Abstract
Endovascular repair (EVAR) is playing an increasingly role in the treatment of abdominal aortic aneurysm. A successful procedure depends on the complete sealing of the aneurysm sac from blood flow to achieve general pressure relief and avoid aneurysm rupture, with a shrinkage of the aneurysm sac. The most common complication of EVAR is endoleak that is the persistence of perigraft flow within the aneurysm sac, which has to be considered the major cause of enlargement and rupture of the aneurysm, and the main indication for surgical late conversion. For this reason, strict surveillance of these patients is mandatory for the early detection of endoleaks and the preferred method of follow-up is represented by CT angiography. However, CTA has limitations. The investigation is repeated several times, making radiation exposure a necessary concern. Therefore, it would be useful to have another reliable diagnostic examination during follow-up. Color duplex ultrasound is non-invasive, does not use radiation or contrast medium, is less expensive, easy to perform and widely available. However, this technique obtained poor results in terms of sensitivity in the detection of endoleaks. In the last years, the introduction of ultrasound contrast agents and contrast-specific imaging has, however, rekindled interest in this modality and its potential for replacing of CTA in routine surveillance. The purpose of this review is to highlight the diagnostic value of CEUS in the post-EVAR endoleaks detection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endovascular abdominal aortic aneurysm repair (EVAR) is today an accepted alternative to open surgery for selected patients with aortic pathology, offering reduced rates of perioperative mortality and procedure-associated complications, and overall reductions in the length of patient hospitalization [1–3]. However, despite these known excellent early results, many patients treated with EVAR require re-intervention during the middle and long-term follow-up due to complications related to the procedure. The most common complication is represented by endoleak, which is defined as the persistence of perigraft flow within the aneurysmal sac excluded by the stent-graft [3–7]; furthermore, it is the major cause of enlargement and rupture of the aneurysm, and the main indication for surgical late conversion [8, 9]. For this reason, strict surveillance of these patients is mandatory for the early detection of endoleaks, in order to determine the long-term performance of these devices [10]. The preferred method of follow-up is represented by multidetector-row computed tomography (MDCT) angiography, which allows the detection of endoleaks and other procedure-related complications with high sensitivity [4, 6, 11]. However, the most important disadvantage of CT is represented by the use of ionizing radiation. This feature is fundamental when dealing with patients who have to undergo 3 or 4 CT exams in the first year, e.g., before treatment, 1, 6 and 12 months after treatment, by using thinner sections and multiple phase acquisitions.
As reported by recent literature [12], and also suggested by the American College of Radiology, unnecessary radiation dose has to be reduced in diagnostic imaging. Therefore, it would be useful to have another reliable diagnostic examination during follow-up.
Color duplex ultrasound (CDUS) is non-invasive, does not use radiation, is less expensive, easy to perform and widely available. However, although the reliability of duplex ultrasound scanning for routine surveillance of abdominal arotic aneurysms (AAA) is well accepted, its accuracy and reliability in evaluating aneurysms after endovascular repair has not been well defined; furthermore, initial studies have limited success [13, 14]. The introduction of ultrasound contrast agents and contrast-specific imaging (CEUS) has, however, rekindled interest in this modality and its potential for replacing CTA in routine surveillance. Based on early published experience, the use of ultrasound contrast agents seem to increase sensitivity in ultrasound surveillance after EVAR by allowing improved blood flow echogenicity for better evaluation [15–19].
The purpose of this review is to highlight the diagnostic value of CEUS in the post-EVAR endoleaks detection.
CEUS imaging technique
The main drawbacks of US include operator dependence, with scan quality and scanning protocols varying greatly from one institution to another. As a matter of fact, standardization of scanning techniques as well as an accurate learning curve of the operator may reduce some of these variabilities.
Ultrasound contrast agents and contrast-specific imaging
Ultrasound contrast agents are characterized by the capacity to be modified by the process used to image them [20], creating contrast-specific signals, with the response depending on the insonation power, i.e., the amplitude of the acoustic pressure wave, indicated by the mechanical index (MI).
With the use of high acoustic powers (high MI) the microbubbles are destroyed producing specific signals, which are of very high intensity over a broad range of frequencies, not requiring contrast-specific imaging software but simply using the normal color Doppler mode.
