Abstract
The main clinically recognized application of contrast-enhanced US (CEUS) with microbubble contrast agents is the characterization of incidental focal liver lesions. CEUS with low transmit power insonation allows the real-time assessment of contrast enhancement in a focal liver lesion after microbubble contrast agent injection, during the arterial (10–25 s), portal venous (from 35 s up to 2 min) and late phase (4–6 min after microbubble injection). During the portal venous and late phase benign lesions appear hyper or iso-enhancing in comparison to the adjacent liver parenchyma, while malignant lesions prevalently present contrast washout with hypo-enhancing appearance. CEUS may provide an added diagnostic value in those incidental focal liver lesions in which contrast-enhanced CT or MR imaging are not conclusive. In particular, CEUS may provide an added diagnostic value in those focal liver lesions appearing indeterminate on single-phase CT scan, or on CT scans performed by an incorrect delay time or also after injection of a low dose of iodinated contrast agent, or also in those focal liver lesions revealing equivocal enhancement patterns on contrast-enhanced CT or MR imaging. CEUS may have an added diagnostic value also in hepatocellular nodules in a cirrhotic liver and can be considered a complementary imaging technique to CT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Unenhanced gray-scale and color Doppler ultrasound (US) present limited accuracy in solid focal liver lesion characterization since benign and malignant lesions may reveal similar echopattern and vascular architecture [1, 2]. Even though tumoral vessels visibility may be improved by color and power Doppler after microbubble contrast agents injection, color signal saturation and motion and blooming artifacts represent important limitations [3, 4]. Contrast-enhanced US (CEUS) with microbubble contrast agents and dedicated US specific contrast modes were introduced to overcome the limitations of unenhanced gray-scale and color Doppler US [5–16].
Several papers have described the general capabilities of CEUS in improving focal liver lesion characterization compared to unenhanced US [1–15]. CEUS provides a reliable benign or malignant diagnosis based on focal liver lesion contrast enhancement [1–11, 16]. This is determined by the high spatial resolution of CEUS due to the high digital image matrix over a limited field of view, its high contrast resolution due to the high sensitivity to harmonic frequencies produced by microbubble insonation, and its high temporal resolution which allows the real-time assessment of contrast enhancement.
The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) guidelines recommends the use of CEUS to diagnose incidental focal liver lesions not characterized on unenhanced US, in lesions or suspected lesions identified in a background of chronic hepatitis or liver cirrhosis, or also in patients with a known history of malignancy [17]. Finally, EFSUMB suggests the use of CEUS also in those focal liver lesions with an inconclusive CT or MR imaging [17]. The uncertain lesion characterization after CT or MR imaging frequently leads to focal liver lesion biopsy or, alternatively, long-term imaging follow-up. The aim of this update article is to describe the added diagnostic value of CEUS in focal liver lesions remaining indeterminate after contrast-enhanced CT or MR imaging.
CEUS and focal liver lesion characterization
CEUS allows the real-time assessment of focal liver lesion perfusion during low transmit power insonation (mechanical index value, 0.1–0.25 according to the US equipment and the employed contrast-specific technique) and it is not limited by motion and blooming artifacts as color and power Doppler US. CEUS is easily performed. The operator has to identify the most suitable acoustic window to evaluate the lesion and simply observe tumoral contrast enhancement after microbubble injection without moving the transducer from the initial scanning position. CEUS is technically a simple imaging method and allows real-time acquisition after microbubble administration without any drawback due to incorrect scanning time in comparison to CT and MR which are limited by image acquisition time issues. Contrast-enhanced CT and MR imaging may be limited in scanning hypervascular tumors owing to difficulties in starting the acquisition at a suitable time point, and are penalized by costs, availability, and patient irradiation in the case of CT. The main limitation of CEUS in comparison to multiphase CT and MR imaging is the fact that only one liver tumor can be scanned at a time as the transducer has to be kept still during the examination, and further microbubble injections are often necessary to characterize additional liver tumors. On CEUS the image planes and different technical parameters (e.g., focal zones position, echo-signal amplification) must be individually optimized for each tumor examined in those cases in which tumors present a different segmental location and depth.
