Abstract
Purpose
To determine the yield of CT urography (CTU) in the surveillance of patients with bladder cancer following cystectomy.
Materials and methods
In this IRB-approved, HIPAA-compliant, retrospective study of 5,404 CT urograms performed at our institution between March 2000 and February 2011, 225 CT urograms were performed in 105 patients [79 men, 26 women; mean age 65 years (43–85)] following cystectomy for bladder cancer. Median follow-up after cystectomy was 63 months (range 1–234), median time between cystectomy and CTU was 39 months (range 0–229), median follow-up after CTU was 34 months (range 1–111). CTU examinations were reviewed by two radiologists in consensus and findings were categorized into those related to surgery, locoregional recurrence, metastases, or metachronous upper tract urothelial tumor (UTT).
Findings
Findings were present in 69 (65.7 %) of 105 patients, including findings related to surgery in 60 (57.1 %) patients, locoregional recurrence or metastatic disease in 21 (20 %) patients, and UTT in 3 (2.9 %) patients. Of surgery-related findings, hydronephrosis (23/105, 21.9 %) and parastomal hernia (17/105, 16.2 %) were the most common findings. Visceral metastases (16/105, 15.2 %) and lymph node metastases (13/105, 12.4 %) were the most common manifestations of recurrent disease.
Conclusion
CTU findings in the surveillance of patients with bladder cancer after cystectomy are common and include those related to surgery, spread of the disease, and metachronous tumors. Our study supports current published guidelines on the use of CTU in these patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
With the exception of prostate cancer, bladder cancer, with an estimated 72,570 new cases in 2013, is the most common malignancy of the urinary tract [1]. Superficial, non-muscle-invasive bladder cancer accounts for 70 %–80 % of cases, and muscle-invasive bladder cancer accounts for the rest. Radical cystectomy and lymphadenectomy, with or without adjuvant chemotherapy, is the standard of care for operable patients with muscle-invasive bladder cancer without metastatic disease [2–4]. Bladder cancer is an important health care problem; 14,880 deaths were attributable to bladder cancer in the United States in 2012, and five-year recurrence-free survival rates following radical cystectomy with lymphadenectomy are only 58 %–68 % [1, 5, 6].
Imaging following cystectomy is needed to assess for surgical complications of urinary diversion procedures, and to detect recurrent disease and metachronous upper tract urothelial tumors (UTT). Complications of urinary diversion procedures are common and include post-operative leaks (in approximately 4 % patients), ureteral or anastomotic strictures leading to obstructive uropathy (in 1 %–30 %), calculi formation (in approximately 10 %), and parastomal hernias (in 10 %–22 %) [7, 8]. Locoregional recurrence of bladder cancer following cystectomy develops in 5 %–15 % patients [9, 10]. While the disease typically recurs within 2 years following cystectomy, a recurrence may not be detected until 5 years, or rarely after 5 years [4]. Distant metastases develop in about 50 % of patients, also usually within 2 years; 5-year survival of patients with metastatic disease is only 7 %–15 % [3, 9, 11]. Early detection of metastatic disease, and the subsequent initiation of chemotherapy can improve the prognosis in patients with invasive bladder cancer [4]. Surveillance imaging is important also to detect upper tract cancers that develop following cystectomy in 0.75 %–7 % of patients [12–14]. While metachronous UTT occur at a median interval of 3–3.3 years after cystectomy, some may occur as late as 9 years [15, 16].
Overall, CT urography (CTU) has been reported to detect 78 %–98 % of renal pelvis tumors and 19 %–53 % of ureteral tumors [17]; however, the yield of CTU in patients after cystectomy is not known [18]. There is also lack of agreement regarding the need of imaging, the imaging test of choice, and the frequency with which patients should be imaged to detect UTT [14]. Nevertheless, the ACR appropriateness criteria recommend CTU as the most appropriate imaging test for follow-up of invasive bladder cancer after cystectomy [19]. The purpose of this study was to determine the yield of CTU in the surveillance of patients with bladder cancer following cystectomy.