As a matter of fact, initial CEUS studies were performed by using the first-generation agent SHU 508A (Levovist, Schering, Berlin, Germany) in combination with color Doppler or tissue harmonic imaging [18, 19, 21, 22]. SHU 508A comprised relatively fragile bubbles of air surrounded by a lipid shell containing palmitic acid and is best imaged with a high MI technique whereby the agent is destroyed as it is imaged. However, signals are only very transient, since the microbubbles are destroyed within a few milliseconds, not allowing a continuous assessment of structures (dynamic imaging).
With the use of a low acoustic powers (low MI) the microbubbles remain static, simply resonating when interrogated with sound, enhancing backscatter and thereby increasing the detected signal and thus blood pool contrast. The oscillation of the microbubbles results in the emission of specific sound waves, which can be detected by the transducer as contrast-specific signals [23].
Contrast-specific imaging, such as pulse inversion (ATL), phase inversion (Siemens), wideband harmonic (Siemens) or coherent contrast imaging (Acuson), provides cancellation and visualization of ultrasound contrast at maximum sensitivity. In detail, the resulting image has very little signal originating from background tissue and high-intensity depiction of echoes from microbubbles. This produces high image contrast between tissue and the microbubble agents within the blood pool.
In addition, movement and blooming artifacts are eliminated allowing real-time dynamic depiction of vessels at the resolutions afforded by gray-scale imaging [24–26].
Second-generation microbubble contrast agent consist of a perfluorocarbon gas surrounded by a phospholipid shell. When low MI techniques are used, they are relatively robust, allowing real-time detection without significant agent destruction. As a matter of fact, this combination allows to visualize a stent-graft in real time, from many different angles and over several minutes. This enables more practical and reproducible evaluation of these patients in a routine clinical setting. Furthermore, in case of doubts, microbubbles can be instantly destroyed by using a brief pulse of high-intensity (highMI) in order to detect/exclude the presence of subtle endoleaks.
Scanning technique
US scans are performed by using a last-generation scanner, equipped with a convex multifrequency 5–2 MHz probe, with contrast-specific software using a nonlinear imaging techniques with a low mechanical index of between 0.1 and 0.2.
Fasting patients are scanned in the supine or lateral position with the head slightly elevated at 10°, with grey scale and color Duplex before the intravenous contrast medium injection.
Both pre- and post-contrast US are performed by using transverse and longitudinal scans focused to the abdominal aorta including the origin of the renal arteries, the whole stent graft from the proximal to the distal anastomosis, including both prosthetic branches or, in the case of an aortic monoiliac prosthesis, only the prosthetic branch and the downstream iliac axis.
At our institution, CEUS is performed after administration of Sonovue (SonoVue®, Bracco, Milan, Italy), a second-generation contrast agent with flexible shells that allow real-time imaging at low acoustic pressure (mechanical index between 0.12 and 0.14). Sonovue is a microbubble preparation that is stable, relatively resistant to pressure, and specifically designed for use as a contrast agent for ultrasound imaging. Stored in the form of a lyophilisate, Sonovue can be reconstituted within seconds by addition of 0.9% saline solution followed by hand agitation. The resulting microbubble suspension is stable for 6 h at room temperature. The microbubble contains sulfur hexafluoride (SF6), a poorly soluble and totally innocuous gas which is eliminated through the lungs. Sonovue is isotonic, and its viscosity is similar to that of blood; furthermore, it does not contain protein-based materials.
The required or optimal dosage for administration of contrast agents after EVAR is not yet clearly defined [15–17]. Our recent published experience [27] performed comparing two different doses of CM (1.2 and 2.4 mL) with the attempt to define the optimal US CM dose, show that 2.4 mL is preferred to 1.2 mL, as it provides significantly better results in intensity and duration of contrast enhancement and, consequently, in visualization than the low dose.
As a matter of fact, we usually inject a bolus of 2.4 mL of CM, followed by flushing with an injection of a 5 mL bolus of saline solution through an 18–20 G cannula placed in an arm vein.
Scanning is started at the beginning of contrast agent injection and the sweep is usually completed within 5 min. The phases of CEUS are defined as arterial (10–40 s after contrast agent injection) and late (90–300 s after injection) phases. In case of uncertain enhancement/doubt diagnosis, a destruction–reperfusion technique can be used. For this technique, a brief pulse of high-intensity (high-mechanical-index) sound is used to confirm the presence of contrast material in an endoleak by its complete destruction. Immediately after this, reperfusion of the endoleaks can also be documented in real time [28].
The image data of CDUS and CEUS are recorded on videotapes in digital format for eventual subsequent analysis.
