Abstract
Objective
The aim of our study was to evaluate the effectiveness of MR imaging for the characterization of small (<2 cm) renal lesions described as indeterminate on prior US or CT
Materials and methods
Sixty-three small renal masses in 51 patients considered indeterminate on prior ultrasound or CT scans were included in the study. A retrospective evaluation of the examinations was performed independently by two body magnetic resonance imaging (MRI) radiologists who were unaware of the final diagnosis. A 3-point confidence scale (1: benign, 2: indeterminate, and 3: malignant) was established to determine the level of suspicion for malignancy. Interobserver agreement was determined with a weighted kappa statistic. The diagnosis was verified by imaging follow-up of at least 24 months (mean 60 months) in 53 lesions and by pathology in 10 lesions.
Results
MRI detected all eight malignancies in the series. There were eight malignant lesions and two benign lesions among those with pathologic follow-up. No interval growth or evidence of malignancy in the remaining 53 lesions was found for a minimum of 24 months by repeat imaging. The sensitivity, specificity, positive predictive value, and negative predictive value of MRI for differentiating benign from malignant small renal lesions were 100% (62.9–100%, 95% CI), 94.5% (84.9–98.8%, 95% CI), 72.7% (39.1–93.6%, 95% CI), and 100% (93.1–100%, 95% CI), respectively. The kappa value for interobserver agreement was 0.77 (95% CI 0.59–0.96, p-value <0.001).
Conclusion
MR imaging is an effective method for characterizing small (<2 cm) renal masses found to be indeterminate by US or CT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The widespread use of cross-sectional imaging has led to a growing number of incidentally detected renal tumors, many of which are found during routine ultrasound (US) and contrast-enhanced computed tomography (CT) examinations [1]. The incidence of these lesions has been rising in the past decades [2]. If any of these lesions are found to be solid by US or enhancing by CT, the likelihood of malignancy is high.
In our experience, when incidentally detected renal lesions are smaller than 2 cm, the characterization of these lesions by US and CT can be challenging. By sonography, technical factors may result in lesions appearing indeterminate in nature due to impaired visualization. For example, a renal cyst may appear to contain internal echoes due to a sonographic window limited by the patient’s body habitus, and thus be called indeterminate in nature, requiring further imaging, either with CT or magnetic resonance imaging (MRI), or with sequential follow-up. By CT, the presence of enhancing soft tissue components in renal lesions is the key determinant of malignancy. However, for lesions measuring less than 2 cm, artifacts related to beam hardening or partial volume averaging can make the assessment for true enhancement difficult, even with dedicated renal mass protocol CT, and render the lesion no better characterized than that found on the initial imaging [3].
With technical advances including improved spatial resolution which allows for the creation of isotropic voxels and multiplanar imaging, and the intrinsic contrast resolution benefits conferred by multiple sequences optimized for tissue specific characterization, MRI is a powerful modality that enables radiologists to diagnose lesions that can be indistinguishable by US and CT. While MRI has been found to be an effective modality for the characterization of small liver lesions (<2 cm) due to its superior contrast resolution [4, 5], the value of MRI for characterizing small renal lesions found to be indeterminate at US or single-phase contrast-enhanced CT is yet to be described. The purpose of our study is to evaluate the performance of MRI for the characterization of small renal lesions (<2 cm) that are found to be indeterminate on a prior CT or US.
Materials and methods
Cohort description
This retrospective study was approved by the institutional review board. Patient consent was waived.
A review of the MRI database for individuals who underwent renal mass MRI between January 2001 and November 2007 using the keywords “renal mass” and “MRI” yielded 647 studies. Of these, 112 patients had a MRI study for the characterization of renal lesions measuring less than 2 cm because the CT or US report declared the renal lesions as “indeterminate” (n = 44) or “too small to characterize” (n = 68). From these 112 patients, 51 patients with 63 lesions were found to have had imaging follow-up for at least 2 years or resection or biopsy yielding a pathology diagnosis. These 63 lesions were the lesions evaluated in this study.
