Abstract
Objective
To identify imaging characteristics that differentiate small (≤2 cm) HCCs from small (≤2 cm) benign nodules in cirrhotic liver on gadoxetic acid-enhanced and diffusion-weighted (DW) magnetic resonance (MR) images.
Materials and methods
On gadoxetic acid-enhanced and DW MR images, we analysed signal intensity of 222 small HCCs and 61 benign nodules (diameter, 0.5–2 cm) at each sequence and rim enhancement during portal or equilibrium phases. Univariate and multivariate logistic regression analyses identified predictors of HCC. Combinations of significant MR findings in multivariate analysis were compared with American Association for the Study of Liver Disease (AASLD) practice guidelines.
Results
In multivariate analysis, arterial enhancement (adjusted odds ratio [aOR], 8.6), T2 hyperintensity (aOR, 5.8), and hyperintensity on DW images (aOR, 3.8) were significant for differentiating small HCCs from benign nodules (p ≤ 0.004). When two or all three findings were applied as diagnostic criteria for differentiating small HCCs from benign nodules, sensitivity and accuracy were significantly higher compared with AASLD practice guidelines (91% vs. 78% and 89% vs. 81%, respectively; each p < 0.0001).
Conclusion
On gadoxetic acid-enhanced MR imaging, arterial enhancement and hyperintensity on T2-weighted and DW MR images are helpful for differentiating small HCCs from benign nodules in liver cirrhosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Hepatocellular carcinoma (HCC) is the sixth most common tumor and the third leading cause of cancer-related death worldwide [1]. Cirrhosis is the strongest predisposing factor for HCC, with approximately 80% of HCCs developing in the cirrhotic liver [2]. Despite many advances in multidisciplinary treatment, complete curative treatment of early-stage HCC remains the only possibility for long-term patient survival. The 5-year survival rates of patients undergoing curative therapies for HCC, including liver transplantation, hepatic resection, and percutaneous ablative techniques, range between 40% and 75% [3]. In liver cirrhosis, HCC develops in a step-wise fashion [4]. Regenerative nodules develop in the following sequence: low-grade dysplastic nodule (DN), to high-grade DN, to small HCC, and finally to large HCC. Therefore, differentiation of small HCCs from benign cirrhotic nodules, including regenerative nodules and DNs, as early as possible in patients with liver cirrhosis is important for deciding the optimal treatment time and decreasing mortality from HCC.
According to current American Association for the Study of Liver Disease (AASLD) practice guidelines, a hepatic mass larger than 1 cm in diameter in the cirrhotic liver or chronic hepatitis B that shows typical features (i.e., a combination of arterial hypervascularity and venous or delayed phase washout) on dynamic contrast-enhanced computed tomography (CT) or magnetic resonance (MR) images, can be diagnosed as HCC without biopsy [5]. Therefore, the role of imaging surveillance in HCC diagnosis is growing. Advanced imaging technologies and imaging surveillance in high-risk patients for HCC enable the detection of small hepatic lesions including HCC and benign cirrhotic nodules [5–8]. However, an accurate characterization and diagnosis of small HCCs remains the most challenging area in imaging of the cirrhotic liver.
In recent years, MR imaging, particularly contrast-enhanced dynamic MR imaging, has played a crucial role in the accurate diagnosis of HCC [9, 10]. Various contrast agents have been developed and utilised for MR imaging of the liver to facilitate the detection and characterization of focal hepatic lesions. Recently developed gadoxetic acid (Primovist®; Bayer Schering Pharma, Berlin, Germany) can provide haemodynamic information on dynamic MR images following bolus injection, as well as functional information on hepatobiliary phase images with approximately 50% of the agent taken up by hepatocytes. These properties of gadoxetic acid have been reported to improve the accuracy of liver MR imaging for lesion detection and characterization [11–17]. Furthermore, the addition of diffusion-weighted imaging (DWI) to gadoxetic acid-enhanced MR imaging has the potential to improve our ability to differentiate HCCs from benign cirrhotic nodules, owing to the differentiation between tissues on the basis of the diffusion properties of water molecules. In tumors, cell density increases considerably as the extracellular space decreases. This is accompanied by a subsequent decrease in mobility of water molecules, which leads to restricted diffusion [18]. However, to the best of our knowledge, there have been only a limited number of studies assessing gadoxetic acid-enhanced MR imaging and DWI findings in the diagnosis of small HCCs in patients with liver cirrhosis [19–21]. Furthermore, there is only one study regarding MR findings to differentiate between small HCCs and benign cirrhotic nodules in patients with hepatitis-induced liver cirrhosis on gadoxetic acid-enhanced and DW MR images [22]. However, in that study, they analysed signal intensities of nodules only on arterial phase, hepatobiliary phases and DW images in a relatively small number of patients. Therefore, the purpose of this study was to identify characteristic imaging features and their diagnostic performance for differentiating small (≤2 cm) HCCs from small benign nodules in the cirrhotic liver on gadoxetic acid-enhanced and DW MR images.
