Abstract
Acute gastrointestinal bleeding is a common medical emergency, which carries a significant mortality. CT Angiography is an important non-invasive diagnostic tool, which can be used to plan subsequent endovascular or surgical management. The cases presented demonstrate that a meticulous and systematic approach to image interpretation is necessary, in particular, to detect focal sites of contrast extravasation and small pseudoaneurysms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Acute gastrointestinal bleeding is a common medical emergency with an incidence of 47.7/100,000 population in the U.S. and accounts for 300,000 hospital admissions a year [1, 2]. It carries a significant mortality of between 5% and 12% [2]. Although many cases are not life threatening and resolve with conservative treatment, a significant proportion of patients require further intervention. CT angiography (CTA) is an important non-invasive diagnostic tool, which can be used to plan further management. With reduced scan times, higher resolution images, and the use of multi-planar reformatting (MPR), CTA is becoming increasingly accurate at detecting the source of active bleeding. The sensitivity of CTA in acute gastrointestinal bleeding is cited as 85% in one review with a specificity of 95%, as compared with digital subtraction angiography (DSA), which has a sensitivity of 47% to 86% [3–5]. When looking at bleeding rates the figures are also comparable. CTA is reported as demonstrating bleeding up to 0.3 mL/min and DSA up to 0.5 mL/min [3]. The aim of this essay is to improve the radiologist’s diagnostic accuracy in image interpretation of CTA.
Upper gastro-intestinal hemorrhage
Upper gastrointestinal hemorrhage is defined as bleeding from a source above the ligament of Treitz/suspensory ligament of the duodenum, i.e., proximal to the duodenojejunal flexure. Upper gastro-intestinal bleeding accounts for approximately 75% of all hospital admissions with gastro-intestinal hemorrhage within the U.K. Mortality is estimated at 14% but rises to 25% when bleeding commences during a hospital admission [6]. Upper GI endoscopy should be performed at presentation in severe life threatening hemorrhage. In a small percentage of cases, the endoscopist will not able to identify or treat a bleeding point, especially if the view is obscured by a stomach full of blood. CTA can then be used to identify the site of hemorrhage and guide subsequent endovascular or surgical management.
Peptic ulcer disease is the commonest cause of upper gastro-intestinal bleeding accounting for between 20% and 39% of cases [6, 7]. Primarily it is managed by the endoscopist, but where endoscopic treatment is not possible or fails to control bleeding CTA should be performed to identify a bleeding source (Figs. 1, 2).
This patient presented with continued bleeding from duodenal ulceration despite over sewing of the ulcer and coil embolization. A Axial CTA image demonstrating active extravasation of contrast into the duodenum (arrow) and high attenuation fluid within the bowel lumen in keeping with blood. B Sagittal MIP demonstrating multiple coils within the gastro-duodenal artery (GDA). A mesenteric angiogram was performed, C selective GDA injection which demonstrates active extravasation of contrast (arrow). The patient was successfully treated by further coil embolization.
This patient presented with upper gastro-intestinal bleeding secondary to gastric ulceration, which could not be controlled at endoscopy. A Axial and B coronal MIPs demonstrating active extravasation of contrast into the stomach (arrows), and C DSA demonstrating active extravasation of contrast from branches of the left gastric artery (arrow). Note the multiple small gastric arterial braches which are well demonstrated on the MIP images. The patient was successfully treated by coil embolization.
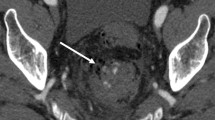
Pancreatitis may be complicated by pseudoaneurysm formation and in the case of acute necrotizing pancreatitis by vessel rupture. Hemorrhage from either of these sources can enter the pancreatic duct and subsequently the gastro-intestinal tract via the ampulla of vater, this process is known as hemosuccus pancreaticus and is a well recognized but rare cause of gastrointestinal bleeding (Figs. 3, 4) [8–10]. Pseudoaneurysms may be only 1–2 mm in size, and review of coronal and sagittal MPRs and maximum intensity projections (MIPs) over a region of interest may be necessary in order not to miss small pseudoaneurysms amenable to endovascular treatment (Fig. 5). Pseudoaneurysms are also a rare but well recognized post surgical complication which may be treated by stent-graft insertion or coil embolization (Fig. 6). [11].