MR imaging protocol
MR imaging was performed on a 1.5-T unit (Signa; General Electric Medical Systems, Milwaukee, WI). The sequences were performed as follows:
-
1.
Coronal heavily T2-weighted single-shot fast spin echo (FSE) without fat suppression (TR/TE, infinite/180 ms; slice thickness, 8 mm/0 interslice gap; matrix, 256 × 128–160)
-
2.
Axial T1-weighted dual-echo spoiled gradient-echo sequence (TR/TE150/2.1 ms; 150/4.2 ms; slice thickness, 6–8 mm with no interslice gap; matrix, 256 × 160)
-
3.
Axial T2-weighted FSE with fat suppression and respiratory triggering (>3000/96 ms; slice thickness, 6–8 mm; matrix, 256 × 224)
-
4.
Coronal T1-weighted pre- and post-dynamic gadolinium-enhanced imaging using three-dimensional (3-D) spoiled gradient echo with fat suppression (TR 2–4 ms/TE range 1–2 ms; flip angle, 12°; section thickness, 3; matrix, 384 × 160). Imaging was performed in the cortico-medullary, nephrographic, and excretory (3 min following contrast administration) phases. Subtraction of pre- from post-gadolinium imaging for all three dynamic phases was performed by the readers on a workstation.
-
5.
Post-contrast delayed excretory phase axial T1-weighted 2-dimensional spoiled gradient echo with fat suppression (TR 100–180 ms/TE range 1.8–2.4 ms; flip angle, 70°; slice thickness, 5–6). Gadolinium was administered up to a maximum dose of 20 mLs at a rate of 2 mL/s followed by 15 mL/s of saline flush.
Imaging interpretation
Each case was reviewed retrospectively and independently by two body MRI radiologists with ten (reader 1) and three (reader 2) years of experience. Case review was performed without prior knowledge of the original interpretation of the studies or the final diagnosis. Each lesion was graded according to a 3-point scale, with grade 1 = benign, grade 2 = indeterminate, and grade3 = malignant. Cases in which the readers disagreed were reviewed and regraded by consensus. Follow-up by imaging was performed either because this was recommended in the original MRI interpretation, or because imaging was performed at a later date for some other reason, and the kidneys were included in the field of view.
The following features were used by the readers to characterize and grade renal lesions:
Grade 1 (Benign)
Simple cyst: a well-demarcated lesion with low signal intensity (SI) relative to the renal cortex on T1-weighted imaging, no visible change in the SI of the lesion between out-of-phase (OP) and in-phase (IP) imaging, high SI on T2-weighted imaging, and no internal enhancement after contrast confirmed on subtraction imaging. Nephrographic and excretory phase images were chosen for subtraction to ensure enhancement of slowly enhancing lesions.
Minimally complicated cyst: a lesion fulfilling the criteria for a simple cyst and additionally possesses 1-3 hairline internal septations which may enhance with contrast (5).
Hemorrhagic or proteinaceous cyst: a non-enhancing cyst with uniform high SI relative to renal cortex on T1-weighted imaging and high or low SI on T2-weighted imaging. The high SI on T1-weighted imaging could be dependent, indicating layering hemorrhage or proteinaceous material.
Classic angiomyolipoma (AML): a lesion with high SI relative to renal parenchyma on in-phase T1-weighted imaging which suppresses on T1-weighted imaging with fat suppression indicating the presence of macroscopic fat shows a complete or incomplete ring of signal loss at its interface with normal renal parenchyma due to phase cancelation (“India ink” or “etching artifact”), and may enhance with contrast.
Grade 2 (Indeterminate)
Complex cyst (indeterminate): a cyst containing minimal thickening (greater than hairline-thin) of the septae or wall.
Non-classic AML: An enhancing lesion showing signal dropout on OP imaging in comparison with IP imaging but not demonstrating suppression with fat suppression, and without an india ink artifact at the lesion to normal renal parenchyma interface, indicating the presence of microscopic but not macroscopic fat. These lesions are generally of low SI relative to renal parenchyma on T2-weighted imaging (6).