Perfluorocarbon or sulphur hexafluoride—filled microbubbles covered by a phospholipid shell, including Definity (Lantheus Medical Imaging, MA, USA) and SonoVue (Bracco, Milan, Italy), present non-linear response with production of harmonic and sub-harmonic frequencies at low acoustic power insonation, allowing the employment of continuous non-destructive imaging and the real-time evaluation of lesion contrast enhancement. Microbubble contrast agents present a pure intravascular distribution and do not leak in the interstitial space but persist in the sinusoids and portal vessels without the evidence of any equilibrium phase. The enhancement resulting exclusively from the hepatic arterial supply is timed from 10 s after the start of the intravenous injection and lasts for 10–15 s [18]. The portal venous phase starts about 35 s from microbubble injection and then lasts for 2 min after the start of injection, whereas the subsequent late phase lasts for up to 4–6 min after injection, until microbubble clearance from the liver parenchyma [18]. After blood pool clearance perfluorocarbon—filled agents, such as Sonazoid (GE Healthcare, UK) and Levovist (Bayer Schering, Germany) were shown to have also a Kupffer cell-specific parenchymal affinity at late phase [19].
CEUS provides a benign or malignant diagnosis based on focal liver lesion enhancement at hepatic arterial, portal venous, and late phase in comparison to the adjacent liver parenchyma. Malignancies typically show a low echo-signal intensity at late phase [4, 5, 8], regardless of whether they are hyper- or hypo-enhancing in terms of their arterial supply, while benign lesions generally present a sustained enhancement with hyper or iso-enhancing appearance to the adjacent liver parenchyma [5–15]. CEUS is a reliable imaging technique in the characterization of liver lesions as benign or malignant with a sensitivity and specificity ranging, respectively, from 85% to 90% and 80% and 99% [8, 10, 20, 21]. Most often in routine clinical practice it is not possible to predict a histological diagnosis by CEUS since many benign lesions appear hyper-enhancing during the hepatic arterial phase and, even after microbubble injection, are often indistinguishable. A small hyper-enhancing hemangioma, a focal nodular hyperplasia, and a hepatocellular adenoma may all appear iso or hyper-enhancing at portal venous and/or late phase; the only exception is the hepatocellular adenomas which may appear hypo-enhancing on late phase [22]. Neither different malignant lesion histotypes can be differentiated since hepatocellular carcinomas, intrahepatic cholangiocarcinomas, and liver metastases prevalently appear hypo- or hyper-enhancing during the arterial phase and hypo-enhancing with contrast washout at portal venous and late phase [8, 23, 24], even though hepatocellular carcinoma may also appear iso-enhancing to the adjacent liver parenchyma during the portal venous-late phase in about 40% of cases [6–9].
In selected cases CEUS may also provide improved lesion histological differential diagnosis provided that a specific enhancement pattern is visualized, as in the case of typical liver hemangiomas showing peripheral nodular enhancement and focal nodular hyperplasia showing centrifugal filling with central spoke wheel-shaped enhancement during the hepatic arterial phase [9, 10]. Between 5% and 25% of focal liver lesions remain indeterminate even after CEUS [2, 3, 5, 8, 10] and need to be characterized by contrast-enhanced CT or MR imaging [25, 26].
CEUS presents a high level of concordance with CT and MR imaging [27] in depicting the contrast enhancement pattern of focal liver lesions during the arterial phase. Concordance in the portal venous phase is generally lower, and discordance in the portal venous phase occurs most often in malignancies reflecting the tendency of CT and MR contrast agents, unlike microbubbles, to diffuse into the tumoral interstitium in cases in which CEUS showed washout [27].
