohio, USA), 1.5T Signa Excite Platform version 11x (GE Healthcare, Cleveland, Ohio, USA), 3.0T Signa HDXt Platform version 16.0 (GE Healthcare, Cleveland, Ohio, USA), 1.5T Ingenia Platform version 4.1.3 (Philips Healthcare, Best, Netherlands), 1.5T Panorama HFO Platform version 3.2.3 (Philips Healthcare, Best, Netherlands), 1.5T Achieva Platform version 3.2.3 (Philips Healthcare, Best, Netherlands), 3.0T Achieva Platform version 3.2.2 (Philips Healthcare, Best, Netherlands), 3.0 T Ingenia Platform version 2.6.3 Philips Healthcare, Best, Netherlands), 1.5T Intera Platform version 3.2.3 (Philips Healthcare, Best, Netherlands), and 1.5T Achieva Platform version 3.2.3.2 (Philips Healthcare, Best, Netherlands). On all scanners, T1-weighted 3D ultrafast gradient-echo sequence with fat suppression was utilized. Fat suppression was achieved with modified Dixon technique on 1.5T Ingenia and 3.0T Signa HDXt scanners, and with frequency-selective fat saturation technique on the remainder of the scanners. Table 1 summarizes the imaging parameters of T1-weighted fat-suppressed sequences for all the scanners.
Image analysis
For each patient in the study group, pre-contrast FS T1-weighted sequences were reviewed by two radiologists (MF and JL) blinded to the enhancement status of the lesion using Centricity Picture Archiving and Communication System (GE Healthcare, Cleveland, Ohio, USA). The following was recorded for the largest T1-High lesion on either side: the laterality (left or right), the maximum axial diameter of the lesion, and lesion heterogeneity. Lesion heterogeneity was assessed subjectively as a presence or absence . Regions of interest (ROIs) were drawn in the lesion and in the adjacent renal cortex (Fig. 1), and mean T1 signal intensities (SI) of the lesion and the renal cortex were recorded. For homogeneous lesions, a round ROI was drawn to cover as much of the lesion’s axial area as possible; for heterogeneous lesions, a round ROI was drawn to cover as much of the T1-hyperintense portion of the lesion as possible. When drawing ROIs in the renal cortex adjacent to the lesions, a round ROI was drawn in such a way as to cover a maximal amount of the renal cortex without extending into the renal medulla or the juxtarenal fat. For all recorded data, the two reviewing radiologists were required to reach a consensus.
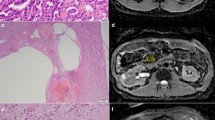
Non-contrast T1-weighted fat-suppressed axial image in a patient with a T1-hyperintense lesion in his left kidney. Regions of interest were drawn in the lesion (solid circle) and in the adjacent renal cortex (dotted circle) so that the mean signal intensity of each could be recorded and their ratio subsequently calculated
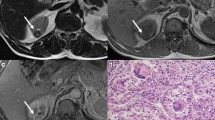
T1 signal ratio (T1-SR) of each lesion was calculated as (mean SI of lesion)/(mean SI of cortex). For each lesion, subtraction images were created manually based on source pre- and post-contrast images using AQNet Client software (TeraRecon, Foster City, CA, USA) in order to eliminate misregistration, and the presence or absence of enhancement within the lesion was recorded from the subtraction images. The absence of enhancement was defined as complete signal void within the lesion on the subtraction images (Fig. 2). Any visually discernible signal within the lesion on the subtraction images was considered as enhancement (Fig. 3).
Statistical analysis
Continuous variables with normal distribution were described as mean ± standard deviation, non-parametric continuous variables were described as medians with interquartile ranges, and categorical variables were described as proportions of the total. Bivariate associations between continuous variables were tested by Student’s t test. Assumptions of the test were verified, and non-parametric counterpart (Mann–Whitney test) was used, where appropriate. χ 2 test was used to evaluate bivariate associations between categorical variables.
Multivariate logistic regression with binary outcome of the presence or absence of lesion enhancement was performed. The covariates with p value less than 0.1 on bivariate associations were included into the model. The final parsimonious model was constructed by using a backward stepwise selection approach, with exit criteria defined as a change in the coefficient of the variable of interest (T1-SR) of 10% or less and lack of statistical significance of the coefficient for the covariate. Receiver operating curve (ROC) analysis was constructed to determine the cut-off T1-SR to maximize specificity for diagnosis of lack of enhancement. Statistical analysis was performed using STATA, version 13 (StataCorp, College Station, TX, USA). Statistical significance was considered at p < 0.05.