Material and methods
This retrospective study was approved by our institutional review board, and informed consent was waived.
Patients
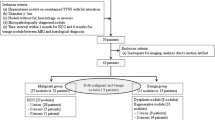
Between November 2009 and June 2011, 786 consecutive patients with liver cirrhosis underwent dynamic contrast-enhanced and DW MR imaging of the liver in our institution. We selected the patients with: (a) a pathology-confirmed, small (≤2 cm) HCC or benign cirrhotic nodule, such as a DN or regenerative nodule, or (b) a radiology-reported small (≤2 cm) benign cirrhotic nodule showing no interval change in size on follow-up images for at least 12 months (19). In total, 280 consecutive patients were eligible according to these inclusion criteria. Among them, we excluded 50 (18%) patients from this study, because a different type of MR contrast agent was used (n = 14), the time interval between MR imaging and pathology procedure was over 90 days (n = 25), patients had more than five nodules (n = 10), or the interpretation of MR imaging was difficult owing to respiratory motion artefact in the arterial phase (n = 1). Therefore, 230 patients with 222 HCCs and 61 benign nodules were included in our study (Fig. 1). The 230 patients (mean age, 57 years; age range, 29–83 years) included 178 men (mean age, 56 years; age range, 29–83 years) and 52 women (mean age, 59 years; age range, 42–77 years). Of the 230 patients, 188 patients had only HCCs (n = 210), 32 patients had only benign nodules (n = 50), and 10 patients had both HCCs (n = 12) and benign nodules (n = 11). The causes of liver cirrhosis were viral hepatitis (hepatitis B [n = 192] or hepatitis C [n = 19]), alcohol (n = 10), or cryptogenic (n = 9). Liver cirrhosis was determined by histology via biopsy (n = 19) or surgery (n = 197), as well as by clinical and radiologic findings and blood chemistry tests (aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, bilirubin, albumin and globulin) (n = 14).
All 222 HCCs were pathologically diagnosed by surgical resection (partial hepatectomy [n = 156] or liver explanation [n = 38]) or by percutaneous biopsy (n = 28). Of the 61 benign nodules, 28 DNs (46%), including 17 low-grade DNs and 11 high-grade DNs, were pathologically confirmed by partial hepatectomy (n = 6), liver explanation (n = 16), or percutaneous biopsy (n = 6). Therefore, pathological confirmation was obtained in 88% (250 of 283) of the cases. The time interval between MR imaging and the pathology procedure ranged from 0 to 90 days (mean, 19 days; median, 11 days). The remaining 33 benign nodules were stable in size for at least 12 months of follow-up MR imaging. The time interval between the initial and the last follow-up imaging ranged from 366 to 718 days (mean, 475 days; median, 428 days). The size of the HCCs ranged from 0.5 to 2.0 cm (median, 1.5 cm) and that of the benign nodules ranged from 0.5 to 2.0 cm (median, 1.1 cm) (p < 0.0001).
MR imaging techniques
MR examinations were performed in all patients using a 1.5-T scanner (Magnetom Avanto; Siemens Medical Solutions, Erlangen, Germany) with a six-element, phased-array torso coil as the receiver. Fat-suppressed T1-weighted fast low-angle shot (FLASH) MR imaging using the three-dimensional volumetric interpolated breath-hold examination (VIBE) technique was performed before and after administration of gadoxetic acid. The contrast material was administered as a bolus at a dose of 0.025 mmol/kg of body weight at a rate of 1 mL/s via a 22-gauge intravenous cubital line followed by a 20-mL saline flush at the same flow rate, using a power injector (Spectris Solaris; MedRad, Indianola, PA, USA). The peak time of time-density curve for determining image acquisition timing was achieved using a test-bolus technique in which 1 mL of gadoxetic acid was injected with a saline flush and the vessel of interest (abdominal aorta) was scanned approximately once per second. Images of the arterial, portal venous, equilibrium and hepatobiliary phases were obtained 5 s after the peak time, 60–70 s, 3 min, and 20 min, respectively, after contrast injection. The images were acquired in the transverse plane and had a section thickness of 4 mm and no gap. Images in the coronal plane were also obtained for a hepatobiliary phase image, just after the axial hepatobiliary phase image at 20 min. Subtraction of the arterial phase images was automatically computed by the software of the MR machine. The software provided a new series by image-by-image subtraction of pre-contrast images from arterial phase images for each patient. DWI was acquired before the administration of gadoxetic acid using a respiratory-triggered single-shot echo-planar imaging sequence with b values of 0, 50, 500 and 900 s/mm2. A spectral attenuated inversion-recovery technique was used for fat suppression on DWI.