Materials and methods
Subjects
In this institutional review board approved, Health Insurance Portability and Accountability Act-compliant, single center retrospective study, we reviewed 5,404 CT urograms performed at our institution between March 2000 and February 2011. Of these, 225 CT urograms were performed to evaluate 105 patients (79 men, 26 women; mean age 65 years, range 43–85 years) with bladder cancer following cystectomy. Median clinical follow-up following cystectomy was 63 months (range 1–234). The median time between cystectomy and CTU was 39 months (range 0–229) and median follow-up after CTU was 34 months (range 1–111).
CT urography technique
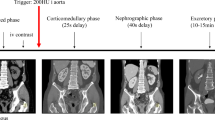
CT urograms were performed using 4-, 16-, or 64-detector CT scanners (Somatom Volume Zoom, Somatom Sensation 16, and Somatom Sensation 64; Siemens Medical Solutions, Forcheim, Germany). All patients were scanned in the supine position, received 900 mL of water orally, and were asked to void prior to the examination. All patients were scanned using a single-bolus, three-phase protocol which included an unenhanced scan (0.6–2.5-mm collimation, pitch of 0.875–1.25, 120 kVp, and 155–280 mA) of the abdomen and pelvis, a nephrographic phase scan (0.6–2.5-mm collimation, pitch of 0.875–1.25, 120 kVp, and 155–280 mA) of the kidneys 100 s after intravenous administration of 100 mL of 300 mgI/mL of iopromide (Ultravist; Bayer Healthcare Pharmaceuticals, Wayne, NJ), and an excretory phase scan (0.6–1.0-mm collimation, pitch of 0.65–1.00, 120 kVp, and 160–280 mA) of the abdomen and pelvis 15 min after contrast medium injection [20]. Automatic exposure control (CareDose4D; Siemens Healthcare, Forchheim, Germany) was used to optimize the radiation dose. The excretory phase scans were reconstructed in the axial plane at 3–5-mm-thick sections, and reformatted in the coronal and sagittal planes at 3-mm-thick sections. CT urograms were supplemented with either 10 mg of intravenous furosemide (Lasix; Abbott Laboratories, North Chicago, IL) administered 2–3 min before contrast medium injection, or 250 mL of intravenous normal saline infused by gravity after administration of intravenous contrast medium, or both.
Of 105 patients examined with 225 CT urograms; 50 had one, 23 had two, 12 had three, 12 had four, four had five, three had six, and one had seven CT urograms during the study period. Thus, 55 (52.4 %) patients had more than one CT urogram, and eight (7.6 %) had five or more CT urograms.
Data collection
All CT urograms were reviewed by two abdominal radiologists in consensus and findings were categorized into those related to cystectomy and urinary diversion, locoregional recurrence, metastatic disease, and new, UTT. All recorded CTU findings were verified by reviewing medical records and using one of the following criteria: stability on follow-up imaging obtained at least 12 months or more after the finding was detected, unequivocal progression or resolution of suspected recurrent or metastatic tumor, histopathology (following percutaneous biopsy or surgery), or other non-imaging tests such as cystoscopy yielding definitive results. The timing of each confirmed CTU finding was noted in relation to the date of the cystectomy.
Results
Of 105 patients, confirmed findings were detected with CTU in 69 (65.7 %) patients. Findings related to surgery were seen in 60 (57.1 %) patients, locoregional or distant recurrence of bladder cancer in 21 (20 %), and UTT in three (2.9 %); 15 patients had findings in more than one category. Of findings related to surgery, new hydronephrosis was seen in 23 (21.9 %) patients, parastomal hernia in 17 (16.2 %), and calculi formation in 11 (10.5 %) patients; these were the most common findings related to cystectomy and urinary diversion (Table 1). Of 60 patients with findings related to surgical complications, five (8.3 %) required surgery (Fig. 1).
72-year-old man following cystectomy for bladder cancer presenting with abdominal pain and high output from the surgical drain. CT urography performed on post-operative day two showed urinary leak. A Axial image in nephrographic phase shows dilated right ureter (arrowhead) and renal calyx. Periureteral fluid and stranding are noted (arrow), with fluid along the inferior vena cava (curved arrow). Perihepatic fluid is present. B Axial image from the excretory phase shows dilated right intrarenal collecting system (long thin arrow), a dilated unopacified right ureter (arrowhead), and contrast material outside the ureter (arrow) and along the inferior vena cava (curved arrow). C Coronal reformatted image in excretory phase again demonstrates dilated unopacified right ureter (arrowhead) with extraluminal contrast material surrounding the ureter (arrows).