Grade 3 (Malignant)
Enhancing lesion regardless of T1-weighted and T2-weighted signal characteristics, with the only exception being a lesion exhibiting the signal characteristics for a classic AML.
Statistical methods
Continuous variables were summarized using means with range given. Sensitivity, specificity, and positive and negative predictive values were calculated with 95% CI to assess the performance of MRI to differentiate benign from malignant lesion. Grade 2 lesions (indeterminate) were presumed to be malignant as they merited serial follow-up or biopsy owing to their equivocal imaging characteristics. A weighted kappa was calculated to assess interobserver agreement on grading of the lesions by MRI. A probability (P) value of 0.05 or smaller was considered significant for all hypothesis tests. The above procedures were performed using SAS 9.2, Cary, NC, and an online calculator (http://www.medcalc.org/calc/diagnostic_test.php, accessed February 1, 2014).
Results
Patient population
The mean age was 58 years (range 18–92 years) with 24 males (mean 62 years, range 18–92 years), 27 females (mean 55 years, range 31–79 years). Forty-five patients had one lesion, two patients had two lesions, two patients had three lesions, and two patients had four lesions, for a total of 63 lesions in 51 patients.
Initial imaging
The initial imaging in which the 63 total lesions were found to be indeterminate was sonography in 31 lesions (49.2%), single-phase (portal venous) contrast-enhanced CT in 16 lesions (25.4%), and renal mass CT (noncontrast, nephrographic, and excretory phases of enhancement) in 16 lesions (25.4%) (Flow Chart 1). The mean size of indeterminate lesions was 13.6 mm (range 3–19 mm) by US, 11.6 mm (range 3–19 mm) by single-phase contrast-enhanced CT, and 12.5 mm (range 3–17 mm) by renal mass CT. Overall, the mean lesion size was 13 mm (range 3–19 mm). 18 lesions measured less than 10 mm (28.5%).
The reasons for calling a lesion indeterminate on US were if it did not fulfill the sonographic criteria for a simple cyst, defined as a rounded anechoic lesion with a thin, imperceptible wall and posterior acoustic enhancement, or it demonstrated irregular margins. On CT, the reasons for an indeterminate renal lesions were an attenuation value higher than simple fluid which was defined as >20 Hounsfield units (HU) on a single-phase contrast-enhanced CT, and evidence of enhancement on renal mass CT which was defined as >10 HU increase from noncontrast to post-contrast images.
MRI imaging for each grade
Grade 1 lesions (n = 52)
For grade 1 lesions, the average maximum dimension was 12 mm. Sixteen of these 41 lesions measured less than 10 mm. 13 were initially imaged by single-phase contrast CT and demonstrated HU > 20. 23 were imaged by US and did not show posterior acoustic enhancement. Five were imaged by renal mass CT and demonstrated enhancement of greater than 10 HU.
All grade 1 lesions were reimaged at a minimum of 24 months after the original MRI without evidence of development of malignancy. 11 of the 52 lesions (21.1%) were identified as AML on the initial MRI due to their typical imaging characteristics including chemical fat suppression (n = 10) or phase cancelation at the margins of the lesion with adjacent renal parenchyma (n = 1) (Fig. 1). The remaining 41 lesions (78.8%) were regarded as simple or minimally complicated cysts which required no imaging follow-up, but which were subsequently imaged for other indications.
Angiomyolipoma. 51-year-old female with a history of salpingo-oophorectomy and right loin pain. An 11 mm lesion with a small focus of enhancement was seen on CT (A) (arrow) in the right kidney and described as “too small to characterize.” In (B) and opposed (C) phase dual-echo T1-weighted imaging shows a complete ring of India ink artifact around the lesion, at the interface of the fat-containing angiomyolipoma and the renal parenchyma (arrows). MRI initial interpretation and review by the authors described this as an angiomyolipoma, or grade 1 lesion. An MRI 31 months later (not shown) demonstrated stability of the lesion.