Results
The initial hospital database search yielded 232 abdominal MR reports containing the word combination #1, 588 abdominal MR reports containing the word combination #2, and 191 abdominal MR reports containing the word combination #3. After the inclusion criteria were applied, 175 patients with a total of 181 T1-hyperintense renal lesions remained in the study group. 80 (44.2%) of 181 T1-hyperintense renal lesions were enhancing and 101 (55.8%) of 181 were not enhancing. Of the 80 enhancing lesions, 62 (77.5%) demonstrated solid/homogeneous enhancement, 12 (15.0%) demonstrated septal enhancement, and 6 (7.5%) demonstrated heterogeneous enhancement not otherwise specified. Table 2 summarizes the bivariate associations between the groups with and without enhancement. There was no statistically significant difference between the groups based on sex, age, race, or laterality of the T1-High lesion. Slightly higher proportion of non-enhancing T1-High lesions was imaged on 1.5T MR scanner (p = 0.048). Compared to enhancing T1-High lesions, non-enhancing T1-High lesions were smaller (median diameter 11.0 vs. 20.5 mm, p < 0.0001) and were less commonly heterogeneous (18.8% vs. 70.0%, p < 0.0001). Mean T1-SR was higher in non-enhancing T1-High lesions as compared to enhancing T1-High lesions (1.77 vs. 1.25, p < 0.0001) (Fig. 4).
Table 3 summarizes the results of the multivariate logistic regression. The main magnetic field strength was neither an independent predictor nor a confounder in the multivariate model and therefore was removed from the final model. For each incremental increase of 0.5 in the T1-SR, the odds of having no enhancement increased by a factor of 3.3 (95% CI 1.8–5.8, p < 0.0001), adjusted for lesion size and heterogeneity.
On ROC analysis, T1-SR alone had area under the curve of 0.88 (95% CI 0.78–0.89) for predicting lack of enhancement (Fig. 5). T1-SR ≥2.15 had positive likelihood ratio of 9.5 for lack of enhancement. In our study group, 2 (2.5%) of 80 enhancing T1-High lesions and 22 (21.8%) of 101 non-enhancing T1-High lesions had T1-SR ≥2.15 (p = 0.001), yielding specificity of 97.5% for lack of enhancement.
Pathologic correlation was available for 28 (35.0%) of 80 enhancing lesions and 0 (0.0%) of 101 non-enhancing lesions. Of the 30 enhancing lesions for which pathology was available, 26 were renal cell carcinomas (mean T1-SR = 1.21; papillary type: 12; clear cell type: 9; chromophobic type: 3; TFE-3 translocation associated: 1; collision lesion consisting of both papillary and chromophobic components: 1), 1 was an oncocytoma (T1-SR = 1.05), and 1 was a benign hemorrhagic lesion (T1-SR = 1.04).
Discussion
Our study demonstrates the usefulness of the lesion-to-cortex signal intensity ratio in predicting lack of enhancement in T1-hyperintense non-fat-containing renal lesions, as T1-SR is a predictor of lack of enhancement independent of both the lesion’s size and heterogeneity. Because non-contrast abdominal MR is routinely performed for a broad range of indications (e.g., MRCP to evaluate suspected biliary pathology) and because incidental renal lesions are an extremely common finding on non-contrast abdominal MR, having a criterion that could eliminate the need of further work-up and/or follow-up would be a useful tool in clinical practice. The criterion of T1-SR ≥2.15 is particularly well suited for practical use in clinical settings, as calculation of T1-SR is neither time intensive nor costly, requiring no special software aside from a calculator. Moreover, due to its high specificity, the criterion of T1-SR ≥2.15 allows a radiologist to determine with great confidence when further post-gadolinium imaging is not necessary. Implementing this criterion in clinical practice could therefore spare many patients from needless expense, inconvenience, and anxiety; it would be especially beneficial for patients with renal insufficiency, who cannot receive gadolinium.
The analogous dilemma of characterization of renal lesions incidentally detected on non-contrast CT scans was addressed in 2012 by Pooler et al., whose research demonstrated that an attenuation of greater than 70 HU on non-contrast CT is highly specific for a diagnosis of a proteinaceous or hemorrhagic cyst [8]. While Pooler’s >70-HU criterion has helped shorten the management of many renal incidentalomas detected on non-contrast CT, no analogous criteria for distinguishing a renal cyst with hemorrhagic or proteinaceous contents from an enhancing lesion on pre-contrast T1-weighted imaging have been presented in the literature. We believe the pathophysiology behind our findings is analogous to that found by Pooler et al., where T1-SR ≥2.15 diagnoses cysts with very high hemorrhagic or proteinaceous content.
The use of apparent diffusion coefficient (ADC) ratios obtained with diffusion-weighted imaging in the characterization of T1-hyperintense renal lesions has been discussed by Kim et al. [10]. Additionally, Mytsyk et al. have shown that ADC values not only vary significantly between benign and malignant renal lesions but also vary between low-grade RCCs and high-grade RCCs [11]. While Kim et al. demonstrated solid lesions to have significantly lower mean ADC values, no validated cut-off ADC value distinguishing a solid lesion from a T1-hyperintense cyst was reported [10]. Moreover, diffusion-weighted imaging may not be routinely performed in some institutions, and determination of actual ADC values may require supplementary software, making ADC a less practical tool. Additionally, the variability in DWI acquisition parameters among various institutions is another factor limiting generalizability [12]. In contrast to this, the T1-SR can be easily calculated in any setting, requiring very little additional effort and time, and no special software aside from a calculator.