A Axial CTA image and B coronal MIP demonstrating active contrast extravasation from a ruptured splenic artery aneurysm (arrows), which occurred as a complication of acute pancreatitis. The patient was treated successfully by coil embolization of the splenic artery. Note also the high attenuation free fluid in keeping with blood, large hematomas and a surgical drain is in situ. The vessels on the coronal MIP are slender irregular and tapering in keeping with hemodynamic compromise.
A Axial CTA image, B Coronal MIP, and C DSA demonstrating a gastroduodenal artery pseudoaneurysm (arrows) which is best appreciated on the coronal MIP. This patient was known to have metastatic neuroendocrine tumor, hence the liver lesions and biliary stents. The pseudoaneurysm occurred as a complication of a duodenal lesion which was bleeding at the time of presentation.
A Axial MIP and B curved MPR demonstrating a common hepatic artery aneurysm (arrows). The patient presented with upper gastro-intestinal bleeding post Whipples procedure. The aneurysm was successfully excluded by placement of a covered stent within the common hepatic artery. C DSA prior to deployment of the stent.
Variceal hemorrhage is an important cause of upper gastro-intestinal bleeding and has a mortality rate of 14.5% to 25% [12, 13]. The most important diagnostic and therapeutic tool is upper GI endoscopy. In patients with cirrhotic portal hypertension and upper gastro-intestinal hemorrhage studies have shown that a high proportion (84%) are due to variceal hemorrhage and the remainder are due to non-variceal causes [14]. CTA is useful when a bleeding point cannot be identified at endoscopy, and there is a suspicion of non-variceal hemorrhage or variceal bleeding involving the small bowel or lower GI tract. It is important to include a delayed portal venous phase study as part of any examination for gastro-intestinal bleeding to obtain good opacification of the portal vein, hepatic veins and any varices (Fig. 7). Variceal bleeding may also present as lower gastro-intestinal hemorrhage [15].
A Axial and B coronal portal venous phase MPRs demonstrating active extravasation of contrast from an SMV varix into the duodenum (arrows). Upper GI endoscopy in this patient was non-diagnostic due to a large amount of blood. Hemorrhage is also seen within the small bowel lumen, transverse and descending colon, and there is a small amount of peri-hepatic free fluid. The patient underwent a TIPS procedure and coil embolization of an actively bleeding SMV varix.
Lower gastro-intestinal hemorrhage
Lower gastrointestinal hemorrhage is defined as bleeding from a source below the ligament of Treitz. The commonest cause is colonic diverticulosis [16, 17]. The mean patient age at presentation is higher than that in patients with upper gastrointestinal hemorrhage and a high proportion of cases will cease spontaneously [17]. In acute life threatening hemorrhage, after resuscitation commences, a proctoscopy and later sigmoidoscopy should be performed to try to identify the bleeding source. A small proportion of apparent lower gastrointestinal hemorrhage will actually be due to a bleeding source above the ligament of Treitz and an upper GI endoscopy should always be considered. Colonoscopic hemostasis may be considered in diverticular and post-polypectomy bleeding in experienced and skilled hands [18]. CTA is an important non-invasive diagnostic tool in this older and higher risk population.