The other MR sequences included: (a) axial dual-echo T1-weighted breath-hold gradient-echo sequence for acquisition of in-phase and out-of-phase images; (b) axial respiratory-triggered turbo-spin echo T2-weighted sequence with fat saturation; and (c) axial half-Fourier acquisition single-shot turbo-spin echo T2-weighted sequence with fat saturation. The MR sequence parameters are summarized in Table 1.
Image analysis
All images were reviewed with a local picture archiving and communication systems (PACS) monitor and digital imaging and communications in medicine (DICOM) image viewing software. A coordinator (J.Y.K., with 8 years of experience in abdominal MR imaging interpretation) selected MR images performed just before biopsy or surgery, and initial MR images in cases of radiology-reported benign cirrhotic nodules showing no change on more than 12 months of follow-up, and also reviewed the medical charts and pathology reports of the patients.
Two board-certified abdominal radiologists (J.H.B. and H.J.K., with 13 and 6 years of experience in abdominal MR imaging interpretation, respectively) retrospectively reviewed the MR images in consensus. They were blinded to the final diagnosis, but were aware of the study concept and design. For each nodule of 2 cm or smaller in diameter on MR images, the longest diameter and location according to the Couinaud classification were recorded. Subsequently, the following seven MR imaging findings were evaluated: T2 hyperintensity, T1 hypointensity, arterial enhancement, washout, hepatobiliary phase hypointensity, rim enhancement, and hyperintensity on DW image with a b value of 900 s/mm2. The signal intensity of a nodule on an MR image was considered hyper-, iso-, or hypointense when it was higher than, comparable to, or lower than that of the adjacent hepatic parenchyma, respectively. Arterial enhancement was defined as hyperintensity on the arterial subtraction images. Washout was defined as when the lesion showed hypointensity during the portal or equilibrium phase. Rim enhancement was considered positive when portal or equilibrium phase images showed a hyperintense rim around the lesion, indicating capsule enhancement. Hyperintensity on DW images was defined as when the nodule showed hyperintensity on the DW image and the apparent diffusion coefficient of the nodule was lower than or equal to that of the liver parenchyma. Nonsolid lesions such as cysts and hemangiomas were excluded for evaluation and their diagnoses were based on typical imaging findings and stability for at least 12 months of follow-up.
Statistical analysis
All statistical analyses were performed using commercially available software (SPSS, version 19.0 for windows; SPSS, Chicago, ILL, USA) and a p value of less than 0.05 was considered statistically significant. The sizes of HCCs and benign nodules, which were compared using Mann–Whitney U test, were not included in the subsequent univariate and multivariate analyses, as size was limited to a small range of equal to or less than 2 cm in our study. For each of the seven MR imaging findings, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated for differentiating small HCCs from benign nodules. Univariate logistic regression analysis was used to determine the findings predictive of a small HCC rather than a benign nodule. The generalized estimating equations were used to adjust for the correlation between multiple nodules within a patient (clustering effect) [23]. Multivariate logistic regression analysis was performed to determine independently significant MR imaging findings for differentiating a small HCC from a benign nodule. In the multivariate analysis, only the variables with p values less than 0.20 in the univariate analysis were included. Results are presented as the odds ratio (OR), 95% confidence interval (CI), and p value. We combined the significant MR imaging findings in the multivariate analysis and determined the number of nodules that fitted that combination. For these combinations, sensitivity, specificity, PPV, NPV, and accuracy were calculated to differentiate a small HCC from a benign nodule, and then the sensitivity, specificity, and accuracy were compared with those of the AASLD practice guidelines (a combination of arterial enhancement and washout) [5] using the McNemar test. All analyses were performed on a per-lesion basis.
Results
Among the 222 HCCs and the 61 benign nodules 2 cm or smaller in diameter, 32 small HCCs (14%) and 16 benign nodules (26%) were less than 1 cm in diameter. The MR findings of the small HCCs and benign nodules are summarized in Table 2. On T2-weighted images, most small HCCs (87%, 192 of 222) were hyperintense, whereas most benign nodules (84%, 51 of 61) were iso- or hypointense. Most small HCCs were hypointense on T1-weighted images (77%, 172 of 222) and on hepatobiliary phase images (94%, 209 of 222), hyperintense on DW images (87%, 193 of 222), and showed arterial enhancement (89%, 198 of 222) and washout (86%, 191 of 222) (Fig. 2). Most benign nodules also showed hypointensity in the hepatobiliary phase (67%, 41 of 61) and washout (66%, 40 of 61) (Fig. 3). Of the seven findings evaluated, hepatobiliary phase hypointensity had the highest sensitivity (94%, 209 of 222) and the lowest specificity (33%, 20 of 61) for diagnosis of small HCC. Rim enhancement showed the highest specificity (97%, 59 of 61) and PPV (96%, 54 of 56), and the lowest sensitivity (24%, 54 of 222), NPV (26%, 59 of 227), and accuracy (40%, 113 of 283) for diagnosing small HCC. Arterial enhancement had the highest NPV (68%, 50 of 74) and accuracy (88%, 248 of 283) for diagnosis of small HCC.