Grade 2 lesions (n = 3)
The first lesion, measuring 12 mm, demonstrated MRI characteristics that were suggestive but not diagnostic of AML. The lesion was hyperintense on T1-weighting, hypointense on non-fat suppressed T2-weighted imaging, and enhanced with contrast. It demonstrated that no fat saturation on chemically fat suppressed T1-weighted imaging or evidence of India ink artifact, but did show signal drop out on opposed-phase imaging indicating the presence of microscopic fat. This lesion was followed at 6 months and then annually for 3 years. There was no growth and the lesion continues to be suggestive of AML.
The second lesion, measuring 16 mm lesion, was initially identified on a standard contrast-enhanced CT that was performed for an unrelated indication (bloody diarrhea). Both the initial standard CT and the subsequent renal mass CT found the lesion to be indeterminate due to an attenuation value of >20 HU and the presence of enhancement, respectively. By MRI, the lesion was isointense relative to renal parenchyma on T1-weighting and hypointense on T2-weighting. There was no fat suppression or definite signal loss on opposed-phase imaging to suggest lipid content. The India ink artifact was incomplete at the interface with adjacent renal parenchyma. With contrast, there was rim enhancement as well as some enhancement peripherally. A biopsy was performed which revealed an AML.
The third lesion, a 12-mm lesion in a 66-year-old male, was initially identified on an unenhanced CT and further evaluated with a renal mass CT. The renal mass CT was unable to distinguish a “pseudoenhancing” lesion from a renal mass, and so an MRI was recommended. This showed a lesion with some central enhancement and raised the possibility of renal cell carcinoma. The clinical service opted for imaging follow-up. For the subsequent 24 months, this lesion remained stable in size but demonstrated persistent enhancement for which continued imaging follow-up was recommended. The patient was lost to follow-up, but reappeared at 61 months after the initial MRI to have a CT study for an unrelated purpose which showed enlargement of the lesion. A second MRI five months later demonstrated growth from 12 to 15 mm in maximum dimension. This was biopsied, found to be a papillary renal cell carcinoma, and treated with cryoablation.
Grade 3 lesions (n = 8)
Of the grade 3 lesions, seven of eight were confirmed malignant by surgical pathology. Five were clear cell carcinoma, one was papillary cell carcinoma, and one was renal cell carcinoma, unclassified. All seven malignant lesions (identified in six patients) demonstrated enhancement on post-contrast imaging were correctly identified by MRI, and successfully resected (Fig. 2).
Renal cell carcinoma. 52-year-old female with a history of abdominal pain. A 16-mm hyperdense lesion was seen on pre- and post-contrast CT in the left kidney and described as demonstrating equivocal enhancement (A, B) (arrows). MRI images show a lesion of intermediate SI on pre-contrast coronal spoiled gradient-echo imaging (C), and mixed iso- or low SI on post-contrast images (D). A subtracted image shows enhancing nodularity and septations within the lesion (E) (arrows). The mass was resected and proved to be a clear cell carcinoma.
The 8th lesion was a 9-mm mass seen on a standard post-contrast CT and found to be indeterminate due to an attenuation value >20 HU. The follow-up renal mass CT was unable to distinguish whether the increase in enhancement following contrast represented true enhancement in a solid mass or pseudoenhancement in a benign lesion. MRI demonstrated faint enhancement in the mass (Fig. 3). Biopsy revealed a metanephric adenoma.
Metanephric adenoma. 47-year-old female who underwent a renal mass protocol CT for a lesion first seen on an outside hospital CT. The CT report indicated that the mass was too small to characterize. Unenhanced CT demonstrates an isodense 9 mm lesion which becomes hypodense on post-contrast CT, and which demonstrates an increase of 16 HU following contrast administration (A, B) (arrows). MRI demonstrated isointensity on the pre-contrast T2-(C) and T1-weighted spoiled gradient-echo images (D), and subtle enhancement post-contrast (E). Subtraction imaging helped to confirm the enhancement (F) (arrows). The mass, biopsied prior to ablation, was a metanephric adenoma.