Our study had several limitations. The study was retrospective and limited to a single institution, and due to the limitations of the word-combination-centered methodology used to identify the study population, our study group may not be representative of all patients with T1-hyperintense renal lesions. Additionally, our study population was imaged on a variety of MR scanners, introducing considerable heterogeneity into the imaging parameters. In our analysis, we did not analyze the effect of MR scanner vendor or fat-suppression method because, for each of these individual qualities, the sample size was not adequate to power a meaningful analysis. However, the proposed criteria use the ratio between the mean SI of the lesion and the cortex rather than the actual mean SI value of the lesion, which helps to diminish the influence of the use of different MR scanners. Furthermore, although magnet strength could theoretically influence the SI ratio, scanner strength was not a confounder of T1-SR in the multivariate model.
Other lesion characteristics, such as T2 signal and diffusion pattern, could have been analyzed in place of, or in addition to, T1-SR. However, the aim of our study was to establish a simple and practical criterion for distinguishing benign and worrisome renal lesions on unenhanced images that could easily be applied to clinical practice. While a complex equation taking into account other characteristics such as T2 signal might more accurately predict non-enhancement, it would be less easily applicable in clinical practice. Additionally, meaningful analysis of T2 signal characteristics would be limited by the variety of MR scanners we used and the variety of different T2 acquisition methods used by each scanner (e.g., BTFE vs. SSFSE vs. TSE). Diffusion characteristics were not analyzed because DWI sequences are not routinely obtained for all patients at our institution. Imaging parameters vary greatly even within our institution, limiting the conclusions that can be drawn.
Our study used lack of enhancement rather than pathology of the lesion as a gold standard to validate T1-SR performance. While this may be unconventional, we deliberately chose this method as it reflects clinical practice where lack of enhancement establishes a diagnosis of a complicated cyst requiring no further work-up. Furthermore, since renal lesions proven to be non-enhancing do not typically undergo biopsy or excision, pathologic correlate is not available in the vast majority of such lesions.
Lastly, the interobserver reproducibility of T1 signal intensity measurements was not assessed by our study. Interobserver variability in the determination of the T1-SR of a lesion may have been present, as different observers might differ in where they choose to place the ROIs, and our study did not assess this potential. These would be worthwhile issues for future follow-up studies to address.
In conclusion, signal ratio of lesion to cortex on non-contrast T1-weighted images of ≥2.15 is a highly specific predictor of lack of enhancement, and therefore benignity, in a T1-hyperintense non-fat-containing renal lesion. Use of this criterion may eliminate the need for further work-up of a subset of T1-hyperintense renal lesions.
References
Glockner JF (2007) Incidental findings on renal MR angiography. AJR Am J Roentgenol 189(3):693–700
Cieszanowski A, Maj E, Kulisiewicz P, et al. (2014) Non-contrast-enhanced whole-body magnetic resonance imaging in the general population: the incidence of abnormal findings in patients 50 years old and younger compared to older subjects. PLoS ONE 9(9):e107840
Sahni VA, Silverman SG (2014) Imaging management of incidentally detected small renal masses. Semin Intervent Radiol 31(1):9–19
Marhuenda A, Martin MI, Deltoro C, et al. (2008) Radiologic evaluation of small renal masses (I): pretreatment management. Adv Urol. doi:10.1155/2008/415848
Luciani LG, Cestari R, Tallarigo C (2000) Incidental renal cell carcinoma-age and stage characterization and clinical implications: study of 1092 patients (1982-1997). Urology 56(1):58–62
Jayson M, Sanders H (1998) Increased incidence of serendipitously discovered renal cell carcinoma. Urology 51(2):203–205
Childs DD, Clingan MJ, Zagoria RJ, et al. (2014) In-phase signal intensity loss in solid renal masses on dual-echo gradient-echo MRI: association with malignancy and pathologic classification. AJR Am J Roentgenol 203(4):W421–W428
Pooler BD, Pickhardt PJ, O’Connor SD, et al. (2012) Renal cell carcinoma: attenuation values on unenhanced CT. AJR Am J Roentgenol 198(5):1115–1120
Berland LL, Silverman SG, Gore RM, et al. (2010) Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 7(10):754–773
Kim S, Jain M, Harris AB, et al. (2009) T1 hyperintense renal lesions: characterization with diffusion-weighted MR imaging versus contrast-enhanced MR imaging. Radiology 251(3):796–807
Mytsyk Y, Borys Y, Komnatska I, Dutka I, Shatynska-Mytsyk I (2014) Value of the diffusion-weighted MRI in the differential diagnostics of malignant and benign kidney neoplasms—our clinical experience. Pol J Radiol. 79:290–295
Kang SK, Zhang A, Pandharipande PV, et al. (2015) DWI for renal mass characterization: systematic review and meta-analysis of diagnostic test performance. AJR Am J Roentgenol 205(2):317–324
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Le, J., Flusberg, M., Rozenblit, A.M. et al. T1-hyperintense renal lesions: can high signal predict lack of enhancement?. Abdom Imaging 40, 3175–3181 (2015). https://doi.org/10.1007/s00261-015-0539-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-015-0539-0