Hepatocellular carcinoma in a 36-year-old man. In hepatic segment III, a 2-cm nodule (arrow) shows hyperintensity on the axial T2-weighted image (A), hypointensity on the axial T1-weighted image (B), hyperintensity on the arterial phase contrast-enhanced T1-weighted image (C), hyperintensity on the subtraction image (D), washout on the equilibrium phase (E), hypointensity on the hepatobiliary phase (F), and hyperintensity on the diffusion-weighted image (b value of 900 s/mm2) (G).
Dysplastic nodule in a 70-year-old woman. No definite focal lesion is delineated on the axial T2-weighted image (A), axial T1-weighted image (B), arterial phase contrast-enhanced T1-weighted image (C), or subtraction image (D). In hepatic segment VII, a 1.2-cm nodule (arrow) shows washout on the equilibrium phase (E) and hypointensity on the hepatobiliary phase (F). On the diffusion-weighted image (b value of 900 s/mm2), the nodule is isointense (G).
According to the univariate logistic regression analysis results, the arterial enhancement (OR 28.7; p < 0.0001) and hyperintensity on DW images (OR 11.0; p < 0.0001) were significantly associated with small HCCs. Multivariate logistic regression analysis showed that arterial enhancement (adjusted OR 8.6; p < 0.0001), T2 hyperintensity (adjusted OR 5.8; p = 0.0002), and hyperintensity on DW images (adjusted OR 3.8; p = 0.004) were independently differentiating variables for small HCC (Table 2).
When two or three findings of T2 hyperintensity, arterial enhancement, and hyperintensity on DW images were applied as diagnostic criteria for differentiating small HCCs from benign nodules, small HCCs were diagnosed with sensitivity, specificity, PPV, NPV, and accuracy values of 91% (201 of 222; 95% CI 87%, 94%), 85% (52 of 61; 95% CI 74%, 93%), 96% (201 of 210; 95% CI 92%, 98%), 71% (52 of 73; 95% CI 59%, 81%) and 89% (253 of 283; 95% CI 85%, 93%), respectively. For all small HCCs, our proposed criteria yielded significantly higher sensitivity (91%) and accuracy (89%) than AASLD practice guidelines (78% [174 of 222; 95% CI 72%, 84%] and 81% [228 of 283; 95% CI 75%, 85%], respectively; p < 0.0001) (Table 3). For HCCs 1–2 cm in diameter, the sensitivity (92% [174 of 190; 95% CI 87%, 95%]) and accuracy (90% [211 of 235; 95% CI 85%, 93%]) achieved using our proposed criteria were also significantly higher than those of the AASLD practice guidelines (80% [152 of 190; 95% CI 74%, 85%] and 81% [191 of 235; 95% CI 76%, 86%], respectively; p ≤ 0.0001) (Table 4). For HCCs smaller than 1 cm in diameter, the sensitivity (84% [27 of 32; 95% CI 67%, 95%]) and accuracy (88% [42 of 48; 95% CI 75%, 95%]) achieved using our proposed criteria were significantly higher than those of AASLD practice guidelines (69% [22 of 32; 95% CI 50%, 84%] and 77% [37 of 48; 95% CI 63%, 88%], respectively; p = 0.03) (Table 5).
Discussion
Although arterial enhancement followed by portal or delayed washout is a conclusive finding of HCC according to AASLD criteria, only 76% (44/58) and 90% (53/59) of small HCCs showed this dynamic enhancement pattern in two previous studies using gadoxetic acid-enhanced MR imaging [20, 24]. This suggests that, in addition to arterial enhancement and portal or delayed washout, other diagnostic imaging criteria are needed for the diagnosis of small HCCs. Therefore, we evaluated the most predictive findings among various parameters of gadoxetic acid-enhanced and DW MR imaging. Our study showed that T2 hyperintensity, arterial enhancement, and hyperintensity on DW images were important imaging features that differentiate small (≤2 cm) HCCs from benign nodules in the cirrhotic liver on MR images with high sensitivity (87%–89%), specificity (79%–84%), PPV (94%–95%), and accuracy (85%–88%). These are well known as important imaging features for distinguishing HCCs from benign nodules [19, 25, 26], as well as being predictors of progression from hypovascular nodules to hypervascular HCCs [27, 28]. Furthermore, when two or all three such findings were applied as new diagnostic criteria for diagnosing small HCCs, high sensitivity (91%), specificity (85%), PPV (96%), and accuracy (89%) were achieved. Compared with AASLD practice guidelines, the sensitivity and accuracy of our proposed criteria were significantly greater, without a significant decrease in specificity. The early diagnosis of small HCCs is a very important factor for determining the survival rate of patients with HCC as it affords a high chance of surgical cure and a low risk of recurrence; therefore, this result is meaningful for managing patients with liver cirrhosis and HCC. Therefore, we believe that two or all three findings of T2 hyperintensity, arterial enhancement, and hyperintensity on DW images have the potential to be an alternative to the AASLD practice guidelines when diagnosing small HCC on gadoxetic acid-enhanced and DW MR images.