Verification of diagnosis
Histological confirmation of diagnosis was obtained for 10 of the 63 lesions, three by percutaneous biopsy under US guidance, and seven by surgical resection. Follow-up imaging for the remaining 53 lesions was performed specifically because the lesions were indeterminate, or because unrelated imaging was performed over the subsequent years (mean 60 months, range 24–114 months). Final imaging follow-up was by US for 11 lesions, by MRI for 22 lesions, and by contrast-enhanced CT for 20 lesions.
MRI test performance and interobserver agreement
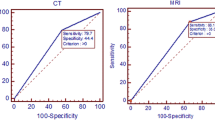
The sensitivity, specificity, and positive and negative predictive values with 95% confidence intervals (CI) for the ability of MRI are summarized in Table 1.
The two readers were in agreement over the grades assigned to 58 of the 63 lesions. Of these 58 lesions, 48 were grade 1, three were grade 2, and seven were grade 3. The readers disagreed over the grade assigned to five lesions. Four of these lesions were graded as a grade 1 by reader 1 and grade 2 by reader 2. All four were regraded as grade 1 on consensus read. One mass was graded as grade 2 by reader 1 and grade 3 by reader 2, and regraded as grade 3 on consensus read. This lesion displayed subtle enhancement on subtraction imaging which were not definitive and proved to be a metanephric adenoma at pathology.
The weighted kappa value was 0.77 (95% CI 0.59–0.96) which was statistically significant (p-value <0.001).
Discussion
Incidentally detected renal lesions are commonly encountered in daily clinical practice. Follow-up imaging is usually recommended if the lesion cannot be fully characterized, and can be performed with renal mass CT or MRI. By the ACR Appropriateness Criteria, although both CT and MRI are both considered appropriate for the assessment of indeterminate renal masses, CT is rated slightly higher than MRI with the recognized limitation that small renal masses (<1.5 cm) can be problematic because the effects of partial voluming averaging can make it difficult to assess the attenuation value and for enhancement [7]. The phenomenon of “pseudoenhancement” describes artificially increased attenuation values in small renal lesions in the presence of intravenous contrast, especially when lesions are centrally located in the parenchyma. These diagnostic dilemmas were observed in our study, as 16 of the lesions remained indeterminate for these reasons despite renal mass CT. Following MRI, three of these lesions remained indeterminate. In addition, 47 of the lesions that were incompletely characterized by US or single-phase enhanced CT were correctly graded following MRI. These results illustrate the diagnostic power of MRI due to its superior tissue contrast characteristics and suggest that MRI may be the preferred modality over US, single-phase contrast-enhanced CT, and even renal mass CT when one is confronted with a small indeterminate renal lesion in routine practice and deciding which modality to recommend.
Almost all malignant renal masses can be distinguished from benign cysts by the presence of enhancement. Most enhancing lesions can be identified on subjective visual comparison of unenhanced and enhanced images [6]. For lesions which are not immediately recognizable as enhancing, regions of interest can be applied to both sequences provided that the imaging parameters are kept constant [8]. This technique can be problematic in small lesions [3]. Image subtraction is widely accepted in both breast and angiographic MRI, and has recently gained acceptance in the evaluation of the renal mass [9]. It is particularly useful in the evaluation of lesions which are hyperintense on pre-contrast imaging [6]. In our study, subjective visual comparison, relative enhancement, and subtraction were all used to help evaluate the renal lesions. However, subtle features of complexity or enhancement can be problematic and lead to interobserver variability in determining whether a lesion is benign.
The Bosniak classification [10, 11] includes two categories (I and II) which describe benign cysts, two categories which merit consideration of surgical intervention (III and IV), and the IIF category which leads to a recommendation for follow-up. Our grading system for MRI echoes the management recommendations of the Bosniak classification, categorizing benign lesions requiring no further follow-up as grade 1, indeterminate lesions recommended for imaging follow-up as grade 2 (similar to the Bosniak category IIF lesions), and lesions suspicious for malignancy and meriting surgical evaluation as grade 3.