CEUS endoleak detection
On CEUS images, the endoleak appears as a high attenuation area beyond the graft but within the aneurysm sac, absent on the baseline unenhanced phase images, due to the presence of contrast enhancement. The evaluation is based on visual assessment, without attenuation measurements, with particular attention to the endoleaks wash-in (defined as the time between beginning of contrast material injection and contrast visualization within the aneurysm sac), endoleaks wash-out (defined as the time between beginning of contrast material injection and disappearance of all contrast from the sac), the origin of the endoleaks as well as the identification of inflow and outflow collateral vessels. The enhancement morphology of endoleaks should be either as “cavity filling” (defined as contrast concentration into a pseudocavity within the sac) or a simple diffuse spreading of contrast agent into the thrombus.
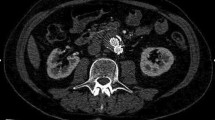
Recent literature [15–18, 21, 22, 28–30] demonstrated that the use of microbubble contrast agents significantly improves the capability of US to detect endoleaks, overcoming its limitations, principally due to echo reflection by the metallic portion of stent-graft, presence of calcifications, and slow endoleak flow, which does not allow distinction of color signals coming from vessel walls and surrounding tissue from those derived from corpuscular hematic components. In detail, in our recent experience performed on 84 consecutive patients treated with EVAR, CEUS significantly improved the visualization of all parts of the endoprosthesis (Fig. 1) as well as the diagnostic performance in endoleaks detection, with an obtained sensitivity and negative predictive value similar to MSCT angiography (97.5% and 97.3%, respectively) [27].
A 75-year-old woman treated with aortobiliac stent-graft implantation (6-month follow-up). CEUS allows an excellent visualization of the stent-graft, recognizing the presence of graft thrombosis (lines in C), as an intraluminal, parietal, non-enhanced area within the stent-graft, as confirmed by CT images (MIP-images: A, B).
Due to the longer duration of enhancement, lack of metallic artifacts and angio-dynamic evaluation of the leak during the dynamic phase, CEUS seems more specific than CTA in detection of small low-flow endoleaks. In the study of Napoli et al. [17], CEUS was able to detect low-flow endoleaks, confirmed with angiography, in all patients with enlarging aneurysm sac classified as endotension (expansion of the aneurysm without the presence of a detected endoleaks) on the basis of triple phase contrast enhanced CT. Bendick et al. [19] also reported two similar cases of endoleaks occult to CTA and detected with CEUS. These authors suggested CTA failure may have resulted from shorter imaging duration than with CEUS supporting the hypothesis that endotension represents a missed or undiagnosed endoleak characterized by a very slow flow rather than true aneurysm expansion in the absence of perigraft flow (Fig. 2). On the other hand, CEUS could also allow an easier identification of small endoleaks with simple diffuse spreading of contrast agent into the thrombus in which the lack of concentration in a defined and confined region of the sac could reduce CTA detection capability (Fig. 3).
An 85-year-old woman treated with EVAR with increase in size of the aneurysm sac in comparison with previous CT exam. No endoleak was detected on both arterial (A) and 60-s delayed (B) phase, with a consequent diagnosis of endotension. However, a small-sized endoleak was detected on contrast-enhanced ultrasonography (D) (arrows). This low-flow endoleak was confirmed by a delayed phase image performed 300 s after contrast medium injection (C) (arrows), justifying the increased aneurysmal sac and excluding the previous diagnosis of endotension.
An 84-year-old man treated with EVAR (12 month follow-up). CEUS allows an easier visualization of a small endoleak with diffuse spreading of contrast agent into the thrombus (arrows in B) in which the lack of concentration in a defined and confined region of the sac could reduce CTA detection capability (arrows in A).
We would like to underline that in order to reduce the false positive diagnosis, an accurate baseline US examination before contrast medium injection has to be performed, mainly to assess the morphology of the aneurysmal sac. As a matter of fact, at the beginning of our experience, three false-positive diagnoses were performed, due to a baseline high attenuation of the thrombus not completely recognized on baseline US (Fig. 4).
A 78-year-old woman treated with EVAR (12-month follow-up). In CEUS image (A) a high attenuation area is present outside the graft but within the aneurysm sac: a diagnosis of endoleak was performed. However, no endoleak is detected on both axial arterial (B) and delayed (C) phase CT images. An accurate evaluation of baseline CDU (D) image allows recognition of a high attenuation of the thrombus outside the stent-graft lumen excluding the previous false-positive endoleak.