There have been a few previous studies reporting MR findings of small HCC [29–31]. In a previous study [29], the most common MR imaging features of small HCC were arterial enhancement (79%) and hypointensity on hepatobiliary phase (68%), similar to our results. They compared imaging features of small HCC with those of large HCC, not benign nodule, and concluded the small HCC less frequently shows hyperintensity on T2-weighted and DW images. However, in clinical practice, we often feel difficulty in differentiating small HCC form benign nodules, not large HCC. In another pervious report [30], authors studied only additional value of DW image to the gadoxetic acid-enhanced MR images in detecting small HCC. A recent study [31] reported that new criteria, namely nodules fitting the AASLD practice guidelines or the presence of three or more findings of T2 hyperintensity, arterial phase hyperintensity, washout, and hepatobiliary phase hypointensity, increased the sensitivity for diagnosing HCC in nodules 1–2 cm in size with specificity comparable to that of the AASLD practice guidelines alone. However, these authors only included HCCs that were 1–2 cm in diameter, used an MR contrast material other than gadoxetic acid (gadobenate dimeglumine), and did not use DWI. Furthermore, they included arterial phase hyperintensity, not arterial enhancement on subtraction images as was done in our study. When a nodule is hyperintense on pre-enhanced T1-weighted images, arterial enhancement is unclear on arterial phase images. Therefore, we included arterial enhancement on subtraction images rather than arterial phase hyperintensity.
According to the currently available imaging criteria for HCC in recent AASLD practice guidelines [5], the diagnosis of malignancy applies only to nodules that are larger than 1 cm and that show typical vascular profiles. Therefore, the diagnosis of HCCs smaller than 1 cm or that show an atypical vascular pattern is still made on the basis of positive biopsy results, or patients with such lesions are recommended for a wait-and-see policy owing to a high false-positive rate [5, 6]. This emphasizes the need to refine new diagnostic criteria for HCC and to include other imaging in addition to conventional dynamic imaging techniques that focus only on enhancement patterns. AASLD criteria showed moderate sensitivity (69%) and accuracy (77%) for the diagnosis of HCC less than 1 cm in diameter in this study, similar to the sensitivity of 77.8% observed in a previous study [19]. However, our proposed diagnostic criteria (two or three findings of T2 hyperintensity, arterial enhancement, and hyperintensity on DW images) showed significantly higher sensitivity (84%) and accuracy (88%) compared with the AASLD guidelines, but with the same specificity (94%). The recent development of MR techniques, including DWI, and MR contrast agents can provide diverse information such as hepatocyte function and tissue diffusivity of lesions as well as their hemodynamics. These MR features are very helpful for diagnosing very small HCCs or HCCs with atypical dynamic enhancement patterns. In our study, T2-weighted and DW images in addition to the dynamic imaging, made it possible to reliably diagnose HCCs smaller than 2.0 cm.
In previous studies and our study, hyperintensity on DW images was helpful in differentiating small HCCs from other benign nodules in the cirrhotic liver [19, 22, 32]. Since DWI can differentiate between tissues according to their degree of cellular density and architectural change [33, 34], it has the potential to improve the detection of small HCCs that do not show typical HCC vascular patterns by allowing more accurate characterization of equivocal lesions. However, it may be difficult to identify HCCs on a background of cirrhotic liver since the parenchyma of the cirrhotic liver also shows restricted diffusion [35, 36]. Furthermore, DWI has inherent limitations, including limited spatial resolution; susceptibility to motion artifacts, especially for lesions located in the left lateral segment and close to the diaphragm because of cardiac motion; and difficulty in differentiation of solid hepatic masses [33, 37, 38]. Although DWI is not generally used as a standalone sequence, combined with contrast-enhanced MR imaging, DWI may provide improved characterization of HCC in liver cirrhosis [21, 39].