The added diagnostic value provided by CEUS in incidental focal liver lesions
There are some evidences in the recent literature showing that CEUS may provide an added diagnostic value in the characterization of incidental focal liver lesions remaining indeterminate after contrast-enhanced CT due to different causes. This may be due to: (a) single-phase CT (usually during the arterial phase on CT angiography or during the portal venous phase for an overall evaluation of the abdomen and pelvis) revealing one or more hypo- or hyper-attenuating incidental focal liver lesions; (b) the small lesion diameter (below 3 mm) which does not allow a reliable density analysis on the different dynamic phases; (c) the incorrect delay time (e.g., too early scanning during the hepatic arterial or portal venous phase); (d) the low injected dose of iodinated contrast agent (low injected volume of iodinated contrast, or low iodine concentration per mL, or low amount of volume of iodinated contrast injected per minute) compared to the patient body weight. Moreover, focal liver lesions may present an equivocal enhancement pattern on contrast-enhanced CT or MR imaging, e.g., those lesions appearing persistently hypo-enhancing after contrast material injection during the different dynamic phases. According to the results of a recent study from our group [28] including fifty-five incidental solid focal liver lesions (1–5 cm in diameter; 40 hemangiomas, 2 hepatic angiomyolipomas, 1 hepatocellular adenoma, and 12 metastases) in 43 non-cirrhotic patients (24 female, 19 male; age ± SD, 55±10 years), CEUS provides improvement in the diagnosis in those focal liver lesions appearing equivocal or uncharacterized on contrast-enhanced CT or MR imaging avoiding biopsy or further contrast-enhanced CT examinations or long-term imaging follow-up in the vast majority of these lesions. In particular, the additional review of CEUS after CT/MR images allows to improve the diagnostic accuracy (before vs. after CEUS review = 48% vs. 85% -reader 1- and 51% vs. 87% -reader 2-) and diagnostic confidence (area under ROC curve before vs after CEUS review = 0.854 vs. 0.974 -reader 1- and 0.868 vs. 0.978 -reader 2-) in focal liver lesion characterization [28].
CEUS improves the characterization of these focal liver lesions by identifying some specific enhancement patterns, namely the peripheral nodular enhancement (Fig. 1) or the focal peripheral dot-like enhancement (Fig. 2) in those liver hemangiomas appearing hypo-attenuating on single-phase CT scan. The focal peripheral dot-like enhancement which may be evident on CEUS resembles the “bright-dot” sign previously described on contrast-enhanced CT in liver hemangiomas and defined as a tiny enhancing dot that did not progress to the globular peripheral enhancement [29]. Small fibrosclerotic hepatic hemangiomas with a persistent hypo-attenuating appearance on contrast-enhanced CT cannot be characterized either by dual-phase CT or by CEUS which confirms the persistent hypo-enhancing pattern (Fig. 3). Solid focal liver lesions appearing hyper-attenuating on single-phase contrast-enhanced CT may be characterized by CEUS which may visualize the complete enhancement pattern during the different dynamic phases (Fig. 4). Another field of application of CEUS, as an imaging technique providing an additional diagnostic value to CT, corresponds to those solid focal liver lesions appearing slightly hypo-attenuating on contrast-enhanced CT during the portal-venous or late equilibrium phase, with a low conspicuity due to the reduced liver-lesion attenuation difference related to the insufficient injected dose of iodinated contrast agent in relation to the large patient habitus or body weight (Fig. 5). Finally, some other solid focal liver lesions appearing indeterminate on contrast-enhanced MR imaging due to the atypical lesion enhancement pattern during the dynamic or hepatobiliary phase may be effectively characterized by CEUS as benign or malignant (Fig. 6). In all these frequent clinical situations CEUS may close the patient diagnostic work-up if a diagnosis of benignancy is achieved.
A–D Liver hemangioma with atypical enhancement on single-phase contrast-enhanced CT. A Contrast-enhanced CT performed only during the portal venous phase, transverse plane. A solid lesion (arrow) appearing hypo-attenuating during the portal venous phase is identified in the right liver. B–D Contrast-enhanced US after sulphur hexafluoride—filled microbubble injection during arterial (B), portal (C), and late phase (D), sagittal scan. The lesion (arrow) presents a peripheral nodular enhancement during arterial phase (B), with a progressive centripetal fill-in during portal-venous (C), and late phase (D). Diagnosis of hemangioma was made after contrast-enhanced US.
A–F Liver hemangioma. A Contrast-enhanced CT performed only during the portal venous phase, transverse plane. A large solid lesion (arrow) appearing hypo-enhancing during the portal-venous phase is identified in the right liver. Some small equivocal enhancing dots are visualized at the periphery of the lesion. B, C Contrast-enhanced US after sulphur hexafluoride—filled microbubble injection during the portal venous (B), and late phase (C). The lesion presents focal dot-like peripheral enhancement (arrow) suggesting hemangioma diagnosis with a higher diagnostic confidence than CT. D–G Contrast-enhanced MR imaging. The lesion (arrow) appears homogeneously hyperintense on T2-weighted MR sequences (D), and presents progressive evidence of peripheral dot-like enhancement (small arrow) on contrast-enhanced fat-suppressed MR imaging sequences during arterial (E), portal venous (F), and late equilibrium phase (G). Based on contrast-enhanced US the diagnosis of hemangioma was provided and it was confirmed by imaging follow-up.