Among patients with locally recurrent or metastatic bladder cancer (30 findings in 21 patients), visceral metastases were seen in 16 (15.2 %) patients, lymph node metastases in 13 (12.4 %), and a pelvic recurrence was seen in one (1 %) patient. Of these 21 patients, seven had coexistent nodal and distant metastases and one had local recurrence with nodal and distant metastases.
An UTT was found in three (2.9 %) patients. Of these, two had tumors in the renal pelvis (Fig. 2) and one had a tumor in the proximal ureter (Fig. 3). Of 105 patients, findings suggestive of UTT were seen in 11 (10.5 %) patients. Of these, seven (63.6 %) were false positive, three (27.3 %) were true positive, and one (9.1 %) was lost to follow-up (Table 2).
61-year-old woman post cystectomy for bladder cancer. A Axial image from nephrographic phase of CT urogram show thickening of the renal pelvis (arrowhead) and heterogeneous and delayed nephrogram (arrow). This proved to be an UTT arising in the renal pelvis and invading the renal parenchyma. B Axial image at a slightly lower level again demonstrates the low attenuation area in the right kidney (arrows). Residual normal renal parenchyma is noted anteriorly (arrowhead). Mildly enlarged retroperitoneal lymph nodes (curved arrows) proved to be metastatic disease.
Median interval between cystectomy and detection of postsurgical findings was 12 months (range 0–156 months) (Table 1). Pelvic recurrence of bladder cancer was seen after a median of 39 months following cystectomy; lymph node metastases after a median of 11 months (range 1–68 months); and distant metastases were seen after a median of 16.5 months (range 0–68 months). UTTs developed after a median period of 43 months (range 16–73 months) following cystectomy. There was a significant overlap of the time intervals to the development of postsurgical complications, recurrent disease, and UTT (Fig. 4).
Surveillance of patients with bladder cancer following cystectomy: findings detected on CTU related to follow-up interval. There is considerable overlap of the time intervals in which each category of findings was detected. Findings related to surgery were detected throughout the study period and as late as 156 months. Recurrent bladder cancer was detected up to 68 months after surgery; metachronous UTT were detected up to 73 months.
Discussion
Although the ACR appropriateness criteria recommend CTU as the test of choice for following patients with bladder cancer after cystectomy [17], there are no data, to our knowledge, that describe the exact yield of CTU in these patients. A small study has demonstrated that CTU in these patients can be used to accurately demonstrate both normal and abnormal post-operative findings [21]; more data are lacking. Indeed, there is no consensus regarding the imaging follow-up of bladder cancer patients after cystectomy; opinions differ regarding the need for imaging follow-up, as well as the recommended modality, frequency, and duration of follow-up, both for the detection of locoregional or distant recurrence of bladder cancer and for metachronous UTT [4, 10, 19, 22–26]. Although CTU is considered the test of choice by some [4, 22, 23], other tests including intravenous urography, renal ultrasound with retrograde pyelography, and MR urography have also been recommended along with CTU [22]. Our study contributes to the growing body of knowledge supporting the use of CTU in the surveillance of patients with bladder cancer following cystectomy. Our results are consistent with the report by Giannarini et al. [25], who used single phase CT of abdomen and pelvis and renal ultrasound with or without intravenous urography for follow-up of patients with bladder cancer after cystectomy and concluded that imaging follow-up of patients with bladder cancer is justified. In our cohort, almost two-thirds of patients had a finding detected with CTU. All of them were important and related to surgical complications, recurrent disease, or metachronous disease. The identification and diagnosis of a broad range of conditions is evidence that CTU is a comprehensive examination of the urinary tract and able to depict changes due to a variety of processes during the entire care continuum.
Surgical complications including trauma and obstructive uropathy were found commonly throughout the study period. Hydronephrosis, parastomal hernias, and urinary tract calculi were the most frequent in this category. Local, regional, and distant spread of tumor were detected in 20 % of patients. The frequency was lower than previous reports which have estimated that recurrent disease occurs in about half of the patients [3, 9–11]. This lower proportion could be partly because prior studies have included follow-up intervals of up to 10 years compared to our shorter median follow-up of 5 years.