The washout on portal or equilibrium phase images is considered to be one of the most reliable findings of HCCs, in contrast to other lesions specified in diagnosis guidelines for HCC, including the AASLD practice guidelines. However, in a previous report [19] and our study, the washout on portal or equilibrium phase was not a statistically significant finding for diagnosing small HCCs on gadoxetic acid-enhanced MR images. In our study, 66% (40 of 61) of benign nodules showed washout on the portal or equilibrium phases. Hepatocyte enhancement can start before the acquisition of late dynamic phase images, the so-called equilibrium phase. Therefore, due to the rapid and strong enhancement of the liver parenchyma on gadoxetic acid-enhanced MR images [40], it may be possible that other benign nodules show hypointensity before hepatobiliary phase images. This difference of signal intensity during the equilibrium phase between gadoxetic acid and other extracellular contrast materials is important when evaluating liver MR imaging, particularly in patients with liver cirrhosis. Therefore, imaging diagnostic criteria for HCC on gadoxetic acid-enhanced MR images should be reevaluated and redefined.
Several studies of gadoxetic acid-enhanced MR imaging demonstrate that the hepatobiliary phase can provide valuable additional information regarding the characterization of focal liver lesions [11, 41–43]. In those studies, HCCs usually exhibited hypointensity on gadoxetic acid-enhanced hepatobiliary phase images. However, some HCCs may retain the contrast agent [11, 41, 44–46], and some benign lesions, such as DNs, may exhibit hypointensity on hepatobiliary phase images [19, 26, 31, 47]. A previous study [19] showed that the hypointensity on hepatobiliary phase images was not statistically significant for diagnosing HCCs 1 cm or smaller in diameter on gadoxetic acid-enhanced MR images. Our results also showed that 67% (41 of 61) of benign nodules 2 cm or smaller in diameter were hypointense on hepatobiliary phase images. Therefore, hepatobiliary phase images with gadoxetic acid as the contrast agent are not specific enough to distinguish small HCCs from benign nodules, albeit they are highly sensitive for small HCCs. Gadoxetic acid is highly liver specific, with uptake from blood sinusoids into hepatocytes by organic anion-transporting polypeptide (OATP) 8, and is gradually excreted through multidrug-resistant protein (MRP) 3. Kitao et al. [48], reported that the expression of OATP 8 and MRP 3 in HCC cells significantly correlates with the signal intensity of HCCs in the hepatobiliary phase of gadoxetic acid-enhanced MR imaging and the degree of HCC differentiation. However, in step-wise carcinogenesis, DN also could show under- or over-expression of these transporters. In our study, hypointensity on gadoxetic acid-enhanced hepatobiliary phase images showed the highest sensitivity as well as the lowest specificity for differentiating small HCCs from benign nodules. Therefore, radiologists should carefully evaluate small lesions using multiple imaging features for the diagnosis of small HCCs, even though hepatobiliary phase images of gadoxetic acid-enhanced MR imaging have the potential to improve the detection and characterization of small hepatic lesions in patients with liver cirrhosis.
There were a few limitations of our study. First, because of its retrospective nature, there may have been selection bias in our patient population. Second, 33 benign nodules were not pathologically confirmed. Although the development of HCC within DN has been reported to occur within as little as 4 months [4, 49], the mean 16-month follow-up period may not be long enough to exclude very slow-growing HCCs. Obtaining a histopathological diagnosis in all cases would be desirable, but the usefulness of biopsy samples for small hepatic nodules is limited because of the difficulties in both carrying out the image-guided biopsy technique and the histopathological diagnosis of borderline lesions, such as DNs and well-differentiated HCCs [50, 51]. Third, the number of investigated benign nodules was relatively small, especially compared with the HCC sample size. Therefore, the results of this study need to be confirmed in a larger series.
In conclusion, for differential diagnosis of small (≤2 cm) HCCs from benign nodules in cirrhotic liver, gadoxetic acid-enhanced and DW MR imaging are valuable. In our study, hyperintensity on T2-weighted images, arterial enhancement on gadoxetic acid-enhanced MR images, and hyperintensity on DW images are helpful for differentiating small HCCs from benign nodules 2 cm or smaller in patients with liver cirrhosis. Two or three such findings have the potential to be an alternative to the AASLD practice guidelines when diagnosing small HCCs.