CEUS endoleak classification
The presence of endoleaks means that the attempt to exclude the aneurysmal sac has failed, but it does not necessarily imply that the procedure itself has failed. As a matter of fact, although the presence of endoleaks may cause the enlargement of the aneurysmal sac with possible caudal migration of the prosthesis or rupture of the aneurysm, a spontaneous solution may also occur. To understand which endoleak needs to be promptly treated, we should consider the following classification based on the etiology of endoleaks, as proposed by White et al. [31–33].
Type I endoleak is due to an incomplete attachment of the proximal and/or distal end of the prosthesis to the aortic walls, due to technical (e.g., suboptimal stent-graft diameter) or anatomical (e.g., short, irregular, ulcerated or angulated proximal neck) problems, or to its caudal migration. On CEUS images, it usually appears as a huge high-flow leak, synchronous with respect to graft enhancement, spreading from the proximal or distal end of the prosthesis into the thrombus with a cranial or caudal direction, respectively (Fig. 5).
Type II endoleak is due to a retrograde filling via aortic collateral arteries such as lumbar arteries, inferior mesenteric artery, or hypogastric artery, if covered. On CEUS images, type II endoleak is most pronounced at the periphery of the aneurysmal sac, with no or a little delayed contact with prosthesis, commonly located in a posterior or lateral position and usually associated with opacification of collateral arteries. Furthermore, CEUS allows an angiodynamic visualization of the agent flow into the aneurysm sac, recognizing its direction and easier differentiating type II (directed from the periphery to the graft) from type III (directed from the graft to the periphery) endoleaks (Fig. 6). With respect to the time of appearance, it is classified as hyperdynamic (Fig. 7) or hypodynamic (Fig. 8) endoleak if wash-in is less or higher than 100 s, respectively [16]. As a matter of fact, type II endoleak is never synchronous with respect to graft enhancement; this characteristic allows an easier differentiation between type I and type II endoleaks.
A 71-year-old man treated with EVAR (1-month follow-up). 3D (A, C) and axial (B) CT images demonstrate a large endoleak located in a postero-lateral position, associated with opacification of a lumbar artery (C), classified as a type II endoleak. However, the leak is also strictly adjacent to the prosthesis, with a consequent possible diagnosis of a concomitant type III endoleak. A clear classification of the endoleak was not performed on the basis of CT images. Dynamic CEUS images (D) demonstrate the back-filling of the excluded aneurysmal sac via lumbar artery, excluding a concomitant type III endoleak, as confirmed by DSA (E–G, arrows in F and asterisk in G).
Type III endoleak arises from a defect of the stent-graft membrane itself, or from modular or graft disconnection; this latter is more likely when multiple prostheses with short overlapping areas are used. On CEUS images, the leak is strictly adjacent to prosthesis, with a delayed contact with margins of the aneurysmal sac, without opacification of collateral arteries. These endoleaks are more frequently synchronous with respect to graft enhancement; as previously described, the opposite direction of the contrast flow allows a differentiation with type II endoleaks (Fig. 6).
Type IV endoleak is due to a porosity of the prosthesis and is usually only detected on conventional angiograms performed at the end of the procedure or in the first week after procedure. On CEUS images, it is similar to a diffuse type III endoleak, involving all the stent-graft; the diagnosis of a type IV endoleaks should be one of exclusion.
Type I and type III endoleaks may cause a substantial increase in aneurysm size putting patients at high risk for aortic rupture, therefore needing an early re-intervention. However, the most common endoleak founded in endovascular stent-grafting is type II endoleak. The treatment of these endoleaks is a source of continuing discussion and debate: based on literature, type II endoleaks require treatment only if associated with an increase in size of the sac.
Bargellini et al. [16] suggested that CEUS could represent a valid tool in the decision making and treatment planning of type-II endoleaks, showing different hemodynamic features which might influence rate of aneurysm enlargement, addressing the need for treatment. In detail, they found that a volume increase might be associated with hypodynamic type II leaks, characterized by a slow wash-in and wash-out. They supposed that these hypodynamic endoleaks are not able to create a way out of the sac, thus causing aneurysm pressurization and progressive enlargement. On the contrary, fast wash-in and wash-out could be interpreted as rapid flow going through the sac without significant effects on pressure and aneurysm increase; in these cases, treatment could be avoided.
CEUS limitations
CEUS also has some limitations: patient habitus (obesity) and bowel gas can interfere with imaging and the patient must cooperate. Moreover, sonographic examination results are operator-dependent and obtaining quality images requires training and specific skills.