A–D Small hypo-enhancing lesion considered indeterminate both on contrast-enhanced CT and contrast-enhanced US. A, B Contrast-enhanced CT. A small lesion (arrow) appears hypo-enhancing during the arterial (A) and portal venous phase (B). C, D Contrast-enhanced US after sulphur hexafluoride—filled microbubble injection during portal venous (C), and late phase (D). Contrast-enhanced US reveal a persistent hypo-enhancing appearance during the arterial (C) and portal venous phase (D). Fibrosclerotic hemangioma was diagnosed based on imaging follow-up.
A–D Incidental focal liver lesions detected on single-phase contrast-enhanced CT performed during the portal venous phase (70 s from iodinated contrast agent injection). A Contrast-enhanced CT, transverse plane. A solid lesion (arrow) appearing slightly hyper-attenuating during the portal-venous phase is identified on the right liver. B–D Contrast-enhanced US after sulphur hexafluoride—filled microbubble injection during arterial (B), portal (C), and late phase (D). The lesion presents diffuse contrast enhancement during arterial phase (B), persistently evident during the portal-venous (C), and late phase (D). Diagnosis of focal nodular hyperplasia was made by US-guided biopsy.
A–D Solid liver metastases in a patient with chronic liver disease and cutaneous melanoma. A, B Contrast-enhanced CT during portal venous (A) and late phase. Some equivocal focal heterogenicities (arrows) are evident in the liver due to a low contrast in comparison to the adjacent liver parenchyma due to insufficient injected dose of iodinated contrast agent if compared to the patient body weight. C, D Contrast-enhanced US after sulphur hexafluoride—filled microbubble injection during portal-venous phase. The multiple metastatic lesions present unequivocal hypo-enhancing appearance (arrows), suggesting the diagnosis of multiple liver metastases.
A Unenhanced gray-scale US reveals a heterogeneous lesion (arrow), revealing spoke wheel-shaped central vascular architecture on color Doppler US (B) with low resistance in the arterial vessels on pulsed Doppler C, D. E–M MR imaging. The lesion (arrow) does not present a fat component on T1 weighted in-phase (E) and out-of-phase (F) fast-field-echo (FFE) MR imaging sequences, while it appears heterogeneously hyperintense on T2-weighted MR imaging sequences (G). Unenhanced (H) and contrast-enhanced (I–M) fat-suppressed 3D MR imaging sequences before and after gadobenate dimeglumine (Gd-BOPTA) injection. The lesion (arrow) appears hyper-enhancing on arterial phase (I) and persists slightly hyper-enhancing if compared to the adjacent liver during portal venous (J), and late equilibrium phase (K, L), and hypo-enhancing during the hepatobiliary phase (M) suggesting a malignant nature. N–Q Contrast-enhanced US after sulphur hexafluoride—filled microbubble injection during arterial (N, O), portal venous (P), and late phase (Q). The lesion (arrow) presents heterogeneous diffuse contrast enhancement and appears hyperenhancing during arterial phase (N, O), with a persistent microbubble uptake during portal-venous (P), and the late phase (Q) suggesting a benign nature. Diagnosis of teleangectatic focal nodular hyperplasia was made by biopsy.
The added diagnostic value provided by CEUS in the cirrhotic liver
One of the key pathological factors for the differential diagnosis between hepatocellular carcinoma and non-malignant hepatocellular lesions in cirrhotic patients is the vascular supply to the nodule. According to the 2001 Barcelona criteria [30] and EASL and AASLD 2005 guidelines [31], the evidence of coincidental arterial hyper-vascularity during arterial phase with contrast washout during the portal venous or late phase on CEUS and CT or MR imaging in nodules ≤2 cm in patients with liver cirrhosis is considered diagnostic of hepatocellular carcinoma and no bioptic procedure is required. If a nodule >2 cm shows arterial hyper-vascularity and washes out in the portal venous or late phase, only a single imaging investigation (CEUS, CT, or MR) is required for HCC diagnosis. If an equivocal pattern is identified (e.g., iso- or hyper-vascularity not followed by hypo-vascularity, or persistent hypo-vascularity) in hepatocellular nodules of any size biopsy should be performed. In patients with cirrhosis with a nodule ≤2 cm detected during surveillance, CEUS showing a typical contrast pattern confidently permits the diagnosis of hepatocellular carcinoma [32].