There were two cases of benign lesions that underwent biopsy. A 16-mm AML demonstrated no evidence of macroscopic or microscopic fat on MRI, making it impossible to assign a diagnosis of AML [12, 13]. The other biopsied benign lesion was a metanephric adenoma which is an uncommon renal tumor that arises in young people and can be regarded as a benign entity with only rare occurrence of malignant transformation [14, 15]. It is unlikely that metanephric adenoma can be prospectively distinguished from renal cell carcinoma based on imaging features [16].
Three AMLs in our series were graded as 2. In each of these cases, there was an absence of a complete india-ink effect between the lesion and the normal renal parenchyma. One of these underwent biopsy. These results illustrate the difficulty which small AML can present. It remains likely that biopsy will be required in a small proportion of these benign tumors [17].
Our study demonstrates that MRI can characterize small renal lesions (<2 cm) as benign or malignant with high sensitivity, specificity, and negative predictive value. Of the 63 lesions, only three were categorized as indeterminate based on MRI. The lower value for positive predictive value reflect the findings in the study in which certain AMLs and rare, benign lesions such as metanephric adenoma are difficult to characterize by MRI and may require biopsy for definitive diagnosis. The substantial interobserver agreement indicates that there is good reliability between radiologists on the interpretation of MRI studies performed for the characterization of small renal lesions that are indeterminate by US or CT.
Eighteen of our 63 lesions measured less than 10 mm at initial imaging. Half of these were solid enhancing lesions (eight AMLs, one metanephric adenoma). The American College of Radiology white paper on incidental imaging findings [18] recommends that solid lesions measuring less than 10 mm be followed at regular intervals for up to 5 years. However, the use of MRI in these nine cases led to the correct categorization of all eight AMLs, and the categorization of the metanephric adenoma as a grade 3 lesion. These patients were, therefore, spared several years of follow-up imaging. The remaining nine lesions measuring less than 10 mm were correctly graded by MRI. These results suggest that the use of MRI to characterize small indeterminate renal lesions may reduce or obviate the need for follow-up imaging which would spare patients the time, cost, and anxiety of following indeterminate lesions. A larger study is needed to better investigate this.
Our retrospective study did not include small renal lesions that were considered frankly benign or malignant on the initial US or single-phase CT, thus creating a selection bias.
Another limitation is that MRI was compared to renal mass CT for only 16 of the 63 (25%) lesions, but compared to US or single-phase contrast-enhanced CT for the remainder of the lesions. However, it is worthy of note that of the 16 cases which were not characterized by renal mass CT, 8 were characterized as grade 1 lesions by both readers and 2 were correctly identified as surgical lesions by both readers, both renal cell carcinoma. Of the remaining 6, two were graded as grade 2 by one reader and grade 1 by the other, and both were determined to be grade 1 by consensus read. The metanephric adenoma was included in this group, initially graded 3 and 2, and by consensus graded as a 3. The three remaining lesions were graded as 2 by both readers. Two of them were AML, and one was an RCC. MRI was, therefore, able to fully characterize 13 of 16 lesions which underwent earlier study by renal mass protocol CT.
It is possible that the lesions that were indeterminate at US or single-phase contrast-enhanced CT may have been adequately characterized by renal mass CT. A larger study would be helpful to further evaluate this and to fully determine the test performance of MRI to renal mass CT.
Although the mean follow-up period was 52 months, the minimum period of follow-up was 24 months. This represents a significant limitation of our study as it is well known that renal cell carcinoma grow indolently with average growth rates of less than 1 mm per year [19–21]. It remains possible that some of the lesions may have been misdiagnosed as benign due to the short-term follow-up. Renal cell carcinoma has been known to lie dormant for up to 5 years before starting to grow again(8, 14,). This was exemplified by the grade 2 lesion that stayed stable in size for 24 months and was ultimately determined to be a papillary renal cell carcinoma 67 months after the initial MR study, confirming the need for careful follow-up.
In conclusion, our study suggests that MR imaging is effective in the characterization of small renal masses (<2 cm) found to be indeterminate at US or single-phased contrast-enhanced CT. Furthermore, MRI may be more effective than renal mass CT for the characterization of small renal masses.