As reported in our published experience [27], it is mandatory to underline that the “low permeability” design Gore Excluder Endoprosthesis cannot be adequately studied with sonographic imaging (US as well as CEUS) at 1-month follow-up. As a matter of fact, this innovative device, introduced in the 2002, is composed of a durable, reinforced ePTFE graft, low permeability material layer, electropolished nitinol stent, and bonding film for stent to graft attachment. Its unique graft design reduces the potential for serous fluid movement through the graft wall with consequent endotension. However, the ePTFE graft material produces significant artifacts with echo reflection at 1-month follow-up (Fig. 9), not allowing a possible stent-graft evaluation as well as endoleaks detection; these artifacts usually disappear at 6-month follow-up.
An 81-year-old woman treated with the implantation of a “low permeability” design Gore Excluder Endoprosthesis (1-month follow-up). At 1-month follow-up (A, B), the ePTFE graft material produces significant artifacts with echo reflection not allowing an adequate stent-graft evaluation as well as endoleaks detection; these artifacts usually disappear at 6-month follow-up.
Finally, in the post-EVAR follow-up, CEUS cannot replace CT-angiography in providing information related to graft anchoring and integrity, aneurysm morphologic changes or visceral vessel patency (renal arteries).
Conclusions
In conclusion, CEUS is a fast, non-invasive, reliable and valid alternative to MSCT angiography for endoleak detection in endovascular aortic stent graft patients.
However, based on its limitations, in the post-EVAR follow-up, CEUS should replace CTA at 6-month follow-up and annually thereafter. In fact, in our opinion, the rationale of post-EVAR follow-up at 1-month and 12-month follow-up should be to detect endoleaks as well as to evaluate intra/peri-procedural complications (related to stent-graft anchoring and visceral vessels patency) and post-procedural complications (related to stent-graft migration/integrity, visceral vessels patency and aneurysm morphologic changes), respectively.
On the basis of this opinion, our suggested post-EVAR follow up is based on CTA at 1 and 12 months after EVAR with CEUS performed at 6 months and annually thereafter, if no complications are detected.
References
Bush RL, Lumsden AB, Dodson TF, et al. Mid-term results after endovascular repair of abdominal aortic aneurysm. J Vasc Surg 2001;33(2 Suppl):S70-76.
May J, White GH, Waugh R, et al. Comparison of first- and second-generation prostheses for endoluminal repair of abdominal aortic aneurysms: a 6-year study with life table analysis. J Vasc Surg 2000;32:124-129.
Zarins CK, White RA, Hodgson KJ, Schwarten D, Fogarty TJ. Endoleak as a predictor of outcome after endovascular aneurysm repair: AneuRx multicenter clinical trial. J Vasc Surg 2000;32:90-107.
Cuypers P, Buth J, Harris PL, Gevers E, Lahey R. Realistic expectations for patients with stent-graft treatment of abdominal aortic aneurysms. Results of a European multicentre registry. Eur J Vasc Endovasc Surg 1999; 17: 507–516.
Gilling-Smith G, Brennan J, Harris P, et al. Endotension after endovascular aneurysm repair: definition, classification, and strategies for surveillance and intervention. J Endovasc Surg 1999; 6: 305–307.
Golzarian J, Struyven J, Abada HT et al. Endovascular aortic stent-grafts: transcatheter embolization of persistent perigraft leaks. Radiology 1997; 202: 731–734.
Gorich J, Rilinger N, Sokiranski R et al. Leakages after endovascular repair of aortic aneurysms: classification based on findings at CT, angiography, and radiography. Radiology 1999; 213: 767–772.
Lumsden AB, Allen RC, Chaikof EL, et al. Delayed rupture of aortic aneurysms following endovascular stent grafting. Am J Surg 1995; 170: 174–178.
Vallabhaneni SR, Harris PL. Lessons learnt from the EUROSTAR registry on endovascular repair of abdominal aneurysm repair. Eur J Radiol 2001; 39: 34–41.
Bown M.J. Fishwick G, et al. (2004) The postoperative complications of endovascular aortic aneurysm repair. J Cardiovascular Surg 45:335-47.
Golzarian J, Dussaussois L, Abada HT, et al. Helical CT of aorta after endoluminal stent-graft therapy: value of biphasic acquisition. AJR Am J Roentgenol 1998;171:329-331.
Brenner DJ, Hall EJ. Computer Tomography – An increasing Source of Radiation Exposure. N Engl J Med 2007; 357(22): 2277-84.