Recently, only contrast-enhanced CT and MR imaging were included as reliable imaging techniques for depicting hepatocellular nodule vascularity in the non-invasive diagnosis of hepatocellular carcinoma in cirrhotic patients according to the revised AASLD guidelines for hepatocellular carcinoma diagnosis [33]. This is determined by a significant proportion of false positives for hepatocellular carcinomas described in patients with intrahepatic cholangiocarcinoma scanned by CEUS [23]. According to Vilana et al. [23], the homogeneous hyper-vascularity on arterial phase followed by hypo-vascularity on the late phase, resembling the hepatocarcinoma pattern, was observed in 10/21 (47.6%) intrahepatic cholangiocarcinomas detected in cirrhotic patients over an observational period of 6 years. Anyway, another paper [24] recently reported that the same pattern was observed only in 3/50 (6%) of intrahepatic cholangiocarcinomas. Moreover, we should consider that the real clinical impact of the occurrence of peripheral cholangiocarcinoma in patients with chronic liver disease on the false-positive-rate for hepatocarcinoma remains unknown [34]. According to these observations, the real impact of false positive rate for hepatocellular carcinoma in cirrhotic patients due to intrahepatic cholangiocarcinomas is probably very low and should not justify the exclusion of CEUS from diagnostic criteria.
CEUS may have an additional diagnostic value to CT even in cirrhotic patients. In a recent paper from our group [35] including 106 cirrhotic patients (68 male, 38 female; mean age ± SD, 70 ± 7 years) with 121 biopsy-proven hepatocellular nodules (72 hepatocellular carcinomas, 10 dysplastic and 15 regenerative nodules, 12 hemangiomas, and 12 other benignancies), it was shown that the combined assessment of hepatocellular nodule vascularity at CT and CEUS improved the diagnostic performance in the diagnosis of malignancy [35] based on the visualization of nodule contrast washing during the arterial phase and contrast washout during the portal venous or late phase [30–32]. The combined assessment of CEUS/CT provided higher sensitivity in the diagnosis of malignancy than did separate assessment of CEUS and CT due to the reduction of false negative findings [35]. Nodules displaying higher, similar or lower enhancement compared to the adjacent liver parenchyma were defined as hyper-, iso- or hypo-vascular, respectively. Concordant vascular profiles on CEUS and CT were not requested and the higher grade of vascularity observed during the arterial phase was considered decisive (e.g., nodules appearing hypervascular on CEUS and iso-vascular on CT were classified as hypervascular), while the lower grade of vascularity in the portal/late phase was considered decisive (e.g., hypervascular nodules appearing iso-vascular on CEUS and hypo-vascular on CT during the portal/late phase were classified as hypo-vascular). The combined CEUS/CT provided a higher diagnostic accuracy (91% -reader 1- and 87% -reader 2-) than did the separate assessment of CEUS (83% -reader 1- and 79% -reader 2-) and CT (83% -reader 1- and 78% -reader 2-; P < 0.05) in the diagnosis of malignancy in patients with liver cirrhosis. This means that contrast washin during the arterial phase and contrast washout during the portal venous phase is depicted in a higher percentage of nodules if CEUS and CT are considered as complementary imaging techniques. According to these data, CEUS should be considered a preliminary examination after unenhanced US to exclude malignancy and a reliable additional imaging technique to CT for characterizing those hepatocellular nodules detected during surveillance (Figs. 7, 8).
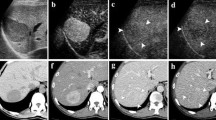
A–G Small hepatocellular carcinoma nodule in cirrhotic liver. A, B Unenhanced gray-scale US (A) reveals a hypoechoic nodular lesion with some peripheral vessels on color Doppler US (B). C, D Contrast-enhanced US. The small liver nodule (arrow) presents contrast washing after microbubble injection during the arterial phase (C) and contrast washout during the portal venous phase (D). E–G Contrast-enhanced CT revealed nodule contrast washin during the hepatic arterial phase (E) and equivocal nodule vascularity (arrow) both during the portal venous (F) and late phase (G) without evidence of contrast washout. Diagnosis of hepatocellular carcinoma was made based on the enhancement pattern depicted by contrast-enhanced US.