Pelvic recurrence of bladder cancer occurred in our study in a single patient at 39 months following cystectomy; lymph node metastases were detected after a median of 11 months and distant metastases after a median of 16.5 months. Our results are consistent with the literature; bladder cancer typically recurs within 2 years, and UTT occur at a median interval of 3–3.3 years [4, 15, 16]. However, recurrent bladder cancer can develop after 5 years. As a result, we do not know of a time point beyond which some form of imaging surveillance would not be needed [4].
Metachronous UTT were found in 2.9 % patients. Previous studies have reported an incidence of metachronous UTT ranging from 2 to 7 % [12, 13, 27–29]. Although suspicious findings were common at CTU and metachronous tumors diagnosed in our patients, most CTU findings were benign. This is consistent with the published high sensitivity and low specificity of CTU for UTT [30]. In fact, in a general population of patients, upper tract findings suspicious for cancer have been shown to be benign in approximately half [30]. In our population, reasons for false positive CT urograms may be related to the propensity for post-operative urinary tracts to reveal inflammatory changes that can mimic cancers, and perhaps to a heightened concern for a second tumor given the prior history of urothelial cancer. Metachronous UTT in our study developed after a median interval of 43 months; a prior study reported a median interval of 29.2 months with most UTT occurring within 3 years after cystectomy [28].
Considering all findings, our results showed a considerable overlap between the time interval to development of postsurgical complications, recurrent or metastatic bladder cancer, and metachronous tumors. Therefore, it may not be possible to choose an optimal time to image patients after cystectomy. We did not evaluate the appropriate frequency or timing of CTU. It is indeed difficult to generalize, as CTU may be indicated for symptoms or other findings suggesting a surgical complication, recurrent disease, or metachronous tumor. More data will be needed to determine whether CTU is indicated, for example, at yearly intervals and whether it would be appropriate to lengthen the interval after a certain period of time.
A limitation of this study included its retrospective design. The follow-up interval was variable (median 60 months) and the number of findings in some categories, such as metachronous UTT, was small. Although we did not codify indications in individual patients beyond surveillance, there was a wide variation in the number of CT urograms obtained in each patient. Despite a median follow-up of 63 months following cystectomy and the burgeoning use of CTU, many patients had only one CT urogram, while more than half of the patients had more than one CT urogram, and some had five or more CT urograms. This variation in clinical practice, i.e., when and how often CTU was used could have been due to several factors including individual differences among patients (e.g., bladder cancer grade, stage, and post-operative course), symptoms, and perhaps the current lack of consensus as to when and how often imaging should be used. We also did not include patients who were examined with other modalities or other CT protocols. We limited our study to patients examined with CTU because CTU is now the recommendation of the ACR and the purpose was to study the yield of CTU [17].
In summary, findings related to surgery, spread of disease, and metachronous tumors are commonly detected with CTU in the surveillance of patients with bladder cancer after cystectomy. Our study supports current published guidelines on the use of CTU in these patients. Future studies will be helpful to evaluate further the value of CTU relative to other modalities and the frequency with which CTU or any imaging modality should be performed.