There are a multitude of causes of lower gastrointestinal hemorrhage and accurate localization of the bleeding point is of fundamental importance in guiding subsequent endovascular or surgical management. With the average small intestine estimated at measuring 7-m long and large intestine 1.5-m long, a meticulous review of the imaging with MPRs is essential to ensure a small bleeding point is not missed. Gastrointestinal hemorrhage where the bleeding point arises from the small bowel is relatively uncommon but CTA plays a very important role in these cases where endoscopy will be non-diagnostic (Fig. 8). Post-operative lower gastrointestinal bleeding may occur at the level of the anastomosis or at a second site distant from the recent surgery (Figs. 9, 10). Careful simultaneous review of the pre- and post-contrast imaging is important in order to not mistake high density suture material at the anastomosis for contrast extravasation [19]. Most cases of lower gastrointestinal bleeding are of colonic origin (Fig. 11) [17]. One of the advantages of CTA compared with DSA is that not only does it demonstrate the bleeding point but it may demonstrate the underlying cause, for example, in the case of a large neoplastic lesion. It may also demonstrate unexpected relevant findings (Fig. 12).
Images from CTA and DSA examinations on a patient who presented with gastrointestinal bleeding post right hemi-colectomy. A Axial CTA image at the level of the anastomosis (arrow), B axial CTA image just below the anastomosis demonstrating active extravasation of contrast (arrow), C coronal MPR demonstrating the bleeding point (arrow). D DSA: selective SMA injection demonstrating contrast extravasation (arrow)
Images from a CTA examination and DSA on a patient who presented with a life threatening lower gastrointestinal hemorrhage. A Axial and B coronal MIPs demonstrating active extravasation of contrast into the cecum (arrows) and adjacent small ileocolic branch vessels. C DSA: SMA injection demonstrating active contrast extravasation from a terminal branch of the ileocolic artery (arrow).
CTA images from a patient who presented with rectal bleeding. A Axial curved MIP and B coronal MIP demonstrating a large common and external iliac artery aneurysm which contains several locules of gas in keeping with infection. The patient was treated by insertion of a bifurcated aortic stent-graft to prevent aneurysm rupture.
Diagnostic pitfalls
Several findings, some of which have already been discussed, can mimic active hemorrhage. It is important not to misinterpret the images as this may lead to unnecessary angiography or surgery. It is essential that no oral contrast is given. Iodinated oral contrast can mimic hemorrhage within the gastrointestinal tract. Hemorrhage within the bowel lumen is a stigma of recent bleeding and angiography should be considered even if no active extravasation of contrast is seen on the CTA (Fig. 13). Oral water will dilute active contrast extravasation, and a bleeding point may be obscured [19]. High attenuation changes within a hematoma can mimic active bleeding and it is always important to perform a pre-contrast study (Fig. 14). Lines, tubes, surgical sutures, and calcification are also important to differentiate from hemorrhage by reviewing the pre-contrast images, and sites of contrast extravasation adjacent to them may be missed without careful review of the images including MPRs (Fig. 15).
A Axial CTA image demonstrating high attenuation fluid within the rectum in keeping with blood. B DSA from the same patient performed shortly afterward: SMA injection demonstrating active extravasation of contrast into the jejunum (arrow). Even in retrospect there is no contrast extravasation on the CTA.
Axial CTA images from a patient who presented with gastrointestinal hemorrhage while on anticoagulants. A Axial pre-contrast image demonstrating high attenuation material within a large hematoma (arrow). Pre-contrast (B) and post-contrast CTA (C) demonstrating the site of active bleeding (arrow), which was confirmed on DSA (D). Note the replaced right hepatic artery arising from the SMA. More selective runs demonstrated multiple bleeding points which were treated by coil embolization.
Conclusion
CT angiography is an important non-invasive diagnostic tool in the management of gastrointestinal hemorrhage. A systematic and meticulous approach to image interpretation using multiplanar reformatting in conjunction with maximum intensity projections should be used, in particular to detect focal sites of extravasation of contrast and small pseudoaneurysms. CTA is a valuable tool for the interventional radiologist as, by demonstrating the site of bleeding, it allows planning of possible endovascular treatment options prior to angiography. Mesenteric angiography remains the “Gold standard” and the role of CTA is not yet proven. If the CTA does not demonstrate a bleeding source but there are stigmata of recent or active hemorrhage then mesenteric angiography should always be considered.