A–J Small indeterminate nodular lesion in a cirrhotic liver. A, B Contrast-enhanced CT during the arterial (A) and portal venous phase (B). The lesion (arrow) appears persistently hypo-attenuating. C Unenhanced and D–F contrast-enhanced US after sulphur hexafluoride—filled microbubble injection during arterial (D), portal-venous (E), and late phase (F). The nodule (arrow) presents peripheral nodular enhancement during hepatic arterial phase (D), persistently evident also during the portal-venous (E), and late phase (F). T2-weighted (G) and T1-weighted fat-suppressed contrast-enhanced (H–J) MR imaging sequences with fat suppression. The nodule (arrow) appears slightly hyperintense on T2-weighted sequences (G). A persistently hypo-enhancing appearance is depicted by contrast-enhanced MR imaging during the arterial (H), portal venous (I), and hepatobiliary phase (J). Diagnosis of fibrotic liver hemangioma was proposed based on contrast-enhanced US and it was confirmed on 2-year imaging follow-up.
Conclusions
CEUS may improve the diagnostic confidence in the characterization of those incidental focal liver lesions remaining indeterminate after contrast-enhanced CT or MR imaging, and may have an added diagnostic value also in hepatocellular nodules detected during surveillance in the cirrhotic liver, being complementary to CT.
References
Reinhold C, Hammers L, Taylor CR, et al. (1995) Characterization of focal hepatic lesions with duplex sonography: findings in 198 patients. Am J Roentgenol 164:1131–1135
Lee MG, Auh YH, Cho KS, et al. (1996) Color Doppler flow imaging of hepatocellular carcinomas. Comparison with metastatic tumors and hemangiomas by three step grading color hues. Clin Imaging 20:199–203
Hosten N, Puls R, Lemke AJ, et al. (1999) Contrast enhanced power Doppler sonography: improved detection of characteristic flow patterns in focal liver lesion. J Clin Ultrasound 27:107–115
Kim TK, Han JK, Kim AY, Choi BI (1999) Limitations of characterization of hepatic hemangiomas using an ultrasound contrast agent (Levovist) and power Doppler ultrasound. J Ultrasound Med 18:737–743
Bryant TH, Blomley MJ, Albrecht T, et al. (2004) Improved characterization of liver lesions with liver-phase uptake of liver specific microbubbles: prospective multicenter trials. Radiology 232:799–809
Nicolau C, Catalá V, Vilana R, et al. (2004) Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: correlation with cellular differentiation. Eur Radiol 14:1092–1099
Giorgio A, Ferraioli G, Tarantino L, et al. (2004) Contrast-enhanced sonographic appearance of hepatocellular carcinoma in patients with cirrhosis: comparison with contrast-enhanced CT appearance. AJR Am J Roentgenol 183:1319–1326
Quaia E, Calliada F, Bertolotto M, et al. (2004) Characterization of focal liver lesions by contrast-specific US modes and a sulfur hexafluoride—filled microbubble contrast agent: diagnostic performance and confidence. Radiology 232:420–430
Quaia E, D’Onofrio M, Cabassa P, et al. (2007) The diagnostic value of hepatocellular nodule vascularity after sulfur hexafluoride - filled microbubble injection in patients with liver cirrhosis: analysis of diagnostic performance and confidence in malignancy characterization. AJR Am Journal Roentgenology 189:1474–1483
Dai Y, Chen MH, Yin SS, et al. (2007) Focal liver lesions: can SonoVue-enhanced ultrasound be used to differentiate malignant from benign lesions? Invest Radiol 42(8):596–603
Luo W, Numata K, Kondo M, et al. (2009) Sonazoid-enhanced ultrasonography for evaluation of the enhancement patterns of focal liver tumors in the late phase by intermittent imaging with high mechanical index. J Ultrasound Med 28:439–448
Lin MX, Xu HX, Lu MD, et al. (2009) Diagnostic performance of contrast-enhanced ultrasound for complex cystic focal liver lesions: blinded reader study. Eur Radiol 19:358–369
Liu GJ, Xu HX, Xie XY, et al. (2009) Does the echogenicity of focal liver lesions on baseline gray-scale ultrasound interfere with the diagnostic performance of contrast-enhanced ultrasound. Eur Radiol 19:1214–1222