References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics. CA Cancer J Clin 63(1):11–30
Stenzl A, Cowan NC, De Santis M, et al. (2009) The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 55:815–825
Bellmunt J, Guix M (2010) New agents for bladder cancer. Ann Oncol 21(Suppl 7):vii56–vii58
Vrooman OPJ, Witjes JA (2010) Follow-up of patients after curative bladder cancer treatment: guidelines vs. practice. Curr Opin Urol 20:437–442
Shariat SF, Karakiewicz PI, Palapattu GS, et al. (2006) Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol 176:2414–2422 (discussion 2422)
Stein JP, Lieskovsky G, Cote R, et al. (2001) Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 19:666–675
El-Nahas AR, Shokeir AA (2010) Endourological treatment of nonmalignant upper urinary. Tract complications after urinary diversion. Urology 76:1302–1308
Catalá V, Solà M, Samaniego J, et al. (2009) CT findings in urinary diversion after radical cystectomy: postsurgical anatomy and complications. Radiographics 29:461–476
Malkowicz SB, van Poppel H, Mickisch G, et al. (2007) Muscle-invasive urothelial carcinoma of the bladder. Urology 69:3–16
Volkmer BG, Kuefer R, Bartsch GC, Gust K, Hautmann RE (2009) Oncological followup after radical cystectomy for bladder cancer—is there any benefit? J Urol 181:1587–1593 ((discussion 1593))
von der Maase H, Sengelov L, Roberts JT, et al. (2005) Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23:4602–4608
Höglund M (2007) On the origin of syn- and metachronous urothelial carcinomas. Eur Urol 51:1185–1193 ((discussion 1193))
Tran W, Serio AM, Raj GV, et al. (2008) Longitudinal risk of upper tract recurrence following radical cystectomy for urothelial cancer and the potential implications for long-term surveillance. J Urol 179:96–100
Picozzi S, Ricci C, Gaeta M, et al. (2012) Upper urinary tract recurrence following radical cystectomy for bladder cancer: a meta-analysis on 13,185 patients. J Urol 188:2046–2054
Sanderson KM, Cai J, Miranda G, Skinner DG, Stein JP (2007) Upper tract urothelial recurrence following radical cystectomy for transitional cell carcinoma of the bladder: an analysis of 1,069 patients with 10-year followup. J Urol 177:2088–2094
Umbreit EC, Crispen PL, Shimko MS, et al. (2010) Multifactorial, site-specific recurrence model after radical cystectomy for urothelial carcinoma. Cancer 116:3399–3407
Sanderson KM, Rouprêt M (2007) Upper urinary tract tumour after radical cystectomy for transitional cell carcinoma of the bladder: an update on the risk factors, surveillance regimens and treatments. BJU Int 100:11–16
Lee EK, Dickstein RJ, Kamta AM (2011) Imaging of urothelial cancers: what the urologist needs to know. AJR Am J Roentgenol 196:1249–1254
ACR appropriateness criteria for follow-up of patients with urothelial cancer. [Internet]. http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/FollowUpImagingBladderCarcinoma.pdf. Accessed 8 Feb 2013
Silverman SG, Akbar SA, Mortele KJ, et al. (2006) Multi-detector row CT urography of normal urinary collecting system: furosemide versus saline as adjunct to contrast medium. Radiology 240:749–755
Sudakoff GS, Guralnick M, Langenstroer P, et al. (2005) CT urography of urinary diversions with enhanced CT digital radiography: preliminary experience. AJR Am J Roentgenol 184:131–138
NCCN clinical practice guidelines in oncology [Internet]. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 8 Feb 2013
Bochner BH, Montie JE, Lee CT (2003) Follow-up strategies and management of recurrence in urologic oncology bladder cancer: invasive bladder cancer. Urol Clin North Am 30:777–789
Slaton JW, Swanson DA, Grossman HB, Dinney CP (1999) A stage specific approach to tumor surveillance after radical cystectomy for transitional cell carcinoma of the bladder. J Urol 162:710–714
Giannarini G, Kessler TM, Thoeny HC, et al. (2010) Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol 58:486–494
Colombo R (2010) Invasive bladder cancer and the role of follow-up: should we consider the match over at radical cystectomy or should we play for extra time? Eur Urol 58:495–497
Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, Huguet-Pérez J, Vicente-Rodríguez J (2000) Upper urinary tract tumors after primary superficial bladder tumors: prognostic factors and risk groups. J Urol 164:1183–1187
Wang P, Luo JD, Wu WF, et al. (2007) Multiple factor analysis of metachronous upper urinary tract transitional cell carcinoma after radical cystectomy. Braz J Med Biol Res 40:979–984
Novara G, De Marco V, Dalpiaz O, et al. (2009) Independent predictors of contralateral metachronous upper urinary tract transitional cell carcinoma after nephroureterectomy: multi-institutional dataset from three European centers. Int J Urol 16:187–191
Sadow CA, Wheeler SC, Kim J, Ohno-Machado L, Silverman SG (2010) Positive predictive value of CT urography in the evaluation of upper tract urothelial cancer. Am J Roentgenol 195:W337–W343
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shinagare, A.B., Sadow, C.A. & Silverman, S.G. Surveillance of patients with bladder cancer following cystectomy: yield of CT urography. Abdom Imaging 38, 1415–1421 (2013). https://doi.org/10.1007/s00261-013-0024-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-013-0024-6