References
Parkin DM, Bray F, Ferlay J, et al. (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
Zaman SN, Melia WM, Johnson RD, et al. (1985) Risk factors in development of hepatocellular carcinoma in cirrhosis: prospective study of 613 patients. Lancet 1(8442):1357–1360
Llovet JM, Schwartz M, Mazzaferro V (2005) Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis 25(2):181–200
Sakamoto M, Hirohashi S, Shimosato Y (1991) Early stages of multistep hepatocarcinogenesis: adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol 22(2):172–178
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53(3):1020–1022
Bruix J, Sherman M (2005) Management of hepatocellular carcinoma. Hepatology 42(5):1208–1236
Sato T, Tateishi R, Yoshida H, et al. (2009) Ultrasound surveillance for early detection of hepatocellular carcinoma among patients with chronic hepatitis C. Hepatol Int 3(4):544–550
Ando E, Kuromatsu R, Tanaka M, et al. (2006) Surveillance program for early detection of hepatocellular carcinoma in Japan: results of specialized department of liver disease. J Clin Gastroenterol 40(10):942–948
Yamashita Y, Mitsuzaki K, Yi T, et al. (1996) Small hepatocellular carcinoma in patients with chronic liver damage: prospective comparison of detection with dynamic MR imaging and helical CT of the whole liver. Radiology 200(1):79–84
Kwak HS, Lee JM, Kim CS (2004) Preoperative detection of hepatocellular carcinoma: comparison of combined contrast-enhanced MR imaging and combined CT during arterial portography and CT hepatic arteriography. Eur Radiol 14(3):447–457
Huppertz A, Haraida S, Kraus A, et al. (2005) Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: correlation with histopathologic findings and spiral CT—initial observations. Radiology 234(2):468–478
Halavaara J, Breuer J, Ayuso C, et al. (2006) Liver tumor characterization: comparison between liver-specific gadoxetic acid disodium-enhanced MRI and biphasic CT—a multicenter trial. J Comput Assist Tomogr 30(3):345–354
Hammerstingl R, Huppertz A, Breuer J, et al. (2008) Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol 18(3):457–467
Huppertz A, Balzer T, Blakeborough A, et al. (2004) Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 230(1):266–275
Zech CJ, Grazioli L, Breuer J, et al. (2008) Diagnostic performance and description of morphological features of focal nodular hyperplasia in Gd-EOB-DTPA-enhanced liver magnetic resonance imaging: results of a multicenter trial. Invest Radiol 43(7):504–511
Zech CJ, Herrmann KA, Reiser MF, et al. (2007) MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent Gd-EOB-DTPA. Magn Reson Med Sci 6(1):43–52
Jung G, Breuer J, Poll LW, et al. (2006) Imaging characteristics of hepatocellular carcinoma using the hepatobiliary contrast agent Gd-EOB-DTPA. Acta Radiol 47(1):15–23
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188(6):1622–1635
Kim JE, Kim SH, Lee SJ, et al. (2011) Hypervascular hepatocellular carcinoma 1 cm or smaller in patients with chronic liver disease: characterization with gadoxetic acid-enhanced MRI that includes diffusion-weighted imaging. AJR Am J Roentgenol 196(6):W758–W765
Kim MJ, Lee M, Choi JY, et al. (2012) Imaging features of small hepatocellular carcinomas with microvascular invasion on gadoxetic acid-enhanced MR imaging. Eur J Radiol 81(10):2507–2512
Park MJ, Kim YK, Lee MW, et al. (2012) Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology 264(3):761–770
Park MJ, Kim YK, Lee MH, et al. (2013) Validation of diagnostic criteria using gadoxetic acid-enhanced and diffusion-weighted MR imaging for small hepatocellular carcinoma (≤2.0 cm) in patients with hepatitis-induced liver cirrhosis. Acta Radiol 54(2):127–136
Bland JM (2004) Cluster randomised trials in the medical literature: two bibliometric surveys. BMC Med Res Methodol 4:21
Hwang J, Kim SH, Lee MW, et al. (2012) Small (≤2 cm) hepatocellular carcinoma in patients with chronic liver disease: comparison of gadoxetic acid-enhanced 3.0 T MRI and multiphase 64-multirow detector CT. Br J Radiol 85(1015):e314–e322
Elsayes KM, Narra VR, Yin Y, et al. (2005) Focal hepatic lesions: diagnostic value of enhancement pattern approach with contrast-enhanced 3D gradient-echo MR imaging. Radiographics 25(5):1299–1320
Kogita S, Imai Y, Okada M, et al. (2010) Gd-EOB-DTPA-enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading and portal blood flow. Eur Radiol 20(10):2405–2413
Kim YK, Lee WJ, Park MJ, et al. (2012) Hypovascular hypointense nodules on hepatobiliary phase gadoxetic acid-enhanced MR images in patients with cirrhosis: potential of DW imaging in predicting progression to hypervascular HCC. Radiology 265(1):104–114