Raman KG, Missig-Carrol N, Richardson T, Muluk SC, Makaroun MS. Color-flow duplex ultrasound versus computed tomographic scan in the surveillance of endovascular aneurysm repair. J Vasc Surg 2003;38:645-51.
Sato DT, Goff CD, Gregory RT, et al. Endoleak after aortic stent graft repair: diagnosis by color duplex ultrasound scan versus computed tomography scan. J Vasc Surg 1998;28:657-663.
Henao EA, Hodge MD, Felkai DD, et al. Contrast-enhanced duplex surveillance after endovascular abdominal aortic aneurysm repair: improved efficacy using a continuous infusion technique. J Vasc Surg 2006;43:259-264.
Bargellini I, Napoli V, Petruzzi P, et al. Type II lumbar endoleaks: hemodynamic differentiation by contrast-enhanced ultrasound scanning and influence on aneurysm enlargement after endovascular aneurysm repair. J Vasc Surg 2005;41:10-18.
Napoli V, Bargellini I, Sardella SG, et al. Abdominal aortic aneurysm: contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology 2004;233:217-225.
Giannoni MF, Palombo G, Sbarigia E, et al. Contrast-enhanced ultrasound imaging for aortic stent-graft surveillance. J Endovasc Ther 2003;10:208-217.
Bendick PJ, Bove PG, Long GW, et al. Efficacy of ultrasound scan contrast agents in the noninvasive follow-up of aortic stent grafts. J Vasc Surg 2003;37(2):381-385.
Wei K, Skyba DM, Firschke C, et al. Interactions between microbubbles and ultrasound: in vitro and in vivo observations. J Am Coll Cardiol.1997; 29(5):1081-8.
McWilliams RG, Martin JM, White D, et al. Detection of endoleak with enhanced ultrasound imaging: comparison with biphasic computed tomography. J Endovasc Ther 2002;9:170-179.
McWilliams RG, Martin JM, White D, et al. Use of contrast-enhanced ultrasound in follow-up after endovascular aortic aneurysm repair. J Vasc Interv Radiol 1999;10:1107-1114.
Greis C. Technology overview: SonoVue (Bracco, Milan). Eur Radiol Suppl (2004) 14[suppl 8]:P11–P15.
Brannigan M, Burns PN, Wilson SR. Blood flow patterns in focal liver lesions at microbubble-enhanced US. Radiographics. 2004;24(4):921–935.
Dill-Macky MJ, Burns PN, Khalili K, et al. Focal hepatic masses: enhancement patterns with SHU 508A and pulse-inversion US. Radiology 2002;222:95–102.
Wilson SR, Burns PN. Liver mass evaluation with ultrasound: the impact of microbubble contrast agents and pulse inversion imaging. Semin Liver Dis. 2001;21(2):147–159.
Iezzi R, Basilico R, Giancristofaro D, et al. Contrast-enhanced ultrasound versus color duplex ultrasound imaging in the follow-up of patients after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2009;49(3):552-60.
Dill-Macky MJ, Wilson SR, Sternbach Y, Kachura J, Lindsay T. Detecting endoleaks in aortic endografts using contrast-enhanced sonography. AJR Am J Roentgenol. 2007;188(3):W262-8.
Giannoni MF, Fanelli F, Citone M, et al. Contrast ultrasound imaging: the best method to detect type II endoleak during endovascular aneurysm repair follow-up. Interact Cardiovasc Thorac Surg 2007;6:359-362.
Carrafiello G, Laganà D, Recaldini C, et al. Comparison of contrast-enhanced ultrasound and computed tomography in classifying endoleaks after endovascular treatment of abdominal aorta aneurysms: preliminary experience. Cardiovasc Intervent Radiol 2006;29:969-974.
White GH, Yu W, May J, Chaufour X, Stephen MS. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis, and management. J Endovasc Surg 1997;4:152-168.
White GH, May J, Waugh RC, Chaufour X, Yu W. Type III and type IV endoleak: toward a complete definition of blood flow in the sac after endoluminal AAA repair. J Endovasc Surg 1998;5:305-309.
White GH, May J, Petrasek P, et al. Endotension: an explanation for continued AAA growth after successful endoluminal repair. J Endovasc Surg 1999;6:308-315.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iezzi, R., Cotroneo, A.R., Basilico, R. et al. Endoleaks after endovascular repair of abdominal aortic aneurysm: value of CEUS. Abdom Imaging 35, 106–114 (2010). https://doi.org/10.1007/s00261-009-9526-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-009-9526-7