Chen LD, Xu HX, Xie XY, et al. (2010) Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol 20:743–753
Bartolotta TV, Taibbi A, Matranga D, et al. (2010) Hepatic focal nodular hyperplasia: contrast-enhanced ultrasound findings with emphasis on lesion size, depth, and liver echogenicity. Eur Radiol 20(9):2248–2256
Quaia E (2007) Microbubble ultrasound contrast agents: an update. Eur Radiol 17(8):1995–2008
Claudon M, Cosgrove D, Albrecht T, et al. (2008) Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)—update 2008. Ultraschall in Med 29:28–44
Bolondi L, Correas JM, Lencioni R, Weskott HP, Piscaglia F (2007) New perspectives for the use of contrast-enhanced liver ultrasound in clinical practice. Dig Liver Dis 39:187–195
Quaia E, Blomley MJK, Patel S, et al. (2002) Initial observations on the effect of irradiation on the liver-specific uptake of Levovist. Eur J Radiol 41:192–199
Tranquart F, Le Gouge A, Correas JM, et al. (2008) Role of contrast-enhanced ultrasound in the blinded assessment of focal liver lesions in comparison with MDCT and CEMRI: results from a multicentre trial. Eur J Radiol 6(11):9–15
von Herbay A, Westendorff J, Gregor M (2010) Contrast-enhanced ultrasound with SonoVue: differentiation between benign and malignant focal liver lesions in 317 patients. J Clin Ultrasound 38(19):1–9
Kim TK, Jang HJ, Burns PN, Murphy-Lavallee J, Wilson SR (2008) Focal nodular hyperplasia and hepatic adenoma: differentiation with low-mechanical-index contrast-enhanced sonography. AJR Am J Roentgenol 190:58–66
Vilana R, Forner A, Bianchi L, et al. (2010) Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 51:2020–2029
Chen LD, Xu HX, Xie XY, et al. (2010) Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol 20(3):743–753
Hammerstingl R, Huppertz A, Breuer J, et al. (2008) Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol 18(3):457–467
Raman SS, Leary C, Bluemke DA, et al. (2010) Improved characterization of focal liver lesions with liver-specific gadoxetic acid disodium-enhanced magnetic resonance imaging: a multicenter phase 3 clinical trial. J Comput Assist Tomogr 34(2):163–172
Burns P, Wilson SR (2007) Focal liver lesions: enhancement patterns on contrast-enhanced images–concordance of US scans with CT scans and MR images. Radiology 242(1):162–174
Quaia E, Pizzolato R, Cavallaro M, Cabibbo B, Cova MA (2010) Characterization of solid tumour detected in a noncirrhotic liver and presenting atypical enhancement pattern on CT or MR: assessment of additional diagnostic value of contrast-enhanced ultrasound. Proceedings of the 96th Scientific Assembly of the Radiological Society of North America, pp 115
Jang HJ, Choi BI, Kim TK, et al. (1998) Atypical small hemangiomas of the liver: “bright dot” sign at two-phase spiral CT. Radiology 208:543–548
Bruix J, Sherman M, Llovet JM, et al. (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL Conference. European association for the study of the liver. J Hepatol 35:421–430
Bruix J, Sherman M (2005) Management of hepatocellular carcinoma. Hepatology 42:1208–1236
Sangiovanni A, Manini MA, Iavarone M, et al. (2010) The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 59:638–644
Bruix J, Sherman M (2010) Management of hepatocellular carcinoma: an update. Hepatology 1–35
Pomfret EA, Washburn K, Wald C, et al. (2010) Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl 16:262–278
Quaia E, Alaimo V, Baratella E, Medeot A, Midiri M, Cova MA (2009) The added diagnostic value of 64-row multidetector CT combined with contrast-enhanced US in the evaluation of hepatocellular nodule vascularity: implications in the diagnosis of malignancy in patients with liver cirrhosis. Eur Radiol 19(3):651–663
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quaia, E. Solid focal liver lesions indeterminate by contrast-enhanced CT or MR imaging: the added diagnostic value of contrast-enhanced ultrasound. Abdom Imaging 37, 580–590 (2012). https://doi.org/10.1007/s00261-011-9788-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-011-9788-8