References
Volpe A, Panzarella T, Rendon RA, et al. (2004) The natural history of incidentally detected small renal masses. Cancer 100(4):738–745
Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK (2006) Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 98(18):1331–1334
Maki DD, Birnbaum BA, Chakraborty DP, et al. (1999) Renal cyst pseudoenhancement: beam-hardening effects on CT numbers. Radiology 213(2):468–472
Mueller GC, Hussain HK, Carlos RC, Nghiem HV, Francis IR (2003) Effectiveness of MR imaging in characterizing small hepatic lesions: routine versus expert interpretation. AJR Am J Roentgenol 180(3):673–680
Gabr AH, Gdor Y, Roberts WW, Wolf JS Jr (2009) Radiographic surveillance of minimally and moderately complex renal cysts. BJU Int 103(8):1116–1119
Israel GM, Bosniak MA (2005) How I do it: evaluating renal masses. Radiology 236(2):441–450
http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IndeterminateRenalMasses.pdf. Accessed Feb 10, 2014
Ho VB, Allen SF, Hood MN, Choyke PL (2002) Renal masses: quantitative assessment of enhancement with dynamic MR imaging. Radiology 224(3):695–700
Hecht EM, Israel GM, Krinsky GA, et al. (2004) Renal masses: quantitative analysis of enhancement with signal intensity measurements versus qualitative analysis of enhancement with image subtraction for diagnosing malignancy at MR imaging. Radiology 232(2):373–378
Bosniak MA (1986) The current radiological approach to renal cysts. Radiology 158(1):1–10
Israel GM, Bosniak MA (2003) Follow-up CT of moderately complex cystic lesions of the kidney (Bosniak category IIF). AJR Am J Roentgenol 181(3):627–633
Bosniak MA (1981) Angiomyolipoma (hamartoma) of the kidney: a preoperative diagnosis is possible in virtually every case. Urol Radiol 3(3):135–142
Uhlenbrock D, Fischer C, Beyer HK (1988) Angiomyolipoma of the kidney. Comparison between magnetic resonance imaging, computed tomography, and ultrasonography for diagnosis. Acta Radiol 29(5):523–526
Picken MM, Curry JL, Lindgren V, Clark JI, Eble JN (2001) Metanephric adenosarcoma in a young adult: morphologic, immunophenotypic, ultrastructural, and fluorescence in situ hybridization analyses: a case report and review of the literature. Am J Surg Pathol 25(11):1451–1457
Drut R, Drut RM, Ortolani C (2001) Metastatic metanephric adenoma with foci of papillary carcinoma in a child: a combined histologic, immunohistochemical, and FISH study. Int J Surg Pathol 9(3):241–247
Fielding JR, Visweswaran A, Silverman SG, Granter SR, Renshaw AA (1999) CT and ultrasound features of metanephric adenoma in adults with pathologic correlation. J Comput Assist Tomogr 23(3):441–444
Sahni VA, Ly A, Silverman SG (2011) Usefulness of percutaneous biopsy in diagnosing benign renal masses that mimic malignancy. Abdom Imaging 36(1):91–101
Berland LL, Silverman SG, Gore RM, et al. (2010) Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol 7(10):754–773
Chawla SN, Crispen PL, Hanlon AL, et al. (2006) The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 175:425–431
Mason RJ, Abdolell M, Trottier G, et al. (2011) Growth kinetics of renal masses: analysis of a prospective cohort of patients undergoing active surveillance. Eur Urol 59(5):863–867
Kato M, Suzuki T, Suzuki Y, et al. (2004) Natural history of small renal cell carcinoma: evaluation of growth rate, histological grade, cell proliferation and apoptosis. J Urol 172(3):863–866
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Willatt, J.M., Hussain, H.K., Chong, S. et al. MR imaging in the characterization of small renal masses. Abdom Imaging 39, 761–769 (2014). https://doi.org/10.1007/s00261-014-0109-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0109-x