References
Van Leerdam ME, et al. (2003) Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol 98:1494–1499. doi:10.1111/j.1572-0241.2003.07517
Friedman LS, Martin P (1993) The problem of gastrointestinal bleeding. Gastroenterol Clin North Am 22(4):717–721
Chua AE, Ridley LJ (2008) (2008) Diagnostic accuracy of CT angiography in acute gastrointestinal bleeding. J Med Imaging Radiat Oncol 52(4):333–338. doi:10.1111/j.1440-1673.2008.01964.x
Fiorito JJ, Brandt LJ, Kozicky O, Grosman IM, Sprayragen S (1989) The diagnostic yield of superior mesenteric angiography: correlation with the pattern of gastrointestinal bleeding. Am J Gastroenterol 84(8):878–881
Hoedema RE, Luchtefeld MA (2005) The management of lower gastrointestinal hemorrhage. Dis Colon Rectum 48:2010–2024. doi:10.1007/s10350-005-0138-1
Rockall TA, Logan RF, Devlin HB, Nothfield TC (1995) Incidence and mortality from acute upper gastrointestinal hemorrhage in the United Kingdom. Steering committee and members of the national audit of acute upper gastrointestinal hemorrhage. BMJ 311(6999):222–226
Boonpongmanee S, et al. (2004) The frequency of peptic ulcer disease as a cause of upper-GI bleeding is exaggerated. Gastrointest Endosc 59(7):788–794
Clay RP, Farnell MB, Lancaster JR, Weiwnd LH, Goustout CJ (1985) An unusual cause of upper gastrointestinal bleeding. Ann Surg 202(1):75–79
Wagnet WH, Cossman DV, Treiman RL, et al. (1994) Haemosuccus pancreaticus from intra-ductal rupture of a primary splenic artery pseudoaneurysm. J Vasc Surg 19(1):158–164
Benz CA, Jakob P, Jakobs R, Riemann JF (2000) Hemosuccus pancreaticus—a rare cause of gastrointestinal bleeding: diagnosis and interventional radiological therapy. Endoscopy 32(5):428–431
Maleux G, Pirenne J, Aerts R, Nevens F (2005) Hepatic artery pseudoaneurysm after liver transplantation: definitive treatment with a stent-graft after failed coil embolization. Br J Radiol 78:453–456
Carbonell N, Pauwels A, Serfaty L, et al. (2004) Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology 40:652–659
Seo YS, et al. (2008) Clinical features and treatment outcomes of upper gastrointestinal bleeding in patients with cirrhosis. J Korean Med Sci 23(4):635–643. doi:10.3346/jkms.2008.23.4.635
Teres J, Bordas JM, Bru C, Bruguera M, Rodes J (1976) Upper gastrointestinal bleeding in cirrhosis: clinical and endoscopic correlations. Gut 17:37–40
Wilson SE, Stone RT, Christie JP, Passaro E Jr (1979) Massive lower gastrointestinal bleeding from intestinal varices. Arch Surg 114(10):1158–1161
Vernava M III, Moore BA, Longo WE, Johnson FE (1997) Lower gastrointestinal bleeding. Dis Colon Rectum 40:846–858
Gostout CJ, et al. (1992) Acute gastrointestinal bleeding experience of a specialized management team. J Clin Gastroenterol 14(3):260–267
Green BT, Rockey DC, Portwood G, et al. (2005) Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: a randomised control trial. Am J Gastroenterol 100(11):2395–2402
Stuber T, Hoffmann M, Stuber G, et al. (2009) Pitfalls in detection of acute gastrointestinal bleeding with multi-detector row helical CT. Abdom Imaging 34:476–482. doi:10.1007/s00261-008-9437-z
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steiner, K., Gollub, F., Stuart, S. et al. Acute gastrointestinal bleeding: CT angiography with multi-planar reformatting. Abdom Imaging 36, 115–125 (2011). https://doi.org/10.1007/s00261-010-9615-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-010-9615-7