Hyodo T, Murakami T, Imai Y, et al. (2013) Hypovascular nodules in patients with chronic liver disease: risk factors for development of hypervascular hepatocellular carcinoma. Radiology 266(2):280–290
Yu MH, Kim JH, Yoon JH, et al. (2014) Small (≤1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology 271(3):748–760
Zhao XT, Li WX, Chai WM, Chen KM (2014) Detection of small hepatocellular carcinoma using gadoxetic acid-enhanced MRI: is the addition of diffusion-weighted MRI at 3.0T beneficial? J Dig Dis 15:137–145
Kim TK, Lee KH, Jang HJ, et al. (2011) Analysis of gadobenate dimeglumine-enhanced MR findings for characterizing small (1–2-cm) hepatic nodules in patients at high risk for hepatocellular carcinoma. Radiology 259(3):730–738
Kim DJ, Yu JS, Kim JH, et al. (2012) Small hypervascular hepatocellular carcinomas: value of diffusion-weighted imaging compared with “washout” appearance on dynamic MRI. Br J Radiol 85(1018):e879–e886
Nasu K, Kuroki Y, Tsukamoto T, et al. (2009) Diffusion-weighted imaging of surgically resected hepatocellular carcinoma: imaging characteristics and relationship among signal intensity, apparent diffusion coefficient, and histopathologic grade. AJR Am J Roentgenol 193(2):438–444
Taouli B, Koh DM (2010) Diffusion-weighted MR imaging of the liver. Radiology 254(1):47–66
Taouli B, Tolia AJ, Losada M, et al. (2007) Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. AJR Am J Roentgenol 189(4):799–806
Luciani A, Vibnaud A, Cavet M, et al. (2008) Liver cirrhosis: intravoxel incoherent motion MR imaging-pilot study. Radiology 249(3):891–899
Kim YK, Kim CS, Han YM, et al. (2011) Detection of liver malignancy with gadoxetic acid-enhanced MRI: is addition of diffusion-weighted MRI beneficial. Clin Radiol 66(6):489–496
Vandecaveye V, De Keyzer F, Verslype C, et al. (2009) Diffusion-weighted MRI provides additional value to conventional dynamic contrast-enhanced MRI for detection of hepatocellular carcinoma. Eur Radiol 19(10):2456–2466
Piana G, Trinquart L, Meskine N, et al. (2011) New MR imaging criteria with a diffusion-weighted sequence for the diagnosis of hepatocellular carcinoma in chronic liver disease. J Hepatol 55(1):126–132
Hamm B, Staks T, Muhler A, et al. (1995) Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology 195(3):785–792
Kim SH, Kim SH, Lee J, et al. (2009) Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol 192(6):1675–1681
Sun HY, Lee JM, Shin CI, et al. (2010) Gadoxetic acid-enhanced magnetic resonance imaging for differentiating small hepatocellular carcinomas (≤2 cm in diameter) from arterial enhancing pseudolesions: special emphasis on hepatobiliary phase imaging. Invest Radiol 45(2):96–103
Ahn SS, Kim MJ, Lim JS, et al. (2010) Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology 255(2):459–466
Frericks BB, Loddenkemper C, Huppertz A, et al. (2009) Qualitative and quantitative evaluation of hepatocellular carcinoma and cirrhotic liver enhancement using Gd-EOB-DTPA. AJR Am J Roentgenol 193(4):1053–1060
Saito K, Kotake F, Ito N, et al. (2005) Gd-EOB-DTPA enhanced MRI for hepatocellular carcinoma: quantitative evaluation of tumor enhancement in hepatobiliary phase. Magn Reson Med Sci 4(1):1–9
Fujita M, Yamamoto R, Takahashi M, et al. (1997) Paradoxic uptake of Gd-EOB-DTPA by hepatocellular carcinoma in mice: quantitative image analysis. J Magn Reson Imaging 7(4):768–770
Motosugi U, Ichikawa T, Sou H, et al. (2010) Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging. Radiology 256(1):151–158
Kitao A, Zen Y, Matsui O, et al. (2010) Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR Imaging—correlation with molecular transporters and histopathologic features. Radiology 256(3):817–826
Takayama T, Makuuchi M, Hirohashi S, et al. (1990) Malignant transformation of adenomatous hyperplasia to hepatocellular carcinoma. Lancet 336(8724):1150–1153
Wanless IR (2007) International consensus on histologic diagnosis of early hepatocellular neoplasia. Hepatol Res 37(Suppl 2):S139–S141
Kojiro M (2007) Diagnostic discrepancy of early hepatocellular carcinoma between Japan and West. Hepatol Res 37(Suppl 2):S121–S124
Acknowledgments
The authors thank Dr. Hwa Jung Kim for help with statistics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwon, HJ., Byun, J.H., Kim, J.Y. et al. Differentiation of small (≤2 cm) hepatocellular carcinomas from small benign nodules in cirrhotic liver on gadoxetic acid-enhanced and diffusion-weighted magnetic resonance images. Abdom Imaging 40, 64–75 (2015). https://doi.org/10.1007/s00261-014-0188-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0188-8